Abstract
Xiongdankaiming tablet (XDKMT), a well-known compound in traditional Chinese medicine, is widely used for the treatment of acute iridocyclitis and primary open-angle glaucoma. In this paper, accurate and reliable methods were developed for the identification of 20 constituents using high-performance liquid chromatography with photo-diode array and electron spray ionization-mass spectrometry (HPLC-PDA/ESI-MSn), and determination of nine of the constituents (chlorogenic acid, gentiopicroside, isochlorogenic acid B, diosmetin-7-O-β-D-glucopyranoside, apigenin, diosmetin, tauroursodeoxycholic acid, acacetin, and taurochenodeoxycholic acid) was developed using HPLC with ultraviolet absorption detector and evaporative light scattering detector (HPLC-UV/ELSD) for the first time. The best results were obtained on a Zorbax SB-C18 column with gradient elution using water (0.1% formic acid) (A) and methanol (0.1% formic acid) (B) at a flow rate of 0.7 ml/min. Tauroursodeoxycholic acid and taurochenodeoxycholic acid, owing to their low UV absorption, were detected by ELSD. The other seven compounds were analyzed by HPLC-UV with variable wavelengths. The calibration curves of all nine constituents showed good linear regression (R 2>0.9996) within the linearity ranges. The limits of detection and quantification were in the ranges of 0.0460–9.90 μg/ml and 0.115–24.8 μg/ml, respectively. The accuracy, in terms of recovery, varied from 95.3% to 104.9% with relative standard deviations (RSDs) less than 4.4%. Precision (with the intra- and inter-day variations less than 4.4%) was also suitable for its intended use. The developed method was successfully applied for the analysis of major components in XDKMT, which provides an appropriate method for the quality control of XDKMT.
Keywords: Traditional Chinese medicine (TCM) compound recipe, Xiongdankaiming tablet, HPLC-UV/ELSD, HPLC-MS, Quality control
1. Introduction
Traditional Chinese medicines (TCMs) and their preparations have been widely used whether in the field of treatment or health care in China for a long time. Such good effects may be attributed to obtaining synergistic effects or diminishing potential adverse reactions among dozens of chemical compounds in TCMs (Li et al., 2011). As for preparations, TCMs are usually composed of several crude drugs, which make these mixtures become more complex. It is challenging and valuable to cover most of the “markers” in quality control of TCMs (Li et al., 2011). More comprehensive strategies are urgently required for the determination of their chemical markers and quality control of a complex TCM system.
Xiongdankaiming tablet (XDKMT), a well-known compound recipe of TCM, is usually used in clinical treatment for acute iridocyclitis and primary open-angle glaucoma. Other clinical uses of XDKMT, including an analgesic (Zhao et al., 1999), prevention of the postoperative complication of phacoemulsification and intraocular lens implantation (Liu et al., 2005), lowering intraocular pressure (Zhao and Qi, 2007), and relieving symptoms of visual fatigue caused by hepatobiliary swelter among young people (Hong et al., 2008), have also been reported. XDKMT is composed of seven medicinal materials or extracts, including bear bile powder, Haliotidis concha, Chrysanthemi flos, Gentianae radix et rhizoma, Lycii fructus, Alismatis rhizoma, and Leonuri fructus. The seven medicinal materials or extracts are used according to the theory of TCM of providing better efficacy through synergistic interaction and causing less severe side effects. However, it is uncertain which components are actually responsible for the therapeutic effects. Therefore, we identified the chemical constituents of XDKMT, which is the basis of investigating the mechanism of XDKMT and monitoring its quality. The pharmacologically active ingredient of XDKMT remains to be further studied.
The chemical constituents for the seven single medicinal materials or extracts have been reported. The main effective components include bile acids (e.g., tauroursodesoxycholic acid and taurochenodesoxycholic acid) (Xie et al., 1981; Cui et al., 2006; Gong et al., 2007), calcium carbonate, flavonoids (diosmetin-7-O-β-D-glucopyranoside, apigenin, diosmetin, acacetin, etc.), organic acids (e.g., chlorogenic acid) (Miyazawa and Hisama, 2003; Kim et al., 2003; Cheng et al., 2005), iridoids (e.g., gentiopicroside) (Kondo et al., 1994; Kumarasamy et al., 2003), terpenoids (e.g., alisol A, alisol B 23-acetate, and alisol C), and amino acid derivatives (e.g., betaine and stachydrine) (Yin et al., 2010). However, to our knowledge no investigation on the chemical constituents of XDKMT has been reported. Whether the constituents from the single medical materials are in fact the ones which make up XDKMT is still unknown.
There have been some reports on the determination of several effective components in the medicinal materials by high-performance liquid chromatography with evaporative light scattering detector (HPLC-ELSD), HPLC with ultraviolet absorption detector (HPLC-UV), or capillary electrophoresis (CE) methods (Zhao et al., 2006; Gao et al., 2007; Zhang et al., 2008; Wang, 2010; Cao and Wang, 2010; Qin and Wen, 2011). However, the combination of medicinal materials increased the complexity of constituents, so the reported methods could not be used for the analysis of XDKMT.
Until now, no method has been developed for the analysis of XDKMT. In this article, an HPLC with photo-diode array and electron spray ionization-mass spectrometry (HPLC-PDA/ESI-MSn) method for the identification of 20 constituents in XDKMT was developed. These compounds have covered almost all the principal types of ingredients in the seven medicinal materials or extracts of XDKMT except calcium carbonate for Haliotidis concha. The content of calcium carbonate in Haliotidis concha should not be less than 93.0% according to the Chinese Pharmacopoeia (2010 Edition). Calcium carbonate is usually analyzed by titrimetry, and was not analyzed in this study. Moreover, an HPLC-UV/ELSD method was developed for quantitative determination of nine of the ingredients, including chlorogenic acid, gentiopicroside, isochlorogenic acid B, diosmetin-7-O-β-D-glucopyranoside, apigenin, diosmetin, tauroursodesoxycholic acid, acacetin, and taurochenodeoxycholic acid. This study represents a detailed investigation of the components in XDKMT for the first time and provides an applicable method for its quality control.
