Abstract
Objective: Labisia pumila var. alata, commonly known as ‘Kacip Fatimah’ or ‘Selusuh Fatimah’ in Southeast Asia, is traditionally used by members of the Malay community because of its post-partum medicinal properties. Its various pharmaceutical applications cause an excessive harvesting and lead to serious shortage in natural habitat. Thus, this in vitro propagation study investigated the effects of different plant growth regulators (PGRs) on in vitro leaf and stem explants of L. pumila. Methods: The capabilities of callus, shoot, and root formation were evaluated by culturing both explants on Murashige and Skoog (MS) medium supplemented with various PGRs at the concentrations of 0, 1, 3, 5, and 7 mg/L. Results: Medium supplemented with 3 mg/L indole-3-butyric acid (IBA) showed the optimal callogenesis from both leaf and stem explants with (72.34±19.55)% and (70.40±14.14)% efficacy, respectively. IBA was also found to be the most efficient PGR for root induction. A total of (50.00±7.07)% and (77.78±16.47)% of root formation were obtained from the in vitro stem and leaf explants after being cultured for (26.5±5.0) and (30.0±8.5) d in the medium supplemented with 1 and 3 mg/L of IBA, respectively. Shoot formation was only observed in stem explant, with the maximum percentage of formation ((100.00±0.00)%) that was obtained in 1 mg/L zeatin after (11.0±2.8) d of culture. Conclusions: Callus, roots, and shoots can be induced from in vitro leaf and stem explants of L. pumila through the manipulation of types and concentrations of PGRs.
Keywords: Auxin, Cytokinin, Labisia pumila, In vitro propagation, Plant growth regulators
1. Introduction
Labisia pumila, also known as Labisia pothonia in Latin, is popularly known as ‘Kacip Fatimah’ (Fatimah’s betal scissors) or ‘Selusuh Fatimah’ (literally Fatimah’s childbirth medicine) in Malaysia (Abdul Kadir et al., 2012). It belongs to the family of Myrsinaceae. L. pumila is a sub-herbaceous plant with creeping stems and is mainly found in the lowland and hill forests in Southeast Asia, particularly Malaysia, Indonesia, Thailand, Laos, Cambodia, and Vietnam (Farouk et al., 2008) at an altitude between 300 and 700 m (Zaizuhana et al., 2006). L. pumila is mostly obtained from the natural tropical forest (Md Ariff et al., 2013) and grows mostly on seasonal wetlands and undulating land under the forest canopy (JIRCAS-SFD Joint Research Project, 2007). In the Malay tradition, a water decoction from the roots or whole plant of L. pumila is often given to pregnant women between one to two months before delivery, as this is believed to induce and expedite labour (Jamia et al., 2003). In addition, it is also used by Malay women as post-partum medicine, for flatulence, dysentery, dysmenorrhoea and gonorrhoea, ‘sickness in the bones’ as well as haemorrhoids (Zaizuhana et al., 2006). The availability of L. pumila in the forest is becoming scarce due to an increase in market demand, its slow growth rate in its natural habitat and logging activities in the forests (Md Ariff et al., 2013). It is anticipated that L. pumila will face extinction and severe genetic loss if necessary cultivation steps are not taken. In this case, plant tissue culture techniques may be able to facilitate the large-scale production of valuable clones to avoid further depletion of this species from its natural habitat (Conde et al., 2008). Significant features of in vitro propagation are the enormous multiplication capacity in a relatively short time span, the production of healthy and disease-free plants, and the ability to generate propagules throughout the year independent of seasonal changes (Christensen and Sriskandarajah, 2008). Additionally, it is also a useful method to propagate endangered plant species with the purpose of optimizing the time and cost required to aid in preservation (Christensen and Sriskandarajah, 2008). In view of the importance and advantages of plant tissue culture, this study aimed to determine the effects of supplementation of various plant growth regulators (PGRs) on in vitro leaf and stem explants of L. pumila.
2. Materials and methods
2.1. Plant materials
The in vitro leaf and stem explants of L. pumila were obtained from plantlets cultured on Murashige and Skoog (1962) medium (MS medium) containing 1 mg/L of benzylamino purine (BAP) (Duchefa, the Netherlands).
2.2. Preparation of medium
Full strength MS medium was used as the basal medium in all the cultures. The basal medium was supplemented with auxin or cytokinin at the concentrations of 0, 1, 3, 5, and 7 mg/L. A total of 30 g/L sucrose was added and the pH of the medium was adjusted with 0.1 mol/L NaOH or 0.1 mol/L HCl to 5.7±0.1. The medium was then supplemented with 8 g/L of plain agar powder (Copens, Malaysia) and autoclaved at 121 °C, 10 342 N/m2 for 15 min. Then, approximately 25 ml medium was poured into Petri dishes. Meanwhile, to prepare MS medium in culture vials, the plain agar added was dissolved completely by heating in a microwave for a few minutes. After that, approximately 15 ml of the medium was distributed into the culture vials and covered with aluminium foil prior to autoclaving. After autoclaving, the media were slanted at 45° and stored in the culture room prior to initiation of treatment.
2.3. Effects of plant growth regulators
The leaf explants were cut into squares (4 mm×4 mm) using sterile scalpels. Three explants were placed on the medium and the Petri dish was then sealed with parafilm. As for the stem explants, they were cut approximately into 5 mm in length using sterile scalpels. Explants were then placed on the culture vials containing MS medium supplemented with either indole-3-butyric acid (IBA) (Duchefa, the Netherlands), naphthaleneacetic acid (NAA) (R&M Marketing, UK), zeatin (R&M Marketing, UK), or kinetin (Duchefa, the Netherlands) at 0, 1, 3, 5, and 7 mg/L. All the cultures were then kept in the culture room at (25±1) °C with the photoperiod of 16 h light and 8 h dark. Cultures were observed daily for eight weeks. The effects of auxins on callus development were evaluated based on the day and degree of callus formed, percentage of callus formation as well as the morphology and texture of the callus formed. In addition, the effects of PGRs on shoot and root development were also evaluated based on the percentage and day of shoot and root formation. Additionally, the numbers of roots and shoots formed as well as the length of the longest root or shoot were recorded. The percentages of rooting and shooting for all the explants were calculated after eight weeks of culture. The number of roots per explant was also counted for each culture.
