Abstract
The in vitro isolation and analysis of pancreatic stem/progenitor cells are necessary for understanding their properties and function; however, the preparation of high-quality single-cell suspensions from adult pancreas is prerequisite. In this study, we applied a cold trypsin-ethylenediaminetetraacetic acid (EDTA) digestion method to disassociate adult mouse pancreata into single cells. The yield of single cells and the viability of the harvested cells were much higher than those obtained via the two commonly used warm digestion methods. Flow cytometric analysis showed that the ratio of ductal or BCRP1-positive cells in cell suspensions prepared through cold digestion was consistent with that found in vivo. Cell culture tests showed that pancreatic epithelial cells prepared by cold digestion maintained proliferative capacity comparable to those derived from warm collagenase digestion. These results indicate that cold trypsin-EDTA digestion can effectively disassociate an adult mouse pancreas into viable single cells with minimal cell loss, and can be used for the isolation and analysis of pancreatic stem/progenitor cells.
Keywords: Mouse, Pancreas, Disassociation, Single cells, Viability, Cold digestion
1. Introduction
The pancreas is a mixed gland with both endocrine and exocrine portions. Endocrine islets are composed of α, β, δ, ε, and pancreatic polypeptide (PP) cells, whereas the exocrine acini secrete digestive enzymes into pancreatic ducts, which drain into the duodenum to digest food. Cell lineage tracing experiments have shown that all endocrine and exocrine lineage cells originate from a common population of stem/progenitor cells during early pancreatic development (Donath and Halban, 2004; Rhodes, 2005). With the growth of an individual, a small population of pancreatic stem/progenitor cells with high proliferative capability and multipotential remains in postnatal and adult pancreata, which function in pancreatic growth and tissue maintenance. Isolation and analysis of adult mouse pancreatic stem/progenitor cells provide a useful model for the discovery of molecular mechanisms underlying proliferation and differentiation of pancreatic stem/progenitor cells and their usage in cell-replacement therapy for type 1 diabetes (Shapiro et al., 2000; Stock and Bluestone, 2004).
Pancreatic stem/progenitor cells are usually enriched from pancreatic single-cell suspensions through flow cytometry using specific markers (Bonner-Weir et al., 2004; Dor et al., 2004; Baeyens et al., 2005; Hao et al., 2006; Xu et al., 2008; Chung et al., 2010). Currently, common procedures for preparation of single-cell suspensions from adult mouse pancreata include subjecting the excised tissue to digestive fluid supplemented with collagenase alone or a combination of collagenase and trypsin at 37 °C, followed by mechanical shearing (Kayali et al., 2003; Suzuki et al., 2004; Inada et al., 2008). Collagenase provides gentle, selective digestion of the intercellular matrix, but is unable to completely disassociate organ tissue into single cells (Gross et al., 1974). Trypsin can disassociate tissue fragments at 37 °C more effectively than collagenase, but can also activate pancreatic zymogens and induce widespread tissue damage and impair viability (Amsterdam and Jamieson, 1974; Ji et al., 2009). These features of warm collagenase and/or trypsin digestion of the adult pancreas cause instability of cell yield and viability, which hampers the isolation and analysis of pancreatic stem/progenitor cells.
Several previous studies reported that the digestion of tissues at temperatures less than 37 °C could improve the viability and multiplication of single cells (Cole and Paul, 1966; McKeehan, 1977; Dono et al., 1992; 1994). Cole and Paul (1966) showed that after liver fragments were soaked in cold trypsin overnight, liver tissue could be completely disassociated into high-viability single-cell populations. Based on their methodology, we prepared pancreatic single-cell suspensions through cold trypsin-ethylenediaminetetraacetic acid (EDTA) digestion of adult mouse pancreas, and compared the effect of cold trypsin-EDTA digestion with that of warm collagenase digestion or combined warm digestion with both collagenase and trypsin-EDTA.