2. Materials and methods
2.1. Apparatus and chromatographic conditions
2.1.1. HPLC-UV/ELSD analysis
The analysis was performed on an Agilent 1100 HPLC system (Agilent Technologies, USA), equipped with a quaternary pump, an on-line degasser, an auto-sampler, a column oven, and an ultraviolet detector coupled with a ChemStation Software Version B.04.01 (Agilent Technologies, USA). An Alltech ELSD-2000ES instrument as well as an Allchrom Plus Client/Server data operator (Alltech, USA) was connected to the liquid chromatography for detection of non-chromophoric substances.
The separation was carried out on a Zorbax SB-C18 column (4.6 mm×250 mm, 5 μm; Agilent Technologies, USA) and temperature was set at 39 °C. The mobile phase consisted of water (0.1% formic acid) (A) and methanol (0.1% formic acid) (B) at a flow rate of 0.7 ml/min, using the following gradient elution: 0–50 min (3%–58% B), 50–80 min (58%–91% B), 80–90 min (91%–100% B). There was a 5-min wash with 100% B after each run and equilibrium time was 15 min. The injection volume was 20 μl. Based on variable wavelength detector (VWD), different detection wavelengths were set for different periods of time: 330 nm for 0–28 min, 280 nm for 28–33 min, or 340 nm for 33–95 min.
The ELSD was set up to a drift tube temperature of 100 °C at gain 1 and the N2 flow rate was 2 L/min.
2.1.2. HPLC-PDA/ESI-MSn analysis
The analysis was performed on a Finnigan LCQ Deca XPplus ion trap mass spectrometer (Thermo Finnigan, USA) connected to the Agilent 1100 HPLC instrument via an electron spray ionization (ESI) interface. The chromatographic conditions were the same as described in Section 2.1.1. The MS spectra were acquired in both negative and positive modes and full scan mass spectra were acquired from the mass-to-charge ratio (m/z) of 100–1000. Parameters for MS were as follows: collision gas, ultrahigh-purity helium (He); nebulizing gas, high-purity nitrogen (N2); sheath gas (N2) and auxiliary gas (N2) at a flow rate of 60 and 20 arbitrary units (AU), respectively; ion spray voltage −4.5 kV; capillary temperature 350 °C; capillary voltages in positive and negative modes 19 and −15 V, respectively; and, tube lens offset voltages 25 and −30 V, respectively.
2.2. Reagents, chemicals, and materials
Deionized water was purified by the Milli-Q System (Millipore, USA) for HPLC assay. Methanol (Merck, Germany) and formic acid (ROE SCIENTIFIC Inc., USA) were both of HPLC grade.
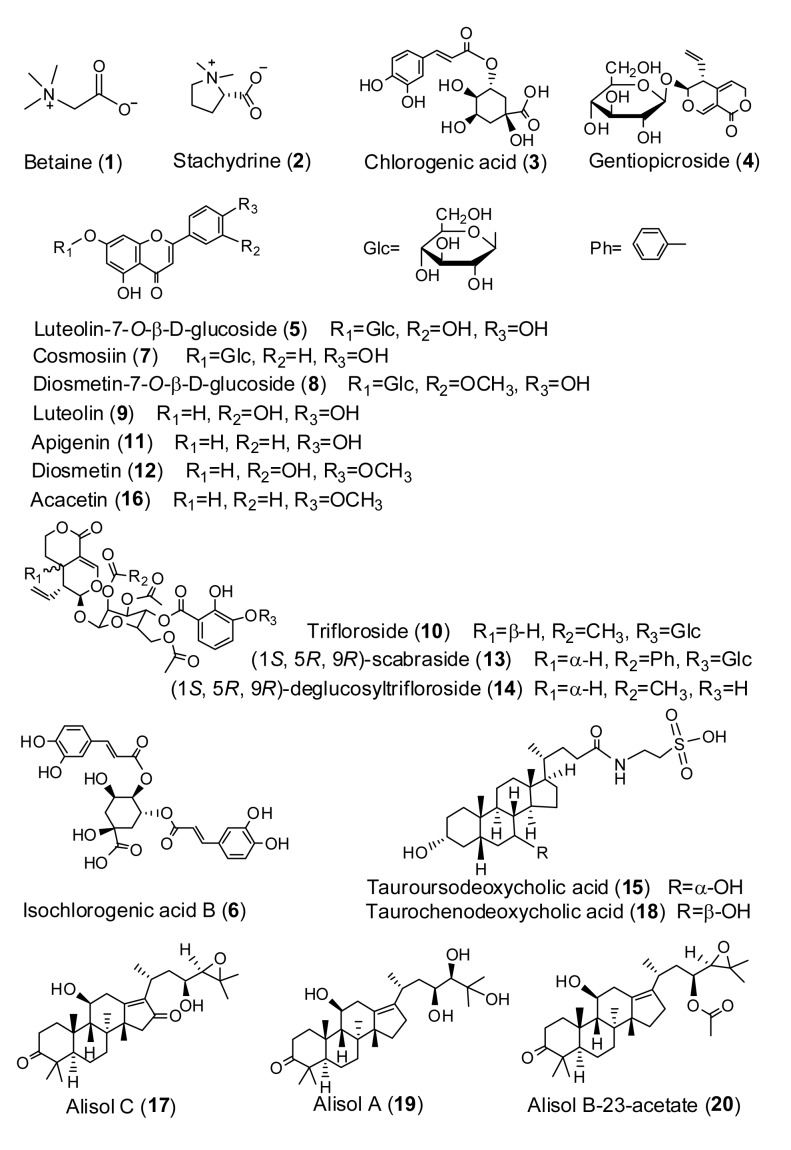
The reference standards are: stachydrine hydrochloride (2; batch No. 32650), chlorogenic acid (3; batch No. 39350), gentiopicroside (4; batch No. 101226), luteolin-7-O-β-D-glucoside (5; batch No. 110426), isochlorogenic acid B (6; batch No. 110317), cosmosiin (7; batch No. 101220), diosmetin-7-O-β-D -glucoside (8; batch No. 101207), luteolin (9; batch No. 110403), trifloroside (10), apigenin (11; batch No. 101207), diosmetin (12; batch No. 110405), (1S, 5R, 9R)-scabraside (13), (1S, 5R, 9R)-deglucosyltri-floroside (14), sodium tauroursodeoxycholate (15; batch No. 110816-200507), acacetin (16; batch No. 110330), alisol C (17), taurochenodeoxycholic acid (18; batch No. 110846-200506), alisol A (19), and alisol B-23-acetate (20; batch No. 110713). Compounds 2 and 3 were purchased from Aladdin reagent database Inc. (Shanghai, China). Compounds 15 and 18 were from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Compounds 4, 5, 6, 7, 8, 9, 11, 12, 16, and 20 were provided by Shanghai Winherb Medical S&T Development Co. Ltd. (China). Compounds 10, 13, 14, 17, 19 were respectively isolated from Gentianae radix et rhizoma and Alismatis rhizoma in our laboratory with the purity higher than 98%. All these five compounds were identified by ESI-MS, proton nuclear magnetic resonance (1H-NMR), and 13C-NMR. The absolute configurations of compounds 13 and 14 were reported in Wang et al. (2013). The chemical structures of the 20 analytes are shown in Fig. 1.