For each treatment, three replicates with three explants were carried out and the experiment was repeated twice. However, due to the limited number of explants, the experiments were carried out in five replicates with one repetition for the in vitro stem explants.
2.4. Statistical analysis
Data collected in the experiments were analyzed using SPSS Version 17.0. The means and the differences within the treatments were compared using one-way analysis of variance (ANOVA). Post hoc Tukey’s honestly significant difference (HSD) test was also performed at P≤0.05.
3. Results and discussion
3.1. Callus induction from in vitro leaf and stem explants
For the callogenesis from the in vitro leaf explants of L. pumila, calli were initiated from the cut margin of the explants in the presence of suitable medium. Among the PGRs tested, only IBA treatments were successful in showing different degrees of callusing whereas NAA, zeatin, and kinetin failed to stimulate any callus formation from the leaf explants. MS medium supplemented with IBA showed the optimal callus induction from the leaf explants (Table 1). The highest percentage of callus formation from the leaf explants was (72.34±19.55)% in the MS medium supplemented with 3 mg/L of IBA. IBA at the concentrations of 1 and 5 mg/L recorded (66.67±0.00)% and (33.34±11.16)% of callus initiation, respectively. Leaf explants in 7 mg/L of IBA did not show any response in callus initiation. Hard, compact, and greenish or whitish calli (Fig. 1a) were induced from swollen leaf explants in the treatments of 1, 3, and 5 mg/L.
Table 1.
Effects of MS medium supplemented with different plant growth regulators (PGRs) at various concentrations on callus formation from the in vitro leaf explants of L. pumila var. alata
| PGR treatment* | Percentage of callus formation (%) | Day of callus formation | Morphology and colour of callus | Degree of callus formation |
| Control | −a | −a | − | − |
| 1 mg/L IBA | 66.67±0.00b | 17.5±5.0b | Greenish, hard, compact | + |
| 3 mg/L IBA | 72.34±19.55b | 17.5±2.3b | Yellowish, hard, compact | + |
| 5 mg/L IBA | 33.34±11.16ab | 9.5±4.5ab | Yellowish, hard, compact | + |
| 7 mg/L IBA | −a | −a | − | − |
No callus formation from the in vitro leaf explants in medium supplemented with NAA, kinetin, and zeatin
Degree of callus formation: −, absence of callus; +, poor callusing. Values represent mean±standard deviation (SD) of two replicates per treatment. Mean followed by the same letter in the same column did not differ significantly at P≤0.05 according to Tukey’s HSD test
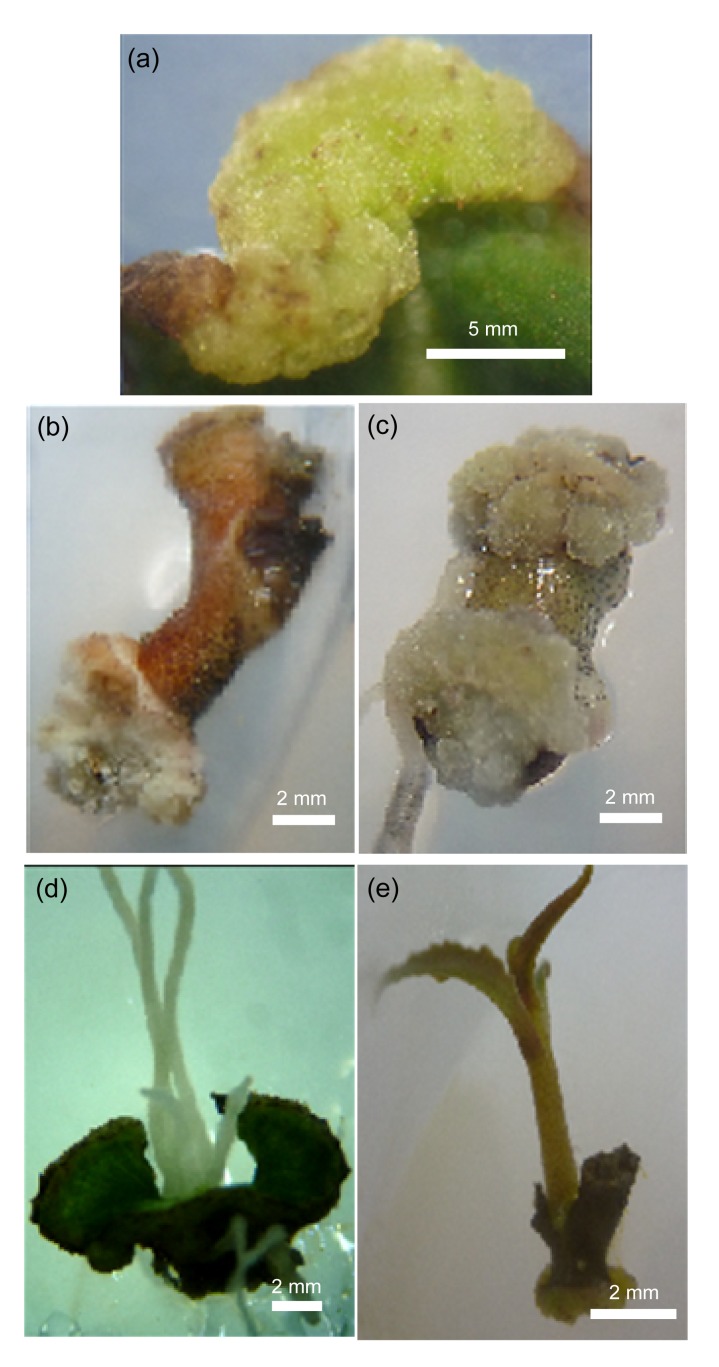
Fig. 1.