2. Materials and methods
2.1. Animals and preparation of pancreatic tissue fragments
CD-1 strain mice at 4–8 weeks of age were purchased from the Experimental Animals Center at the Medical College of Jilin University (Changchun, China) and were maintained in compliance with the protocols established and approved by the Animal Care and Ethics Committee of Northeast Forestry University (Harbin, China). Pancreata from mice were removed carefully and weighed. Fat, blood vessels, mesentery, and lymph nodes were carefully isolated with pointed tweezers under a dissecting microscope. The pancreatic tissues were cut into 1-mm³ fragments and washed with phosphate-buffered saline (PBS).
2.2. Warm digestion with collagenase IV alone or with both collagenase IV and trypsin-EDTA
For warm digestion with collagenase IV, pancreatic tissue fragments were incubated in 0.8 mg/ml collagenase IV (Invitrogen, Carlsbad, California, USA) and DNase I (to a final concentration of 10 μg/ml; Roche, Basel, Switzerland) at 37 °C for 20 min and were gently pipetted every 5 min. At the conclusion of the digestion period, the fragments were gently pipetted and washed to maximize the release of single cells. The single-cell suspension was transferred to a new tube and centrifuged for 5 min at 800–1 000 r/min. The supernatant, upper cell debris, DNA floc winding, and the middle layer of blood cells were discarded. The milky white cells in the lower layer were resuspended in PBS and subjected to cell viability analysis and cell counting.
For warm digestion with a combination of collagenase IV and trypsin-EDTA, the tissue fragments were first subjected to warm collagenase digestion. After the single-cell suspension was removed, the remaining fragments were incubated with 0.25% trypsin-EDTA (2.5 g/L trypsin, 1 g/L EDTA) at 37 °C for 5 min. After gentle pipetting and washing, the single-cell suspension was combined with that isolated from warm collagenase digestion, and then centrifuged and resuspended in PBS.
2.3. Cold trypsin-EDTA digestion
Tissue fragments were soaked for 6–24 h in 0.25% trypsin-EDTA pre-cooled at 4 °C. After the upper enzymatic fluid was removed, the digestion was continued for 10 min with residual trypsin-EDTA and DNase I (to a final concentration of 10 μg/ml) in 2 ml of Dulbecco’s modified Eagle medium (DMEM) preheated to 37 °C. The digestion was terminated by adding DMEM medium containing 5% fetal bovine serum (FBS), and the fragments were gently pipetted and washed several times to maximize the release of single cells. The single cells were collected as described above. The percentage of the digested tissue was calculated by substracting from 100% the percentage of the undigested (the weight of the undispersed portion divided by that of the total pancreatic tissues).
2.4. Cell viability staining and cell counting
Cell density was adjusted to 1×106 cells/ml, and then 0.1 ml of 0.4% trypan blue solution (4 g/L) was added to 0.9 ml of the cell suspension in a sterile tube and mixed thoroughly. The numbers of living and dead cells were microscopically counted and recorded. At least 500 cells were counted per sample, and the results were calculated from at least three independent experiments.
2.5. Fluorescence-activated cell sorting (FACS) analysis
Single cells isolated from the cold trypsin-EDTA digestion method were rinsed with washing buffer (PBS containing 5 g/L bovine serum albumin (BSA)). The isolated cells were then incubated with the appropriate primary antibody for 20 min. The primary antibodies that were used included biotin-DBA (Jackson Immuno Research, West Grove, PA, USA; 1:2 500) and rat anti-BCRP1 (Novus Biologicals, Littleton, Colorado, USA; 1:20). Rat anti-IsoR antibody served as an isotype control. The cells were then washed twice and incubated with secondary antibodies (cy5-conjugated anti-rat IgG, 1:200; cy5-conjugated streptavidin, 1:250) for 20 min. Each sample was washed and suspended in 1 ml of washing buffer and then analyzed using an FACS Vantage SE flow cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA). The data were analyzed with CELLQuest software (BD Biosciences, Franklin Lakes, New Jersey, USA).