Fig. 1.
Structures of the 20 compounds identified in Xiongdankaiming tablets
XDKMTs (Xiuzheng Pharmaceutical Group, batch Nos. 110103, 110601, and 120102) were provided by the manufacturer.
2.3. Preparation of standard solutions
A mixed standard stock solution containing compounds 3, 4, 6, 8, 11, 12, 15, 16, and 18 were prepared in 50% methanol. The working standard solutions were obtained by diluting the mixed standard stock solution with 50% methanol to a series of appropriate concentrations for the calibration curves. Tauroursodeoxycholic acid and taurochenodeoxycholic acid were analyzed by an HPLC-ELSD method and the other seven analytes were analyzed by an HPLC-UV method with variable wavelengths. The standard stock and working solutions were all stored at 4 °C until use and centrifuged prior to injection.
2.4. Preparation of sample solutions
Twenty tablets were accurately weighed and finely powdered after removing the coating. The powder of XDKMT (0.5 g) was weighed accurately into a 25-ml volumetric flask, and 50% methanol was added to the volume mark. After accurately weighing, the mixed solution was ultrasonicated (40 kHz) at room temperature for 40 min. After that, the same solvent was added to compensate for the lost weight during the extraction. The extraction was centrifuged at 12 000 r/min for 10 min. The supernatant was prepared for injection.
For MS analysis, the pretreatment of sample was the same as described above. To get higher detection sensitivity, the supernatant was concentrated to 5 ml by vacuum evaporation process. A 1.0-g aliquot of powder of bear bile powder, Chrysanthemi flos, Gentianae radix et rhizoma, Lycii fructus, Alismatis rhizoma, and Leonuri fructus were separately sonicated in 10 ml of 80% methanol for 60 min followed by centrifugation at 12 000 r/min for 10 min. The supernatant was prepared for analysis.
2.5. Validation of the HPLC method
2.5.1. Calibration curves and limits of quantification and detection
The working standard solutions were first restored to room temperature, and then eight concentrations were analyzed by plotting the peak area versus concentration for LC-UV. For ELSD, the response is usually a non-linear function, so it was calculated by the double logarithmic plots of the peak area versus the concentration of the reference solution injected. The limit of quantification (LOQ) and the limit of detection (LOD) were determined by diluting the standard solution progressively until the signal-to-noise ratios (S/N) of analytes were approximately 10 and 3, respectively.
2.5.2. Precision, stability, and accuracy
The precision of the developed method was determined by intra- and inter-day variability tests, which were investigated by determining the nine analytes for six parallel samples during one day and by repeating the experiments on six consecutive days, respectively. Variations of the concentrations of analytes were taken as the measure of precision and delivered as percentage relative standard deviations (RSDs). The sample solution was injected into the apparatus at 0, 4, 8, 12, 16, 20, and 24 h, respectively, to evaluate the stability of the solution. Recovery test was used to evaluate the accuracy of the method, which was performed by adding accurate amounts (high, medium, and low levels) of the nine standards into 0.25 g of XDKMT powder separately with three replicates at each level. The spiked samples were then extracted according to the method mentioned in Section 2.4, and quantified under the condition in Section 2.1.
3. Results and discussion
3.1. Optimization of sample preparation procedure
To achieve maximum extraction efficiency of the nine compounds, four important factors, namely, extraction solvents, the amount of extraction solvent, extraction time, and extraction number of times were investigated independently. XDKMT was extracted with different levels of each factor including extraction solvent, the amount of extraction solvent, extraction time, and extraction number of times, that were investigated individually by one-factor experimental design. The results revealed that for extraction solvents, methanol had significantly better extraction efficiency than ethanol and acetonitrile. Furthermore, 50% methanol reached the highest point of the extraction efficiency. Thus, 50% methanol was chosen as the suitable extraction solvent. Moreover, using 25 ml 50% methanol and 40 min extracting for one time was found to be adequate for the analysis. We decided that ultrasonic extraction for 40 min by 25 ml 50% methanol was appropriate for the analysis. Optimization process can be seen in the supplementary material.