Effects of plant growth regulators (PGRs) on leaf and stem explants of L. pumila after eight weeks of culture
(a) Callus induced from leaf explants treated with 5 mg/L IBA; (b) Callus formed in stem explants cultured in basal MS medium; (c) Callus induced from stem explants treated with 3 mg/L IBA; (d) Root formation in leaf explants treated with 5 mg/L IBA; (e) Shoot formation in stem explants treated with 1 mg/L zeatin
The results revealed that there was (10.00±4.71)% of callusing on stem explants of L. pumila var. alata in basal MS medium which was used as the control (Table 2). The stem explants swelled after one week of culture and yellowish, friable calli were successfully induced from the cut edge of the stem explants after (9.5±4.5) d of culture (Fig. 1b). MS medium supplemented with IBA showed the greatest callus induction in stem explants. The maximum percentage ((70.40±14.14)%) of callus formation was achieved in the MS medium supplemented with 3 mg/L of IBA followed by 1 and 7 mg/L IBA which attained (60.04±0.00)% and (60.00±9.43)% of callus formation, respectively. Treatment with 5 mg/L IBA showed the earliest callus initiation ((17.5±5.7) d) on stem explants with relatively low percentage of callus formation ((50.00±14.14)%) as compared to other IBA treatments. Callus formation was initiated after approximately 20 d of culture from the remaining three concentrations. The calli initiated from swollen stem explants in the medium with 1 and 7 mg/L IBA appeared to be greenish, compact and hard, while whitish, compact and hard calli were induced from swollen stem explants in the medium with 3 mg/L of IBA (Fig. 1c). Meanwhile, light green, compact and hard calli were observed from the stem explants placed in the medium supplemented with 5 mg/L of IBA. The degree of callus formation from the stem explants placed in the medium supplemented with IBA was higher than that of the calli initiated from the leaf explants.
Table 2.
Effects of MS medium supplemented with different plant growth regulators (PGRs) at various concentrations on callus formation from the in vitro stem explants of L. pumila var. alata
| PGR treatment* | Percentage of callus formation (%) | Day of callus formation | Morphology and colour of callus | Degree of callus formation |
| Control | 10.00±4.71a | 9.5±4.5ab | Yellowish, compact, friable | + |
| 1 mg/L IBA | 60.04±0.00a | 20.0±4.2ab | Greenish, compact, hard | ++ |
| 3 mg/L IBA | 70.40±14.14a | 20.5±5.0ab | Whitish, compact, hard | +++ |
| 5 mg/L IBA | 50.00±14.14a | 17.5±5.7ab | Light green, compact, hard | +++ |
| 7 mg/L IBA | 60.00±9.43a | 22.0±3.8ab | Greenish, compact, hard | ++ |
| 1 mg/L zeatin | 40.00±18.86a | 7.0±3.3ab | Greenish, compact, hard | + |
| 3 mg/L zeatin | 40.00±0.00a | 24.0±4.9b | Greenish, compact, hard | + |
| 5 mg/L zeatin | −a | −a | − | − |
| 7 mg/L zeatin | −a | −a | − | − |
No callus formation from the in vitro stem explants in medium supplemented with NAA and kinetin
Degree of callus formation: −, absence of callus; +, poor callusing; ++, moderate callusing; +++, good callusing. Values represent mean±standard deviation (SD) of two replicates per treatment. Mean followed by the same letter in the same column did not differ significantly at P≤0.05 according to Tukey’s HSD test
For the callus induction, an explant is normally cultured on medium containing growth-regulating substances. The in vitro stem explants of L. pumila cultured in the control medium became swollen after three weeks of culture and eventually signs of callus formation were observed in the following week, though with a low frequency of callogenesis. Typically, this was a wound reaction where the mitosis was induced in the cells from the cut surface leading to callus formation (Pérez-Francés et al., 1995). In contrast, no sign of callus formation was observed on leaf explants of L. pumila in the control medium after eight weeks of cultivation. The leaf explants on the control medium become brown, unhealthy, and eventually died after five weeks of cultivation.
IBA is a synthetic auxin that is used commercially worldwide to initiate root growth in many species (Ludwig-Müller et al., 2005). Auxins play an important role in the mobilization of carbohydrates in leaves and upper stem as well as in the increase of their transport to the rooting zone (Husen and Pal, 2007). The induction of adventitious roots of L. pumila from both leaf and stem explants was preceded by callus formation in the medium supplemented with IBA. A similar observation was made by Ludwig-Müller et al. (2005) on Arabidopsis stem segments. Moreover, the low concentration (3 mg/L) of IBA was more efficient in callus induction among the four concentrations tested on both leaf and stem explants. A possible explanation for this result is that the high concentration of IBA in the medium may inhibit callus growth from both leaf and stem explants. A similar viewpoint has been reported by Khalafalla et al. (2007) where they noticed that the MS medium supplemented with 1 mg/L IBA promoted rapid growth and produced the highest callusing percentage (92.6%) whereas higher concentration (2 mg/L) induced lower callusing percentage (80.0%) on Azadirachta indica A. Juss. Further results obtained indicated that calli were formed from the stem explants in the MS medium supplemented with zeatin. Approximately 40% of the stem explants incubated in the MS medium supplemented with 1 and 3 mg/L of zeatin showed callus initiation. Calli were initiated after (7.0±3.3) and (24.0±4.9) d, respectively, on the medium supplemented with 1 and 3 mg/L of zeatin. As the concentration of zeatin increased, the degree of callus formation decreased. Greenish and compact calli were observed from the edge of the stem, without swelling. There was no callus formation from the stem explants which were cultured in the medium supplemented with 5 and 7 mg/L of zeatin. In short, zeatin showed relatively low percentages of callus induction response as compared to IBA. Zeatin is a naturally occurring cytokinin that was isolated from Zea mays and promotes cell division in callus tissue. In addition to functioning as a shoot growth inducer, zeatin also promotes callus growth, as seen in tobacco (Yamada et al., 1972). In the present study, both shoot induction and callus formation were induced from the in vitro stem explants of L. pumila cultured in medium containing zeatin. Cunha and Ferreira (1996) also concluded that several auxins and zeatin promoted the growth of Euphorbia characias calli.