2.6. Immunofluorescent staining
The isolated or cultured cells were fixed in 0.04 g/ml polyformamide for 20 min, and then treated with 0.3% Triton X-100 for 40 min. Having been blocked with 5% normal animal serum and 5 g/L bovine serum albumin, the cells were incubated with appropriate primary antibodies (rabbit anti-amylase (Sigma Aldrich, 1:500), rabbit anti-insulin (Santa Cruz, 1:100), rabbit anti-E-cadherin (Santa Cruz, 1:50), or rabbit anti-vimentin (Boaoshen, China, 1:100), for 60 min. The cells were then washed and incubated with secondary antibodies (fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG, 1:200; TRITC-conjugated anti-rabbit IgG, 1:200) for 60 min. Results were observed with a fluorescence microscope (Axiovert 200FL, Carl Zeiss) and photographed with a cool charge coupled device (CCD).
2.7. Cell cultivation
Cells isolated using either the cold trypsin-EDTA or warm collagenase digestion methods were seeded at a density of 5×105 cells/ml in a 35-mm plastic culture dish containing DMEM supplemented with 10% FBS and incubated at 37 °C in an atmosphere of 5% CO2 and 95% air. After 40 min of incubation, most of the attached cells were identified as fibroblasts by immunofluorescent staining using the antibody against vimentin, and the unattached cells were transferred into new dishes. Following an overnight incubation, the attached cells were given proliferative media consisting of DMEM/F12 supplemented with 2% FBS, 1×B27 (Invitrogen, Carlsbad, California, USA), 10 ng/ml epidermal growth factor (EGF; R&D, Minneapolis, Minnesota, USA), 10 μg/ml insulin (Sigma-Aldrich, St. Louis, Missouri, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. The cultured cells were subsequently observed under a phase contrast microscope. The cell proliferation capacity was evaluated as follows: 1.5×104 cells were seeded in a 6-cm dish. After exclusion of unattached cells and fibroblasts by twice changing dishes, the attached cells (fewer than 300 cells/cm2) were cultured in proliferation media. The cell colonies formed through proliferation were counted after 7–10 d. The colony-forming assay was replicated three times.
2.8. Statistical analysis
All results were reported as mean±standard deviation (SD). The level of significance was determined by one-way analysis of variance (ANOVA) using SPSS Statistics 17.0. P values less than 0.05 were considered statistically significant.
3. Results and discussion
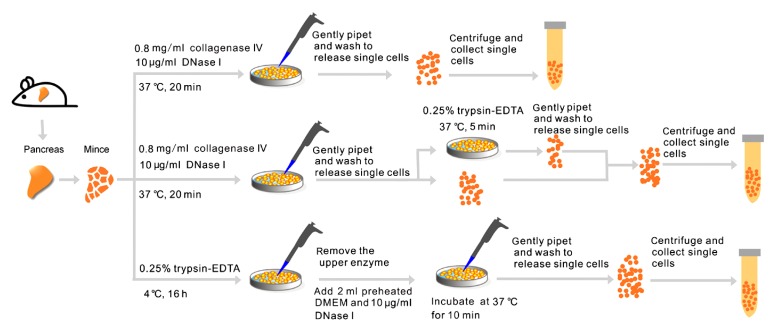
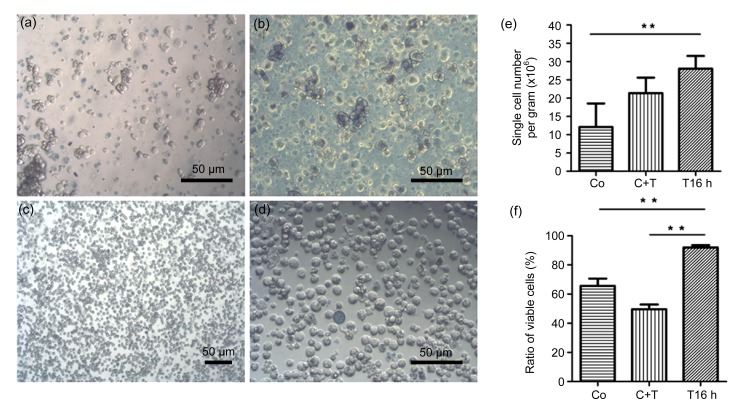
In this study, mouse pancreatic fragments were subjected to warm digestion with collagenase, combined warm digestion with both collagenase and trypsin-EDTA, or cold digestion with trypsin-EDTA (Fig. 1). In the warm collagenase digestion group, having been incubated in collagenase IV at 37 °C for 20 min, pancreatic fragments were relatively less dispersed, and only a few single cells were released. After being pipetted and washed 8–10 times, 29.4% of tissues were still not dispersed, and the cell suspension consisted primarily of clumps of loosely associated cells and several single cells. The single-cell yield was (1.21±0.65)×107 per gram of pancreatic tissue (n=5), in which the ratio of viable cells to total single cells obtained was (65.66±4.96)%. Cell clusters, debris, and dead cells were abundant in the cell suspension (Fig. 2a). This result coincided with that of a previous report in which cell viability was reduced to 70% after adult mouse pancreata underwent warm digestion with 1.5 g/L collagenase (Suzuki et al., 2004).