3.2. Optimization of the HPLC chromatographic conditions
To optimize the condition of separation, different reverse-phase HPLC columns were compared to separate the target compounds, such as Zorbax SB-C18 (4.6 mm×250 mm, 5 μm) and Zorbax Eclipse Plus C18 (4.6 mm×100 mm, 1.8 μm). The latter had a shorter analysis time but a poor resolution. The better separation efficiency and peak shape were achieved by the Zorbax SB-C18. As for the mobile phase, we tested acetonitrile and methanol with different modifiers, such as formic acid and acetic acid. It was found that using methanol and water, both containing 0.1% formic acid achieved the optimal chromatographic conditions. Methanol can simultaneously separate more compounds in the samples than acetonitrile. And formic acid was used to improve the peaks shape and separation, which was also compatible to ELSD and MS detectors. Column temperature had a great effect on the resolution. Temperatures between 20 and 40 °C were tested, and 39 °C was deemed appropriate. Flow rate was also optimized. We found that under the flow rate of 0.7 ml/min, the nine marker constituents were successfully separated. To make each compound to achieve the maximum absorption wavelength, different detection wavelengths were set for different durations: 330 nm for 0–28 min (compound 3); 280 nm for 28–33 min (compound 4); 340 nm for 33–95 min (compounds 6, 8, 11, 12, and 16).
Compounds 15 and 18 were detected by ELSD. As for ELSD, the drift tube temperature, flow rate of nebulizing gas, and detector gain are crucial to improve the S/N ratio. HPLC-ELSD analysis of the sample solution at different drift tube temperatures (110, 100, 90 °C), different gas flow rates (2.5, 2.0, 1.5 L/min), and different detector gains (3, 2, 1) were performed respectively, and optimal parameters were then selected for obtaining the optimal sensitivity. We found that when the drift tube temperature was set to 100 °C at gain 1 and the nitrogen flow rate was 2 L/min, S/N ratios achieved the maximum values.
3.3. Identification of the 20 constituents in XDKMT by HPLC-PDA/ESI-MSn
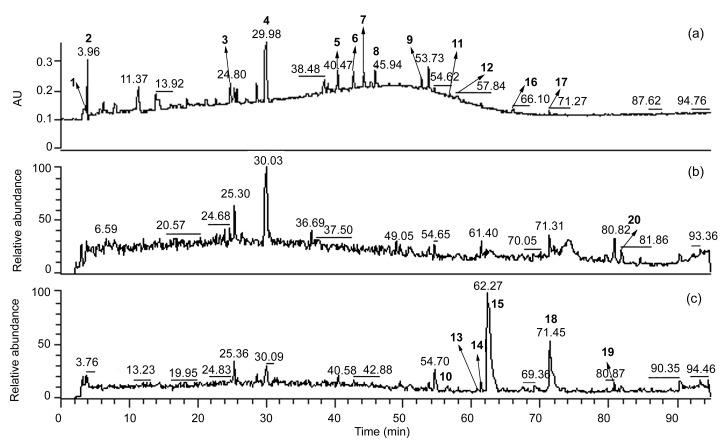
The sample of XDKMT was analyzed by HPLC-PDA/ESI-MSn in both negative and positive ionization modes. The total scan PDA chromatograms and total ion chromatograms (TIC) are shown in Fig. 2, and the mass spectra of the 20 compounds are in the supplementary material. By comparing the retention time, UV spectra and MSn spectra of peaks in sample chromatogram with those of reference standards, 20 peaks were unambiguously identified as stachydrine (2), chlorogenic acid (3), gentiopicroside (4), luteolin-7-O-β-D-glucoside (5), isochlorogenic acid B (6), cosmosiin (7), diosmetin-7-O-β-D-glucoside (8), luteolin (9), trifloroside (10), apigenin (11), diosmetin (12), (1S, 5R, 9R)-scabraside (13), (1S, 5R, 9R)-deglucosyltrifloroside (14), tauroursodeoxycholic acid (15), acacetin (16), alisol C (17), taurochenodeoxycholic acid (18), alisol A (19), and alisol B-23-acetate (20). As for peak 1, the quasi-molecular ions was 118 [M+H]+ and dimer 235 [2M+H]+. The quasi-molecular ion [M+H]+ was odd number, which indicated that the chemical structure should contain one nitrogen atom. The fragment ions of 59 [M+H−N(CH3)3]+ and 58 [M+H−HCOOH]+ were observed in the MS2 data. The MS data of peak 1 were consistent with those of Wang et al. (2011), so it was deduced to be betaine (compound 1). The other peaks were not identified yet without accurate molecular weight data or reference compounds.
Fig. 2.
Total scan PDA chromatogram (a), total ion chromatograms (TIC) in positive mode (b) and negative mode (c) of a Xiongdankaiming tablet sample (numbers of the compounds were the same as those in Fig. 1)
It is useful to observe the fragmentation patterns in the mass spectrum for the characterization of compounds. The quasi-molecular ions and characteristic fragment ions for the 20 compounds are all listed in Table 1.
Table 1.