3.2. Root induction from in vitro leaf and stem explants
Different explants responded differently to the PGRs. Among the explants used in the present study, leaf explants were found to be the best explants in adventitious root induction. This was demonstrated in terms of higher percentage of rooting, average number of roots formed per explants, and the average length of root formed on MS medium supplemented with auxins at various concentrations. Though roots were successfully induced from the stem explants, the frequency and amount of roots induced were relatively low when compared with the leaf explants. It was observed that, root generation always took place near the cut surface of the basal ends of the leaf and stem explants and roots grew into the air column above the medium. This might be due to the strong polarity in the regeneration of roots (Pierik and Steegmans, 1975).
Among the four types of PGRs used, only auxins (IBA and NAA) were successful in inducing rooting response. Based on the results obtained, the highest percentage ((77.78±16.47)%) of root induction resulted from leaf explants in the medium supplemented with 3 mg/L IBA. The percentages of root induced from the leaf explants in the medium supplemented with 1, 5, and 7 mg/L IBA were (66.67±5.24)%, (27.78±13.10)%, and (6.67±2.62)%, respectively. Meanwhile, the highest percentage of root formation ((33.34±15.71)%) among the four different concentrations of NAA tested was recorded in the MS medium supplemented with 1 mg/L NAA. The percentages of root formation from the leaf explants on the medium with 3 and 5 mg/L NAA were (22.22±0.00)% and (11.11±5.24)%, respectively. The percentage of root induced from the leaf explants declined as the concentration of NAA increased (Table 3). Data also showed that high concentration (7 mg/L) of NAA inhibited the growth of roots from leaf explants. Roots formed from leaf explants appeared as whitish, strong, and healthy (Fig. 1d).
Table 3.
Effects of MS medium supplemented with different plant growth regulators (PGRs) at various concentrations on root induction from the in vitro leaf explants of L. pumila var. alata
| PGR treatment* | Percentage of root formation (%) | Day of root formation | Number of root per explant | Length of root (cm) |
| Control | −a | −a | −a | −a |
| 1 mg/L IBA | 66.67±5.24ab | 30.0±2.8a | 2.0±0.1a | 0.89±0.20a |
| 3 mg/L IBA | 77.78±16.47b | 30.0±4.3a | 2.0±0.2a | 1.13±0.26a |
| 5 mg/L IBA | 27.78±13.10ab | 18.0±2.8a | 2.8±0.4a | 0.62±0.29a |
| 7 mg/L IBA | 6.67±2.62ab | 27.0±0.5a | 8.0±0.9a | 0.68±0.06a |
| 1 mg/L NAA | 33.34±15.71ab | 8.5±2.5a | 2.9±1.4a | 0.48±0.23a |
| 3 mg/L NAA | 22.22±0.00ab | 18.0±8.5a | 7.0±0.9a | 0.88±0.11a |
| 5 mg/L NAA | 11.11±5.24ab | 10.5±4.9a | 3.0±1.4a | 0.25±0.12a |
| 7 mg/L NAA | −a | −a | −a | −a |
No root formation from the in vitro leaf explants in medium supplemented with kinetin and zeatin
Values represent mean±standard deviation (SD) of two replicates per treatment. Mean followed by the same letter in the same column did not differ significantly at P≤0.05 according to Tukey’s HSD test
In the present study, roots were formed directly around the midvein of the leaf explants. This might be due to the presence of cells associated with the leaf veins, which could be readily stimulated by the addition of auxins. The use of leaf explants in tissue culture has the advantage of being a system where phytochromes can be easily manipulated to direct pluripotent cells to a particular cell fate (Nolan et al., 2003; Thomas et al., 2004; Imin et al., 2005). Vein-associated cells are then stimulated to divide in response to auxins and grow distinctive sheet cells that originate from the vein of the leaf explants. These vein-derived cells are procambial-like and function as pluripotent stem cells with a tendency to form root meristems or vascular tissues in response to added auxin (Rose et al., 2006). A series of experiments with Cyclamen persicum led to the conclusion that an increased density of vascular tissue will increase root regeneration as a result of the level of phytochromes and other metabolites that are present in the leaf midvein (Sreedhar et al., 2008). Furthermore, the ability of leaf explants in producing its endogenous auxins might also contribute to the more effective adventitious root formation from the leaf than that of stem explants which lack this ability (Vesperinas, 1998).
Among the four types of PGRs tested, only IBA and NAA were successful in showing a positive rooting response on the stem explants. Among four different concentrations of IBA tested, the highest percentage ((50.00±7.07)%) of roots was induced from stem explants in the medium supplemented with 1 mg/L IBA (Table 4). The medium supplemented with 3, 5, and 7 mg/L of IBA, achieved (30.00±7.07)%, (10.00±4.71)% and (20.00±0.00)% of rooting, respectively. The medium with 5 mg/L IBA showed the earliest root initiation ((15.0±7.7) d) from the stem explants while around 26 to 28 d were required for the stem explants to induce root growth in the medium supplemented with 1, 3, and 7 mg/L IBA. Roots that were formed in 1 mg/L IBA appeared to be slimmer, yellowish and blackish in color, while roots grown from the medium with 3, 5, and 7 mg/L IBA were stronger and healthier. Apart from IBA, a total of (40.00±9.43)% of the stem explants in the MS medium containing 1 mg/L NAA also showed rooting response. The percentage of root formation from the stem explants declined as the concentration of NAA increased from 1 to 3 mg/L. Besides that, higher concentrations (5 and 7 mg/L) of NAA inhibited the growth of roots from the stem explants. A significant difference in the percentage of roots formation from the stem explants between IBA and NAA tested at various concentrations was observed based on Tukey’s HSD test. At 1 mg/L NAA, a longer period of (40.0±6.4) d was needed to initiate the root from the stem explants as compared to 3 mg/L which only required (15.5±7.3) d. Whitish, strong and healthy roots were formed from the stem explants in the medium with 1 and 3 mg/L of NAA. The longest roots formed from stem explants were (0.35±0.07) cm and (0.05±0.01) cm long at the concentrations of 1 and 3 mg/L NAA, with 1.8±0.4 and 0.5±0.2 roots per explants, respectively.