Fig. 1.
Scheme for the isolation of single cells from adult mouse pancreas through warm collagenase digestion, warm digestion with combined collagenase and trypsin-EDTA, or cold digestion with trypsin-EDTA
Fig. 2.
Comparison of the single-cell suspensions prepared by warm collagenase digestion, warm digestion with combined collagenase and trypsin-EDTA, or cold digestion with trypsin-EDTA
(a) The cell suspension prepared by warm collagenase digestion; (b) The cell suspension prepared by warm digestion with combined collagenase and trypsin-EDTA; (c, d) The cell suspension prepared via cold digestion with trypsin-EDTA; (e) Yields of single cells prepared by warm collagenase digestion (Co), warm digestion with combined collagenase and trypsin-EDTA (C+T), or cold digestion with trypsin-EDTA for 16 h (T16 h) (mean±SD, n=5; ** P<0.01); (f) The ratio of viable single cells to total single cells in suspensions prepared by warm collagenase digestion (Co), warm digestion with combined collagenase and trypsin-EDTA (C+T), or cold digestion with trypsin-EDTA for 16 h (T16 h) (mean±SD, n=5; ** P<0.01)
For the warm digestion with a combination of collagenase IV and trypsin-EDTA group, pancreas fragments were first subjected to warm digestion with collagenase IV for 20 min, and the residual tissues were subsequently digested with trypsin-EDTA at 37 °C for 5 min (Fig. 1). The majority of tissues (81.24%) were completely digested, and (2.14±0.42)×107 single cells were obtained from each gram of pancreatic tissue (n=5). The ratio of viable cells to total single cells was (49.60±3.22)%. A large amount of cell debris was also observed in the cell suspension for this experimental group (Fig. 2b).
Pancreatic fragments in the cold trypsin-EDTA digestion group were incubated in trypsin-EDTA at 4 °C for 16 h and then digested in residual trypsin-EDTA fluid (with 10 μg/ml DNase I) at 37 °C for 10 min (Fig. 1). During the incubation at 4 °C, few single cells were liberated, though the tissues were loosened and took on a milky white appearance. Following digestion at 37 °C and mechanical blowing, 93.08% of pancreatic tissues were completely disassociated with very little aggregation and adhesion (Figs. 2c and 2d), with the exception of a few remaining tubular-like tissues that were identified as vascular tubes following microscopic evaluation. Although a few 2–3-cell clusters remained, the proportion of single cells in the suspension was over 95%. The single-cell yield was (2.81±0.35)×107 cells per gram of tissue (n=5), and the cell viability was (91.99±1.59)%.
From the comparison of the three digestion methods for isolating single cells from adult mouse pancreata, the yield and viability of single cells prepared through cold trypsin-EDTA digestion were significantly higher than those obtained via warm enzymatic digestion in this (Figs. 2e and 2f) and other studies (Suzuki et al., 2004; Kikugawa et al., 2009). Moreover, the yield and ratio of viable cells in the cold trypsin-EDTA digestion group were consistent throughout the course of multiple repeated tests (n>10).