Identification of the twenty chemical constituents in XDKMT
| No. | t R (min) | MS (m/z) |
MS2 (m/z) | MS3 (m/z) | Identification | Plant source | |
| ESI+ | ESI− | ||||||
| 1 | 3.5 | 118 (59) [M+H]+ | – | 118@40: | – | Betaine | a |
| 235 (100) [2M+H]+ | 59 (100) [M+H−N(CH3)3]+ | ||||||
| 58 (86) [M+H−HOAc]+ | |||||||
| 2 | 4.0 | 144 (100) [M+H]+ | – | 144@40: | – | Stachydrine | |
| 287 (87) [2M+H]+ | 98 (32) [M+H−HCOOH]+ | ||||||
| 84 (100) [M+H−HOAc]+ | |||||||
| 58 (53) [M+H−C4H6O2]+ | |||||||
| 3 | 24.8 | – | 353 (74) [M−H]− | 353@25: | 353@25→191@35: | Chlorogenic acid | b |
| 399 (53) [M+HCOO−]− | 191 (100) [M−H−C9H6O3]− | 173 (34) [M−H−C9H6O3−H2O]− | |||||
| 707 (100) [2M−H]− | 179 (6) [M−H−C7H10O5]− | 127 (46) [M−H−C9H6O3−H2O−HCOOH]− | |||||
| 85 (44) [M−H−C9H6O3−H2O−HCOOH−C2H2O]− | |||||||
| 4 | 30.1 | 357 (5) [M+H]+ | 401 (100) [M+HCOO−]− | 401@30: | 401@30→179@20: | Gentiopicroside | c |
| 355 (61) [M−H]− | 161 (84) [M−H−C10H8O3−H2O]− | ||||||
| 225 (28) [M+HCOO−−C10H8O3]− | |||||||
| 179 (100) [M−H−C10H8O3]− | |||||||
| 5 | 40.6 | 449 (100) [M+H]+ | 447 (85) [M−H]− | 449@35: | 447@30→285@48: | Luteolin-7-O-β-D-glucoside | b |
| 287 (100) [M+H−Glc]+ | 241 (63) [M−H−Glc−CO2]− | ||||||
| 447@30: | |||||||
| 285 (100) [M−H−Glc]− | |||||||
| 6 | 42.9 | – | 515 (100) [M−H]− | 515@30: | 515@30→353@35: | Isochlorogenic acid B | b |
| 353 (100) [M−H−C9H6O3]− | 191 (32) [M−H−2C9H6O3]− | ||||||
| 179 (100) [M−H−C9H6O3−C7H10O5]− | |||||||
| 173 (79) [M−H−2C9H6O3−H2O]− | |||||||
| 7 | 44.4 | 433 (100) [M+H]+ | 431 (100) [M−H]− | 433@25: | 433@25→271@43: | Cosmosiin | b |
| 863 (80) [2M−H]− | 271 (100) [M+H−Glc]+ | 225 (8) [M+H−Glc−H2O−CO]+ | |||||
| 431@35: | 153 (15) [M+H−Glc−H2O−2CO−CO2]+ | ||||||
| 269 (100) [M−H−Glc]− | |||||||
| 8 | 46 | 463 (100) [M+H]+ | – | 463@25: | 463@25→301@38: | Diosmetin-7-O-β-D-glucoside | b |
| 301 (100) [M+H−Glc]+ | 286 (71) [M+H−Glc−CH3]+ | ||||||
| 9 | 52.8 | – | 285 (100) [M−H]− | 285@50: | 285@50→241@55: | Luteolin | b |
| 241 (50) [M−H−CO2]− | 213 (77) [M−H−CO2−CO]− | ||||||
| 175 (32) [M−H−C3O2−C2H2O]− | 197 (88) [M−H−2CO2]− | ||||||
| 10 | 54.7 | – | 781 (100) [M−H]− | 781@35: | 781@35→739@40: | Trifloroside | c |
| 739 (69) [M−H−C2H2O]− | 697 (70) [M−H−2C2H2O]− | ||||||
| 619 (100) [M−H−Glc]− | |||||||
| 577 (41) [M−H−C2H2O−Glc]− | |||||||
| 11 | 57 | 271 (100) [M+H]+ | 269 (100) [M−H]− | 269@50: | 269@50→225@55: | Apigenin | b |
| 315 (31) [M+HCOO−]− | 225 (100) [M−H−CO2]− | 197 (47) [M−H−CO2−CO]− | |||||
| 181 (100) [M−H−2CO2]− | |||||||
| 12 | 57.9 | 301 (100) [M+H]+ | – | 301@40: | 301@40→286@45: | Diosmetin | b |
| 286 [M+H−CH3]+ | 258 (100) [M+H−CH3−CO]+ | ||||||
| 13 | 60.9 | – | 843 (100) [M−H]− | 843@35: | 843@35→681@40: | (1S, 5R, 9R)-scabraside | c |
| 801 (56) [M−H−C2H2O]− | 639 (100) [M−H−Glc−C2H2O]− | ||||||
| 681 (100) [M−H−Glc]− | 621 (16) [M−H−Glc−C2H2O−H2O]− | ||||||
| 639 (27) [M−H−C2H2O−Glc]− | |||||||
| 14 | 61.4 | 621 (74) [M+H]+ | 619 (100) [M−H]− | 619@35: | 619@35→577@40: | (1S, 5R, 9R)-deglucosyltrifloroside | c |
| 643 (100) [M+Na]+ | 577 (100) [M−H−C2H2O]− | 535 (100) [M−H−2C2H2O]− | |||||
| 15 | 62.4 | – | 498 (100) [M−H]− | 498@55: | – | Tauroursodeoxycholic acid | f |
| 997 (67) [2M−H]− | 480 (52) [M−H−H2O]− | ||||||
| 432 (100) [M−H−H2SO2]− | |||||||
| 414 (43) [M−H−H2SO2−H2O]− | |||||||
| 16 | 66.1 | 285 (100) [M+H]+ | 283 (100) [M−H]− | 285@45: | 285@45→270@50: | Acacetin | b |
| 329 (55) [M+HCOO−]− | 270 (100) [M+H−CH3]+ | 242 (100) [M+H−CH3−CO]+ | |||||
| 283@45: | |||||||
| 268 (100) [M−H−CH3]− | |||||||
| 17 | 71.3 | 487 (38) [M+H]+ | – | 487@25: | 487@25→469@30: | Alisol C | d |
| 995 (100) [2M+Na]+ | 469 (99) [M+H−H2O]+ | 451 (98) [M+H−2H2O]+ | |||||
| 451 (37) [M+H−2H2O]+ | 397 (100) [M+H−H2O−C4H8O]+ | ||||||
| 397 (100) [M+H−H2O−C4H8O]+ | |||||||
| 18 | 71.4 | – | 498 (100) [M−H]− | 498@55: | – | Taurochenodeoxycholic acid | f |
| 997 (48) [2M−H]− | 480 (70) [M−H−H2O]− | ||||||
| 432 (69) [M−H−H2SO2]− | |||||||
| 414 (100) [M−H−H2SO2−H2O]− | |||||||
| 19 | 80.9 | – | 489 (6) [M−H]− | 535@25: | 535@25→471@30: | Alisol A | d |
| 535 (100) [M+HCOO−]− | 471 (100) [M−H−H2O]− | 413 (32) [M−H−H2O−C3H6O]− | |||||
| 395 (89) [M−H−H2O−C3H6O−H2O]− | |||||||
| 339 (100) [M−H−H2O−C3H6O−H2O−C3H4O]− | |||||||
| 20 | 82.0 | 515 (100) [M+H]+ | – | 515@25: | 515@25→383@30: | Alisol B-23-acetate | d |
| 497 (18) [M+H−H2O]+ | 497 (29) [M+H−H2O]+ | 365 (100) [M+H−H2O−C4H8O−HOAc]+ | |||||
| 437 (75) [M+H−H2O−HOAc]+ | |||||||
| 419 (29) [M+H−2H2O−HOAc]+ | |||||||
| 383 (81) [M+H−C4H8O−HOAc]+ | |||||||
| 365 (100) [M+H−H2O−C4H8O−HOAc]+ | |||||||
Plant source: a, Lycii fructus; b, Chrysanthemi flos; c, Gentianae radix et rhizoma; d, Alismatis rhizoma; e, Leonuri fructus; f, bear bile powder; –. No MS response; t R: retention time
For two amino acid derivatives, compounds 1 and 2, the quasi-molecular ions were both [M+H]+ and dimer [2M+H]+. The [M+H]+ ions of compounds 1 and 2 produced fragment ions of 58 and 84 after the loss of acetic acid, respectively. The fragment ion of [M+H−HCOOH]+ was observed in the MS2 data of compound 2 due to the side chain formyl group in the chemical structure.