Table 4.
Effects of MS medium supplemented with different plant growth regulators (PGRs) at various concentrations on root induction from the in vitro stem explants of L. pumila var. alata
| PGR treatment* | Percentage of root formation (%) | Day of root formation | Number of root per explant | Length of root (cm) |
| Control | 10.00±4.71ab | 16.0±7.53a | 0.5±0.2a | 0.50±0.23ab |
| 1 mg/L IBA | 50.00±7.07b | 26.5±1.7a | 4.3±0.2b | 1.12±0.15b |
| 3 mg/L IBA | 30.00±7.07ab | 28.0±6.6a | 2.0±0.5ab | 0.60±0.07ab |
| 5 mg/L IBA | 10.00±4.71ab | 15.0±7.7a | 0.5±0.5a | 0.10±0.05a |
| 7 mg/L IBA | 20.00±0.00ab | 28.5±6.8a | 1.0±0.0a | 0.30±0.05ab |
| 1 mg/L NAA | 40.00±9.43ab | 40.0±6.4a | 1.8±0.4a | 0.35±0.07ab |
| 3 mg/L NAA | 20.00±3.54ab | 15.5±7.3a | 0.5±0.2a | 0.05±0.01a |
| 5 mg/L NAA | −a | −a | −a | −a |
| 7 mg/L NAA | −a | −a | −a | −a |
No root formation from the in vitro stem explants in medium supplemented with kinetin and zeatin
Values represent mean±standard deviation (SD) of two replicates per treatment. Mean followed by the same letter in the same column did not differ significantly at P≤0.05 according to Tukey’s HSD test
Stem explants that showed some degree of rooting could be due to the presence of procambial-like tissue in the structure surrounding the vascular tissue. Although stem explants are capable of inducing adventitious roots, the efficiency of rooting was optimized by using leaf explants. It was found that IBA was a much better PGR in inducing roots from L. pumila var. alata stem explant compared to NAA. Studies by Marks and Simpson (2000) found that stem explants from in vitro culture could induce root formation when treated with IBA. Similarly, the effect of IBA on root induction has been reported in plants like Caltropis gigantean (Sahoo and Chand, 1998) and Tridax procumbens (Roy and De, 1990).
IBA has been known as a synthetic auxin for a long time and is the major auxin used commercially for the induction of adventitious roots in stem and leaf cuttings (Srivastava, 2001). IBA is derived from indole acetic acid (IAA) via a chain elongation reaction similar to that found in fatty acid biosynthesis. Besides that, IBA can be converted to IAA after being broken down by peroxisomes through the process of β-oxidation (the same process used to metabolize fatty acids) (Roberts, 2007). Thus, IBA may also be part of the machinery that maintains IAA homeostasis (Srivastava, 2001).
As different concentrations of IBA were applied, different results in rooting efficiency were obtained. It showed that a specific concentration was vital in inducing rooting. In this study, the MS medium supplemented with 3 mg/L IBA demonstrated a better response with the highest percentage of rooting as well as the longest root formation on leaf explants as compared to the other concentrations of IBA tested. Meanwhile, as the concentration of IBA increased from 3 to 7 mg/L, lower rooting efficiency from the leaf explants was observed. This could be explained by the fact that auxins at high concentration may possess herbicidal properties which inhibit the adventitious root induction from leaf explants (Evans et al., 2003).
IBA was believed to be the most suitable PGR in inducing roots from leaf explants. This was verified by the higher rooting percentage as well as number of roots formed per explant. Thus, results of this study showed that IBA was more effective than NAA in adventitious root induction from L. pumila var. alata leaf explants. Khalafalla et al. (2009) have similar results in Vernonia amygdalina. However, in another study by Leonardi et al. (2001), the effects of IBA and NAA on in vitro rooting were similar in Grevillea rosmarinifolia. Contrary to this study, Watas et al. (1992) reported that NAA was more effective than IBA in promoting root formation in some Grevillea species. A similar observation was reported by Cozza et al. (1997), where NAA was the best rooting hormone in Olea europaea. However, the effect of auxin on adventitious root induction and elongation is highly dependent on the plant type (Nandagopal and Kumari, 2007).
The lower efficiency of NAA in rooting could be explained by the connection between levels of endogenous IAA and adventitious root formation. It might be due to the exogenously applied synthetic auxin (NAA) that has not been efficiently oxidized to IAA for plant cell utilization. Hence, due to inadequate supply of IAA, the explants showed lower ability in root initiation. This was evidenced by Liu and Sanford (1988) who mentioned that endogenous IAA was detected in root explants on media supplemented with NAA. Furthermore, more energy may be needed by the explants to convert the absorbed synthetic NAA from the medium to a natural form of IAA before being used by the explants. This condition could likely explain the low efficiency in root induction on explants placed in medium added with NAA. Consequently, additional energy would be used up and might eventually lead to insufficient energy needed for cell growth and development. Zolman et al. (2008) demonstrated that energy is needed in converting NAA to IAA, hence, displaying reduced responses of NAA in root elongation.