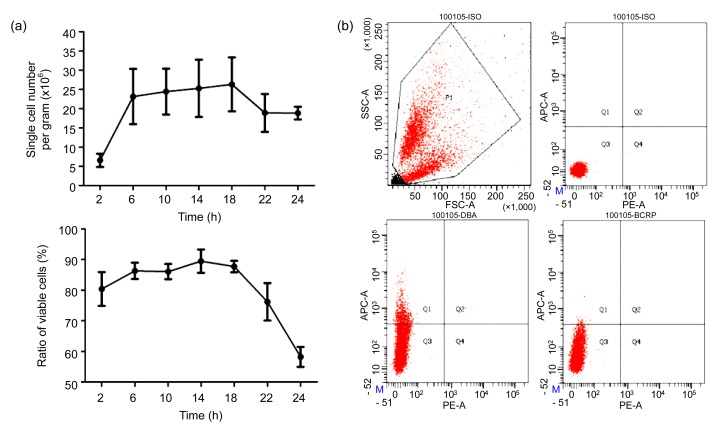
To determine the optimum incubation time, pancreatic fragments were incubated in the trypsin-EDTA fluid for 2–24 h at 4 °C. The cell yield and viability were tested at 4-h intervals. When incubated in cold trypsin-EDTA for 2 h at 4 °C, the pancreatic fragments were not completely disassociated, and the cell yield was only (0.66±0.17)×107 per gram of tissue, although the percentage of viable cells was still (80.38±5.53)% (n=3). Once the fragments were incubated in cold trypsin-EDTA for longer than 6 h, the cell yield was significantly increased, and the cell viability remained high (Fig. 3a). However, when the incubation lasted 22 h, the cell yield and ratio of viable cells decreased significantly (Fig. 3a). The percentage of dead cells was more than 40% after pancreatic tissue was incubated in cold trypsin-EDTA for over 24 h. Incubation of tissue fragments in trypsin-EDTA for 14–18 h was optimal for the cold trypsin-EDTA digestion of adult mouse pancreas.
Fig. 3.
Effect of incubation time on cell yield and viability in cold trypsin-EDTA digestion and flow cytometry analysis of single cells
(a) Yields of single cells and ratios of viable single cells to total single cells after incubation of pancreas fragments in trypsin-EDTA fluid for 2, 6, 10, 14, 18, 22, or 24 h at 4 °C (n=5). (b) Flow cytometry analysis of the percentages of the whole cell population (upper left), isotype control (upper right), and ductal (lower left), and BCRP1-positive (lower right) cells in single-cell suspensions prepared by cold trypsin-EDTA digestion for 16 h
To evaluate whether the single-cell suspension prepared by cold-trypsin digestion was suitable for fluorescence-activated cell analysis and sorting, we analyzed these cells via flow cytometry with a biotin-conjugated dolichos biflorus agglutinin (DBA), which specifically labels ductal cells. The results indicate that little cell clusters existed in the cell suspension, and the DBA-positive cells comprised 11.2% of the total single-cell population (Fig. 3b), which was consistent with a previous report (Githens, 1988). BCRP1 is a multidrug-resistance protein present on the membrane of side population cells, and considered to be a potential marker of multipotent stem cells from various adult sources (Zhou et al., 2001). FACS analysis showed that 0.2% of pancreatic cells were BCRP1-positive (Fig. 3b), which is consistent with the analysis of side population cells in the human pancreas (Poliakova et al., 2004). The other cell types present in the cell population after cold trypsin-EDTA digestion were analyzed through immunofluorescent staining. A total of 87.5% of the isolated cells were amylase-positive acinar cells, whereas 0.7% of cells were insulin-positive β cells. These results indicate that the cell suspension prepared by cold trypsin-EDTA digestion retained a cell composition similar to that seen in vivo.