The quasi-molecular ion 353 [M−H]− of peak 3 produced the fragment ions of 191 [M−H−C9H6O3]−, 179 [M−H−C7H10O5]−, 173 [M−H−C9H6O3−H2O]− after loss of caffeoyl group, quininyl group, or H2O. The MS2 data of peak 6, 353 [M−H−C9H6O3]− indicated that two caffeoyl groups exist in the chemical structure. The characteristic fragment ions of 191 [M−H−2C9H6O3]−, 179 [M−H−C9H6O3−C7H10O5]−, and 173 [M−H−2C9H6O3−H2O]− were also observed.
Though compounds 4, 10, 13, and 14 are all iridoid glycosides, the quasi-molecular ion of them were different. In the negative mode, the quasi-molecular ion of peak 4 was [M+HCOO−]−, but those of the other three peaks were all [M−H]−. The fragment ion of [M−H−C2H2O]− was observed in the MS2 data of peaks 10, 13, and 14 due to the existing of the acetyl group in the chemical structures. In addition, the fragment ion of [M−H−Glc]− was observed in MS2 data of peaks 10 and 13, while it was not found in those of peaks 4 and 14. This indicated that the glucoside of R3 was easy to lose.
For the flavonoid glycosides, compounds 5, 7, and 8, [M+H−Glc]+ or [M−H−Glc]− was observed in the MS2 data of them. The fragment of [M+H−CH3]+ or [M−H−CH3]− was observed for the flavonoid containing methoxy group, for example, peaks 12, 16, and 8. The fragment ions of [M−H−CO2]− in the MS2 data of peaks 9 and 11 could be formed from the neutral loss of CO2 on C-ring.
Compounds 15 and 18 are epimers. They yielded the same quasi-molecular ions and fragment ions but different retention time at 62.4 and 71.4 min, respectively, so it was easy to distinguish these two peaks.
Compounds 17, 19, and 20 are protostane type triterpenoids. The quasi-molecular ions and fragment ions formed from the loss of one or two molecules H2O. Another significant feature was the fragment ions form the loss of C4H8O or C3H6O at the alkly chain.
Furthermore, six medicinal materials or extracts, including bear bile powder, Chrysanthemi flos, Gentianae radix et rhizoma, Lycii fructus, Alismatis rhizoma, and Leonuri fructus were also analyzed by HPLC-PDA/ESI-MSn in both negative and positive ionization modes to confirm the attribution of the 20 compounds. As shown in Table 1, compound 1 was from Lycii fructus; compound 2 belonged to Leonuri fructus; compounds 3, 5, 6, 7, 8, 9, 11, 12, and 16 were derived from Chrysanthemi flos; compounds 4, 10, 13, and 14 came from Gentianae radix et rhizoma; compounds 17, 19, and 20 were from Alismatis rhizom; and, compounds 15 and 18 were from bear bile powder.
3.4. Validation of the HPLC method
3.4.1. Linearity, LOD, and LOQ
As shown in Table 2, all calibration curves of the nine compounds showed good linear regression (R 2>0.9996) within the test ranges. The LODs and LOQs were in the range of 0.0460–9.90 and 0.115–24.8 μg/ml, respectively.
Table 2.
Linearity, limits of detection (LOD), and limits of quantitation (LOQ) of nine compounds by HPLC-UV/ELSD method
| Compound | Calibration curvea | R 2 | Linear range (μg/ml) | LOQb (μg/ml) | LODc (μg/ml) |
| 3. Chlorogenic acid | y=84.6944x−20.3658 | 0.9999 | 0.644–103 | 0.129 | 0.0516 |
| 4. Gentiopicroside | y=36.4927x+2.5304 | 0.9999 | 0.919–147 | 0.149 | 0.0596 |
| 6. Isochlorogenic acid B | y=89.5979x−47.1398 | 0.9999 | 0.625–100 | 0.186 | 0.0744 |
| 8. Diosmetin-7-O-β-D-glucopyranoside | y=57.9145x−2.3414 | 0.9998 | 0.323–51.6 | 0.190 | 0.0760 |
| 11. Apigenin | y=124.4984x−27.0302 | 0.9998 | 0.212–34.0 | 0.130 | 0.0520 |
| 12. Diosmetin | y=120.2592x−17.9481 | 0.9999 | 0.223–35.7 | 0.115 | 0.0460 |
| 15. Tauroursodeoxycholic acid | Y=1.5284X+3.0893 | 1.0000 | 39.5–158 | 20.0 | 7.98 |
| 16. Acacetin | y=138.2031x−25.7620 | 0.9999 | 0.238–38.0 | 0.180 | 0.0720 |
| 18. Taurochenodeoxycholic acid | Y=1.4636X+3.1675 | 0.9996 | 50.5–202 | 24.8 | 9.90 |
y and x stand for peak area and concentration (μg/ml), respectively; Y=lg y, X=lg x
S/N=3
S/N=10
3.4.2. Accuracy, precision, and stability
The accuracy, in terms of recovery, was from 95.3% to 104.9% with RSD less than 4.4%. The intra- and inter-day variations that were to determine the precision of the method were less than 4.4%. For the stability test, the same sample solution was analyzed every 4 h in a day (n=7). The RSD values of the peak area and retention time were less than 3.8% and 0.1%, respectively. The solution was therefore proved to be stable within 24 h.