3.3. Shoot induction from in vitro stem explants
Among the explants tested, only stem explants showed shoot formation in the MS medium supplemented with IBA, zeatin, and kinetin. Zeatin and kinetin showed a better shoot induction response as compared to IBA (Table 5). From the observation, shoot formation begins at the cut margin of the stem explant. Explants treated with zeatin showed the best response in shoot induction. A total of 100% of shoot induction was observed in the MS medium containing 1 mg/L of zeatin (Fig. 1e). However, as the concentration of zeatin increased from 1 to 3 and 5 mg/L, there was 20% of decrease on the percentage of shoot induction to (80.00±7.07)% in both concentrations. Based on the parameters tested in this study, kinetin was found to be a superior PGR in terms of shoot formation stimulation as compared to IBA, though it did not show stimulation similar to that seen with zeatin. The percentage of shoot induction declined as the concentration of kinetin was increased, with the greatest percentage ((90.00±14.14)%) corresponding to the medium supplemented with 1 mg/L kinetin. Among all the concentrations of IBA tested, only 3 and 5 mg/L showed shoot development from the stem explants. A total of (70.00±4.71)% of the stems cultured in 3 mg/L formed shoots while in 5 mg/L, (40.00±0.00)% of them formed shoots.
Table 5.
Effects of MS medium supplemented with different plant growth regulators (PGRs) at various concentrations on shoot induction from the in vitro stem explants of L. pumila var. alata
| PGR treatment* | Percentage of shoot induction (%) | Day of shoot initiation | Number of shoot per explant | Length of shoot (cm) |
| Control | −a | −a | −a | −a |
| 1 mg/L zeatin | 100.00±0.00d | 11.0±0.9a | 1.4±0.3abc | 0.63±0.14a |
| 3 mg/L zeatin | 80.00±7.07d | 15.5±1.1a | 1.8±0.2abcd | 0.68±0.10a |
| 5 mg/L zeatin | 80.00±7.07cd | 15.5±3.1a | 2.3±0.1bcd | 0.36±0.05a |
| 7 mg/L zeatin | 90.00±3.53cd | 15.5±0.7a | 3.3±0.4de | 0.35±0.04a |
| 1 mg/L kinetin | 90.00±14.14cd | 17.5±0.7a | 1.4±0.1abc | 0.70±0.09a |
| 3 mg/L kinetin | 60.00±7.07bcd | 20.0±1.4a | 1.3±0.1abc | 0.44±0.03a |
| 5 mg/L kinetin | 50.00±4.71abcd | 18.5±2.1a | 1.0±0.0ab | 0.43±0.08a |
| 7 mg/L kinetin | 20.00±0.00ab | 29.0±2.8a | 1.0±0.0ab | 0.30±0.05a |
| 1 mg/L IBA | −a | −a | −a | −a |
| 3 mg/L IBA | 70.00±4.71bcd | 30.5±7.8a | 2.8±0.1ced | 0.48±0.18a |
| 5 mg/L IBA | 40.00±0.00abc | 30.5±7.8a | 4.5±0.2e | 0.10±0.00a |
| 7 mg/L IBA | −a | −a | −a | −a |
No shoot formation from the in vitro stem explants in medium supplemented with NAA
Values represent mean±standard deviation (SD) of two replicates per treatment. Mean followed by the same letter in the same column did not differ significantly at P≤0.05 according to Tukey’s HSD test
In the present study, stem explants were found to be the most suitable explants in adventitious shoot induction of L. pumila. The reason could be that in all mature tissues, cytokinins of zeatin-type were predominant and they were the major translocatable form of hormone in xylem (Nandi et al., 1990; Dieleman et al., 1997). Thus, formation of adventitious shoot was easier from the stem explants. Zeatin is a naturally occurring isoprenoid cytokinin which exists in two isoforms, trans- and cis-zeatin (Roberts, 2007). Both isoforms have been found in a wide range of plant species, also indicating a biological function for both forms. Dieleman et al. (1997) suggested that zeatin in the xylem might be involved in the onset of axillary bud growth. It was also found to be effective for shoot initiation in Vaccinium species (Reed and Esquivel, 1991), and for shoot proliferation of lingonberry (Debnath and McRae, 2005) and highbush blueberry (Eccher and Noe, 1989).
Zeatin was found to be the best PGR among the other PGRs tested in inducing shoots from the stem explants of L. pumila. Similarly, it was evidenced by Molina et al. (2007) that zeatin, instead of kinetin, was most effective in shoot formation of Troyer citrange. However, the developmental response of the explants to exogenous hormones is related to the hormone concentration in the medium. This might be due to the cells within the same plant having different levels of endogenous PGRs and additional variations in receptor affinity or cellular sensitivity to PGRs (Minocha, 1987). The developmental response could also be affected by the hormone uptake, metabolism and transport within the explant (Auer et al., 1999). In this study, 1 mg/L of zeatin was the best cytokinin in inducing shoot growth from the stem explants. However, as the concentration of zeatin increased from 1 to 7 mg/L, the shoot length decreased from (0.63±0.41) cm to (0.35±0.04) cm. This might be due to the high concentration of zeatin that may slow down the growth of the shoot induced by changing the normal morphology of the regenerated plant. Debnath (2008) discovered that a high concentration of zeatin was more effective for the shoot proliferation of highbush blueberry.
In kinetin treatment, a trend of decrease in shooting efficiency as well as in the number of shoot formation per explant was recorded in response to increasing kinetin concentration from 1 to 7 mg/L. Medium containing higher concentration of kinetin may be ascribed to toxic effects and eventually gave rise to the lowest regeneration response (Wang et al., 2007). This statement was supported by an earlier study which stated that a low concentration of cytokinin was more effective for inducing shoot growth in Tridax procumbens L. (Sahoo and Chand, 1998).
Despite the fact that IBA is an endogenous auxin, IBA did stimulate the initiation of adventitious shoot formation from the stem explants in this study. In plants, cytokinin is usually translocated from root to shoot in the transpiration stream, the uninterrupted stream of water which is taken up by the roots and via xylem vessels (Kuroha and Satoh, 2007). Hence, the presence of endogenous cytokinin in the stem tissues (Nandi et al., 1990) with the addition of auxin into the medium might have eventually promoted the formation of shoot from the explants. This was shown by Su et al. (2011) who found that a low concentration of auxin combined with cytokinin might aid in shoot initiation. Hence, the ratio of cytokinin to auxin is a critical determinant of organogenesis in plant tissue culture (Xu et al., 2008). Aggarwal et al. (2010) found that the combination of auxin and cytokinin is effective in inducing shoot from the leaf explant of Eucalyptus tereticornis.