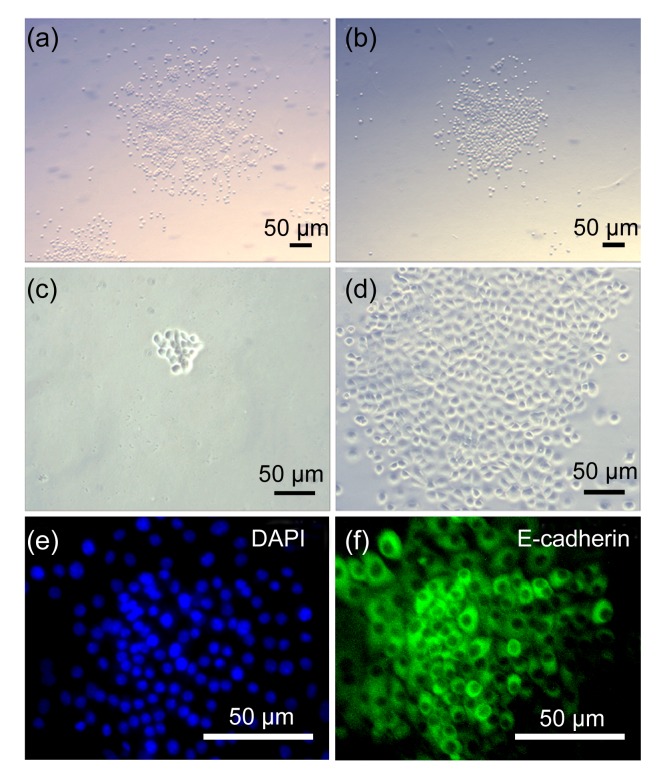
Pancreatic tissue originates from primitive gut endoderm during early embryonic development; therefore, all pancreatic parenchymal cells are derived from endoderm epithelium. To explore the effect of cold trypsin-EDTA digestion on the proliferative capability of pancreatic epithelial cells, the cells isolated through cold trypsin-EDTA or warm collagenase digestion were cultured in proliferative medium. The fibroblasts were removed through isolation of attached cells 40 min after initial seeding. The cells attached at a low density (<300 cells/cm2) and grew quickly to form over 60 colonies of 100 to 300 E-cadherin-positive epithelial cells after 7 d (Figs. 4a, 4c–4f). The proliferation and colony-formation capacities of the cells derived from cold trypsin-EDTA digestion were similar to those of cells derived from warm collagenase digestion (Fig. 4b). Combinatorial digestion with collagenase and trypsin at 37 °C also resulted in a small number of pancreatic single cells; however, this technique was not amenable to flow cytometric analysis of single cell events because of the large degree of cell loss and the abundance of cell clumps in the heterogeneous suspension, which led to distorted results.
Fig. 4.
Proliferation capacity of epithelial cells isolated by cold trypsin-EDTA digestion
Colonies grown from the isolated cells through cold trypsin-EDTA digestion (a) were similar to those grown from the isolated cells through warm collagenase digestion (b). Single cells isolated through cold trypsin-EDTA digestion formed a colony after being cultured for 5 d (c) or 10 d (d); The colonial cells (e) were E-cadherin-positive (f) epithelial cells identified by immunofluorescent staining
The cold trypsin digestion method was first described by Cole and Paul (1966) in disassociation of fetal liver, which produced single-cell suspensions of 80%–90% viability at all stages of liver development studied. In our study, the method was used to disassociate adult pancreata into single cells and acquired over 90% viability through minor modifications. First, the chelating agent EDTA was used in combination with trypsin to disassociate adult pancreatic tissues. EDTA treatment was found to remove some of the connective tissue and basement membrane and to increase the permeability of the tissue to the solutions in the subsequent steps (Oliver, 1980). Immersion of pancreatic fragments in the 0.25% trypsin fluid with EDTA at 4 °C ensured that the trypsin could infiltrate tissues and reach deeply into the extracellular matrix surrounding cells. Second, DNase I was added during the following warm incubation process, which served to degrade DNA released by broken cells, and thereby minimized the loss of cell viability (Seglen, 1976).