3.5. Quantitative determination of major constituents in XDKMT by HPLC-UV/ELSD
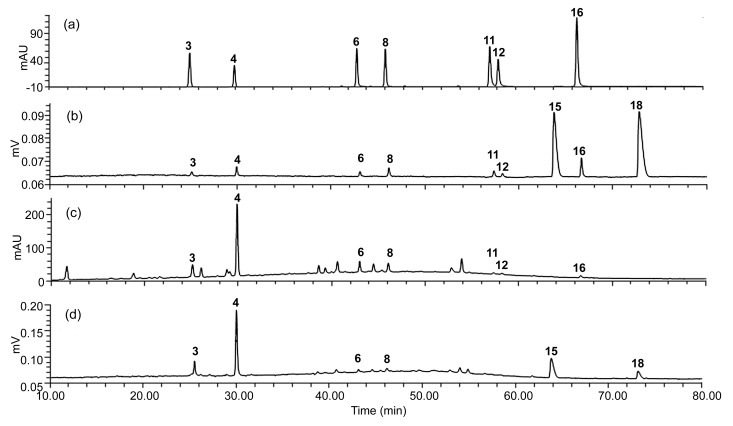
The proposed HPLC-UV/ELSD method was subsequently applied to simultaneous determination of nine components including chlorogenic acid, gentiopicroside, isochlorogenic acid B, diosmetin-7-O-β-D-glucopyranoside, apigenin, diosmetin, tauroursodeoxycholate acid, acacetin, and taurochenodeoxycholic acid in XDKMT of three batches. The HPLC-UV/ELSD chromatograms of the standard mixture and sample are presented in Fig. 3. In addition, the peak purity of nine compounds was proved using HPLC-PDA/ESI-MSn. The contents of the nine components are listed in Table 3. It can be seen from the average contents that gentiopicroside (0.800–1.60 mg/tablet), tauroursodeoxycholic acid (1.61–1.76 mg/tablet), and taurochenodeoxycholic acid (0.864–0.992 mg/tablet) were the predominant components in XDKMT; and, there was much fluctuation in the component contents between different batches. Therefore, it is necessary to monitor the inter-batch variations of components.
Fig. 3.
HPLC chromatograms of standard mixture (a, b) and XDKMT (c, d) by LC-UV/ELSD method
(a, c) refer to UV detection; (b, d) refer to ELSD. The concentrations (μg/ml) of the standards: 3: 9.42; 4: 11.9; 6: 9.42; 8: 13.3; 11: 8.51; 12: 6.19; 15: 63.3; 16: 12.7; 18: 80.7. Numbers of the compounds were the same as those in Fig. 1
Table 3.
Contents of nine components in three batches of XDKMT
| Compound | Batch No. 110103 (n=3) |
Batch No. 110601 (n=3) |
Batch No. 120102 (n=3) |
|||
| Content (mg/tablet) | RSD (%) | Content (mg/tablet) | RSD (%) | Content (mg/tablet) | RSD (%) | |
| 3. Chlorogenic acid | 0.1520 | 0.6 | 0.2270 | 0.3 | 0.2820 | 0.9 |
| 4. Gentiopicroside | 1.6000 | 0.1 | 1.1400 | 0.3 | 0.8000 | 0.5 |
| 6. Isochlorogenic acid B | 0.1090 | 1.6 | 0.1160 | 2.1 | 0.1110 | 1.7 |
| 8. Diosmetin-7-O-β-D-glucopyranoside | 0.1310 | 1.0 | 0.1040 | 1.6 | 0.0599 | 2.3 |
| 11. Apigenin | 0.0148 | 2.5 | 0.0328 | 1.2 | 0.0341 | 2.5 |
| 12. Diosmetin | 0.0123 | 2.2 | 0.0175 | 3.5 | 0.0106 | 2.9 |
| 15. Tauroursodeoxycholic acid | 1.7600 | 0.5 | 1.6200 | 1.0 | 1.6100 | 2.7 |
| 16. Acacetin | 0.0175 | 0.6 | 0.0335 | 1.6 | 0.0216 | 2.1 |
| 18. Taurochenodeoxycholic acid | 0.9920 | 0.8 | 0.8640 | 2.3 | 0.8880 | 2.7 |
4. Conclusions
This is the first report for the identification of 20 constituents by HPLC-PDA/ESI-MSn and the simultaneous determination of the nine major components in XDKMT using HPLC-UV/ELSD. These compounds have covered almost all the principal types of active ingredients in XDKMT, such as bile acids, calcium carbonate, flavonoids, organic acids, iridoids, terpenoids, and amino acids. Therefore, selecting these active constituents as “markers”, we will obtain more comprehensive information for the quality control of XDKMT. The quantitative method has the excellent linearity, precision, stability, and accuracy, and it was proven to be a simple and powerful tool for the quality control of XDKMT.
List of electronic supplementary materials
The electronic supplementary materials mainly cover: (1) identification of 20 constituents in XDKMT by HPLC-PDA/ESI-MSn; (2) optimization of sample preparation procedure; and, (3) optimization of the HPLC chromatographic conditions.