4. Conclusions
In the present study, four types of PGRs IBA, NAA, zeatin, and kinetin, were tested on the in vitro leaf as well as stem explants of L. pumila var. alata. Based on the results, the explants responded differently towards the types of PGRs. Calli were induced in MS medium supplemented with IBA on leaf explants, as well as in MS medium with IBA and zeatin on stem explants. Roots were induced from explants in MS medium supplemented either with IBA or NAA. Meanwhile, MS medium added with IBA, zeatin, and kinetin promoted shoot induction from the stem explants.
Footnotes
Compliance with ethics guidelines: Anna Pick Kiong LING, Kinn Poay TAN, and Sobri HUSSEIN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abdul Kadir A, Nik Hussain NH, Wan Bebakar WM, Mohd DM, Wan Mohammad WMZ, Hassan II, Shukor N, Kamaruddin NA, Wan Mohd WN. The effect of Labisia pumila var. alata on postmenopausal women: a pilot study. Evid Based Complement Alternat Med. 2012;2012:216525. doi: 10.1155/2012/216525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal D, Kumar A, Reddy MS. Shoot organogenesis in elite clones of Eucalyptus tereticornis . Plant Cell Tiss Org Cult. 2010;102(1):45–52. doi: 10.1007/s11240-010-9703-y. [DOI] [Google Scholar]
- 3.Auer CA, Motyka V, Březinová A, Kamínek M. Endogenous cytokinin accumulation and cytokinin oxidase activity during shoot organogenesis of Petunia hybrida . Physiol Plant. 1999;105(1):141–147. doi: 10.1034/j.1399-3054.1999.105121.x. [DOI] [Google Scholar]
- 4.Christensen B, Sriskandarajah S. In vitro culture of Hibiscus rosa-sinensis L.: influence of iron, calcium and BAP on establishment and multiplication. Plant Cell Tiss Org Cult. 2008;93(2):151–161. doi: 10.1007/s11240-008-9354-4. [DOI] [Google Scholar]
- 5.Conde P, Sousa A, Costa A, Santos C. A protocol for Ulmus minor Mill. micropropagation and acclimatization. Plant Cell Tiss Org Cult. 2008;92(1):113–119. doi: 10.1007/s11240-007-9310-8. [DOI] [Google Scholar]
- 6.Cozza R, Turco D, Bati CB, Bitonti MB. Influence of growth medium on mineral composition and leaf histology in micropropagated plantlets of Olea europaea . Plant Cell Tiss Org Cult. 1997;51(3):215–223. doi: 10.1023/A:1005966404642. [DOI] [Google Scholar]
- 7.Cunha ACGD, Ferreira MF. Somatic embryogenesis, organogenesis and callus growth kinetics of flax (Linum usitatissium L.) Plant Cell Tiss Org Cult. 1996;47(1):1–8. doi: 10.1007/BF02318959. [DOI] [Google Scholar]
- 8.Debnath SC. Zeatin-induced one-step in vitro cloning affects the vegetative growth of cranberry (Vaccinium macrocarpon Ait.) micropropagules over stem cuttings. Plant Cell Tiss Org Cult. 2008;93(2):231–240. doi: 10.1007/s11240-008-9366-0. [DOI] [Google Scholar]
- 9.Debnath SC, McRae KB. A one-step in vitro cloning procedure for cranberry (Vaccinium macrocarpon Ait.): the influence of cytokinins on shoot proliferation and rooting. Small Fruits Rev. 2005;4(3):57–75. doi: 10.1300/J301v04n03_05. [DOI] [Google Scholar]
- 10.Dieleman JA, Verstappen FWA, Nicander B, Kuiper D, Tillberg E, Tromp J. Cytokinins in Rosa hybrida in relation to bud break. Physiol Plant. 1997;99(3):456–464. doi: 10.1111/j.1399-3054.1997.tb00560.x. [DOI] [Google Scholar]
- 11.Eccher T, Noe N. Comparison between 2iP and zeatin in the micropropagation of highbush blueberry (Vaccinium corymbosum) Acta Hort. 1989;241:185–190. [Google Scholar]
- 12.Evans DE, Coleman JOD, Kearns A. Basics Plant Tissue Culture. New York: BIOS Scientific Publishers; 2003. Callus Cultures; pp. 63–67. [Google Scholar]
- 13.Farouk AE, Nawi MN, Hassan S. Antibacterial peptides from Euycoma longifolia (Tongkat Ali) and Labisia pumila (Kacip Fatimah) leaves in Malaysia. Sci Brun. 2008;9:55–63. [Google Scholar]
- 14.Husen A, Pal M. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxins (IBA and NAA) treatment. New Forests. 2007;33(3):309–323. doi: 10.1007/s11056-006-9030-7. [DOI] [Google Scholar]
- 15.Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG. Proteomic analysis of somatic embryogenesis in Medicago truncatula explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol. 2005;137(4):1250–1260. doi: 10.1104/pp.104.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamia AJ, Houghton PJ, Milligan SR, Ibrahim J. The oestrogenic and cytotoxic effects of the extracts of Labisia pumila and Labisia pumila var. pumila in vitro. J Sains Kesihatan Malaysia. 2003;1:53–60. [Google Scholar]
- 17.JIRCAS-SFD Joint Research Project. Agroforestry Approach to the Rehabilitation of Tropical Lands by Using Nurse Trees. Malaysia: JIRCAS-Sabah Forestry Department (SFD); 2007. [Google Scholar]
- 18.Khalafalla MM, Gaali EEI, Abbas FM, Ali HA. Neem (Azadirachta indica A. Juss) callus induction and its larvaecidal activity against Anopheles mosquito . Int J Biotechnol Biochem. 2007;3(1):85–94. [Google Scholar]
- 19.Khalafalla MM, Daffalla HM, El-Shemy HA, Abdellatef E. Establishment of in vitro fast-growing normal root culture of Vernonia amygdalina—a potent African medicinal plant. Afr J Biotechnol. 2009;8(2):5952–5957. [Google Scholar]