4. Conclusions
Our study demonstrates that digestion of adult mouse pancreas tissues via a cold trypsin-EDTA method can yield a large quantity of single cells with minimal cell debris and cluster, while preserving the integrity and viability of single pancreatic cells and retaining a cell composition similar to that observed in vivo. Therefore, this method is suitable for preparation of single-cell suspensions and may be useful for the characterization, enrichment, and isolation of pancreatic stem/progenitor cells and other pancreatic cells in future studies.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31272520) and the Special Fund for Scientific and Technological Innovation Talents in Harbin, China (No. 2012RFXXS048)
Compliance with ethics guidelines: Dan LI, Shi-yun PENG, Zhen-wu ZHANG, Rui-cheng FENG, Lu LI, Jie LIANG, Sheng TAI, and Chun-bo TENG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Amsterdam A, Jamieson JD. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeyens L, de Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing β cells from adult exocrine pancreatic cells. Diabetologia. 2005;48(1):49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5(S2):16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28(9):1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 5.Cole RJ, Paul J. The effects of erythropoietin on haem synthesis in mouse yolk sac and cultured foetal liver cells. J Embryol Exp Morphol. 1966;15(2):245–260. [PubMed] [Google Scholar]
- 6.Donath MY, Halban PA. Decreased β-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 7.Dono K, Gotoh M, Fukuzaki T, Ohzato H, Kanai T, Monden M, Mori T. Low-temperature collagenase digestion: an improved islet isolation method from cold preserved pancreas. Transplant Proc. 1992;24(4):1511–1512. [PubMed] [Google Scholar]
- 8.Dono K, Gotoh M, Monden M, Kanai T, Fukuzaki T, Mori T. Low temperature collagenase digestion for islet isolation from 48-hour cold-preserved rat pancreas. Transplantation. 1994;57(1):22–26. doi: 10.1097/00007890-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 10.Githens S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr. 1988;7(4):486–506. doi: 10.1097/00005176-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gross J, Harper E, Harris ED, McCroskery PA, Highberger JH, Corbett C, Kang AH. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem Biophys Res Commun. 1974;61(2):605–612. doi: 10.1016/0006-291X(74)91000-6. [DOI] [PubMed] [Google Scholar]
- 12.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. β-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12(3):310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 13.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. PNAS. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-κB activation. J Biol Chem. 2009;284(26):17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayali AG, van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G, Flodstrom-Tullberg M, Zhang YQ, Sarvetnick N. The stromal cell-derived factor-1α/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163(4):859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikugawa R, Katsuta H, Akashi T, Yatoh S, Weir GC, Sharma A, Bonner-Weir S. Differentiation of COPAS-sorted non-endocrine pancreatic cells into insulin-positive cells in the mouse. Diabetologia. 2009;52(4):645–652. doi: 10.1007/s00125-009-1260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeehan WL. The effect of temperature during trypsin treatment on viability and multiplication potential of single normal human and chicken fibroblasts. Cell Biol Int Rep. 1977;1(4):335–343. doi: 10.1016/0309-1651(77)90063-7. [DOI] [PubMed] [Google Scholar]
- 18.Oliver C. Isolation and maintenance of differentiated exocrine gland acinar cells in vitro. In Vitro. 1980;16(4):297–305. doi: 10.1007/BF02618335. [DOI] [PubMed] [Google Scholar]
- 19.Poliakova L, Pirone A, Farese A, MacVittie T, Farney A. Presence of nonhematopoietic side population cells in the adult human and nonhuman primate pancreas. Transplant Proc. 2004;36(4):1166–1168. doi: 10.1016/j.transproceed.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes CJ. Type 2 diabetes–a matter of β-cell life and death? Science. 2005;307(5708):380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 21.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/S0091-679X(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 23.Stock PG, Bluestone JA. β-cell replacement for type I diabetes. Annu Rev Med. 2004;55(1):133–156. doi: 10.1146/annurev.med.55.091902.103539. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53(8):2143–2152. doi: 10.2337/diabetes.53.8.2143. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, D'Hoker J, Stange G, Bonne S, de Leu N, Xiao X, van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]