Footnotes
Project (No. 2011FZA7005) supported by the Fundamental Research Funds for the Central Universities of China
Electronic supplementary materials: The online version of this article (doi:10.1631/jzus.B1200295) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Shu-fang WANG, Jing LENG, Yi-min XU, and Mei-ling FENG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Cao XY, Wang ZZ. Simultaneous determination of four iridoid and secoiridoid glycosides and comparative analysis of Radix gentianae Macrophyllae and their related substitutes by HPLC. Phytochem Anal. 2010;21(4):348–354. doi: 10.1002/pca.1206. [DOI] [PubMed] [Google Scholar]
- 2.Cheng WM, Li J, You TP, Hu CM. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J Ethnopharmacol. 2005;101(1-3):334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 3.Cui H, Liu JJ, Fu SY, Liu HL, Hou YS, Jin D. Experimental study of the permeability of Fel Ursi’s in eye. Chin J Ophthalmol. 2006;42(11):1023–1025. (in Chinese) [PubMed] [Google Scholar]
- 4.Gao YM, Song Q, Chen HL, Wei SH, Yang YQ, Zhang LY, Wang JK. HPCE determination of gentiopicroside in Radix gentianae . Chin J Pharm Anal. 2007;27(10):1572–1574. (in Chinese) [Google Scholar]
- 5.Gong RY, Zhang D, Lv ZL, Du LP, Zhang LD, Li JR, Qiao XL. Extration of sodium taurochenodeoxycholate and comparison of its pharmacological effect with sodium chenodeoxycholate. Fine Chem. 2007;24(10):975–978. (in Chinese) [Google Scholar]
- 6.Hong L, Li YJ, Qu B. Observation of the influence of Xiongdankaiming tablet on relieving the visual fatigue symptoms of young people. Chin J Modern Drug Appl. 2008;5(2):62–63. (in Chinese) [Google Scholar]
- 7.Kim KJ, Kim YH, Yu HH, Jeong SI, Cha JD, Kil BS, You YO. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale . Planta Med. 2003;69(3):274–277. doi: 10.1055/s-2003-38479. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Takano F, Hojo H. Suppression of chemically and immunologically induced hepatic injuries by gentiopicroside in mice. Planta Med. 1994;60(5):414–416. doi: 10.1055/s-2006-959521. [DOI] [PubMed] [Google Scholar]
- 9.Kumarasamy Y, Nahar L, Sarker SD. Bioactivity of gentiopicroside from the aerial parts of Centaurium erythraea . Fitoterapia. 2003;74(1-2):151–154. doi: 10.1016/S0367-326X(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Wang SW, Zhang BL, Xie YH, Yang Q, Cao W, Wang JB. Simultaneous quantitative determination of 9 active components in traditional Chinese medicinal preparation ShuangDan oral liquid by RP-HPLC coupled with photodiode array detection. J Pharm Biomed Anal. 2011;56(4):820–824. doi: 10.1016/j.jpba.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Li SP, Zhao J, Yang B. Strategies for quality control of Chinese medicines. J Pharm Biomed Anal. 2011;55(4):802–809. doi: 10.1016/j.jpba.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Li X, Wang H. Clinical observation of Xiongdankaiming tablet on prevention and treatment of the postoperative complications caused by phacoemulsification intraocular lens implantation. Lishizhen Med Mat Med Res. 2005;4(16):341. (in Chinese) [Google Scholar]
- 13.Miyazawa M, Hisama M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium . Biosci Biotech Biochem. 2003;67(10):2091–2099. doi: 10.1271/bbb.67.2091. [DOI] [PubMed] [Google Scholar]
- 14.Qin S, Wen XS. Simultaneous determination of 6 active components in Chrysanthemum morifolium by HPLC. J Chin Mat Med. 2011;36(11):1474–1477. (in Chinese) [PubMed] [Google Scholar]
- 15.Wang JY, Zhang TT, Xu QX, Sun H, Zhou Q, Wu YM. Study on betaine by electrospray ionization mass spectrometry. Sugar Crops China. 2011;3(3):23–25. (in Chinese) [Google Scholar]
- 16.Wang LM. Determination of ridoids in Radix gentianae from various habitats. Chin Tradit Patent Med. 2010;32(11):1941–1945. (in Chinese) [Google Scholar]
- 17.Wang SF, Xu YM, Jiang W, Zhang YF. Isolation and identification of constituents with anti-inflammatory activities from Gentiana triflora Pall. Planta Med. 2013;79(8):680–686. doi: 10.1055/s-0032-1328460. [DOI] [PubMed] [Google Scholar]
- 18.Xie PS, Liang GH, Mao WZ. Analysis and quality investigation of bear bile. Chin J Pharm Anal. 1981;1(3):137. (in Chinese) [Google Scholar]
- 19.Yin J, Zhang ZW, Yu WJ, Liao JY, Luo XG, Shen YJ. Stachydrine, a major constituent of the Chinese herb Leonurus heterophyllus sweet, ameliorates human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Am J Chin Med. 2010;38(1):157–171. doi: 10.1142/S0192415X10007737. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Dong SQ, Chi LZ, He PG, Wang QJ, Fang YZ. Simultaneous determination of flavonoids in Chrysanthemum by capillary zone electrophoresis with running buffer modifiers. Talanta. 2008;76(4):780–784. doi: 10.1016/j.talanta.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Qi Y. Experimental studies of Xiongdankaiming tablet on lowering the intraocular pressure. J Changchun Univ Tradit Chin Med. 2007;1(23):27–28. (in Chinese) [Google Scholar]
- 22.Zhao M, Qi Y, Han Y. Animal experiments on analgesic and anti-inflammatory effects of Xiongdankaiming tablet. J Norman Bethune Univ Med Sci. 1999;3(25):44. (in Chinese) [Google Scholar]
- 23.Zhao Y, Zan LX, Sun WJ. HPLC-ELSD determination of tauroursodeoxycholic acid and taurochenodeoxycholic acid in bear biliary drainage powder. Chin J Pharm Anal. 2006;26(1):127–129. (in Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The electronic supplementary materials mainly cover: (1) identification of 20 constituents in XDKMT by HPLC-PDA/ESI-MSn; (2) optimization of sample preparation procedure; and, (3) optimization of the HPLC chromatographic conditions.