- 20.Kuroha T, Satoh S. Involvement of cytokinins in adventitious and lateral root formation. Plant Root. 2007;1:27–33. doi: 10.3117/plantroot.1.27. [DOI] [Google Scholar]
- 21.Leonardi C, Ruggen A, Malfa SI. Hormone effects on in vitro proliferation and rooting of Grevillea explants. Sci Hort. 2001;90(3-4):335–341. doi: 10.1016/S0304-4238(01)00228-X. [DOI] [Google Scholar]
- 22.Liu ZR, Sanford JC. Plant regeneration by organogenesis from strawberry leaf and runner tissue. HortScience. 1988;23:1056–1059. [Google Scholar]
- 23.Ludwig-Müller J, Vertocnik A, Town CD. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J Exp Bot. 2005;56(418):2095–2105. doi: 10.1093/jxb/eri208. [DOI] [PubMed] [Google Scholar]
- 24.Marks TR, Simpson SE. Rhizogenesis in Forsythia intermedia and Syringa vulgaris: application of a simple internode experimental system. Plant Cell Rep. 2000;19(12):1171–1176. doi: 10.1007/s002990000264. [DOI] [PubMed] [Google Scholar]
- 25.Md Ariff FF, Hashim SS, Haja M, Osman M. An assessment of genetic relationship among superior accessions of Labisia pumila analyzed by amplified fragment length polymorphism (AFLP) markers. Open Sci Reposit Agric. 2013:e70081945. doi: 10.7392/Agriculture.70081945. [DOI] [Google Scholar]
- 26.Minocha SC. Cell and Tissue Culture in Forestry. Dordrecht: Martinus Nijhoff Publisher; 1987. Plant Growth Regulators and Morphogenesis in Cell and Tissue Culture of Forest Trees; pp. 50–66. [DOI] [Google Scholar]
- 27.Molina RV, Castello S, Luis GA, Guardiola JL. Light cytokinin interaction in shoot formation in epicotyl cuttings of Troyer citrangr cultured in vitro. Plant Cell Tiss Org Cult. 2007;89(2-3):131–140. doi: 10.1007/s11240-007-9221-8. [DOI] [Google Scholar]
- 28.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 29.Nandagopal S, Kumari RBD. Effectiveness of auxin induced in vitro root culture in chicory. J Cent Eur Agric. 2007;8(1):73–80. [Google Scholar]
- 30.Nandi SK, de Klerk GJM, Parker CW, Palni Nandi LMS. Endogenous cytokinin levels and metabolism of zeatin riboside in genetic tumour tissue and non-tumorous tissues of tobacco. Physiol Plant. 1990;78(2):197–204. doi: 10.1111/j.1399-3054.1990.tb02081.x. [DOI] [Google Scholar]
- 31.Nolan KE, Irwanto RR, Rose RJ. Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenesis cultures. Plant Physiol. 2003;133(1):218–230. doi: 10.1104/pp.103.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Francés JF, Valdés F, Msrtín R. Callus induction and culture from explants of Erysimum scoparium in a growth regulator-free medium. Plant Cell Tiss Org Cult. 1995;43(3):223–228. doi: 10.1007/BF00039948. [DOI] [Google Scholar]
- 33.Pierik RLM, Steegmans HHM. Analysis of adventitious root formation in insolated stem explants of Rhododendron . Sci Hort. 1975;3(1):1–20. doi: 10.1016/0304-4238(75)90031-X. [DOI] [Google Scholar]
- 34.Reed BM, Esquivel AA. The used of zeatin to initiate in vitro cultures of Vaccinium species and cultivars. HortScience. 1991;26:1320–1322. [Google Scholar]
- 35.Roberts K. Handbook of Plant Science. USA: John Wiley & Sons Ltd; 2007. Auxin; pp. 352–360. [Google Scholar]
- 36.Rose RJ, Wang XD, Nolan KE, Rolfe BG. Root meristems in Medicago truncatula tissue culture arise from vascular-derived procambial-like cells in a process regulated by ethylene. J Exp Bot. 2006;57(10):2227–2235. doi: 10.1093/jxb/erj187. [DOI] [PubMed] [Google Scholar]
- 37.Roy AT, De DN. Tissue culture and plant regeneration from immature embryo explants of Calotropis gigantean (L.) R. Br. Plant Cell Tiss Org Cult. 1990;20:229–233. doi: 10.1007/BF00041886. [DOI] [Google Scholar]
- 38.Sahoo Y, Chand PK. In vitro multiplication of a medicinal herb, Tridax procumbens L. (Mexican daisy, Coatbuttons): influence of explanting season, growth regulator synergy, culture passage and planting substrate. Phytomorphology. 1998;48(2):195–205. [Google Scholar]
- 39.Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS, Ravishankar GA. Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol Plant. 2008;52(2):355–360. doi: 10.1007/s10535-008-0073-9. [DOI] [Google Scholar]
- 40.Srivastava LM. Plant Growth and Development: Hormones and Environment. USA: Academic Press; 2001. Auxins; pp. 155–171. [Google Scholar]
- 41.Su YH, Liu YB, Zhang XS. Auxin-cytokinin interaction regulates meristem development. Mol Plant. 2011;4(4):616–625. doi: 10.1093/mp/ssr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas C, Meyer D, Himber C, Steinmetz A. Spatial expression of sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem. 2004;42(1):35–42. doi: 10.1016/j.plaphy.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Vesperinas ES. In vitro root induction in hypocotyl and plumule explants of Helianthus annuus . Env Exp Bot. 1998;39(3):271–277. doi: 10.1016/S0098-8472(98)00019-7. [DOI] [Google Scholar]
- 44.Wang B, Peng DX, Liu LJ, Sun ZH, Zhang N, Gao SM. An efficient adventitious shoot regeneration system for ramie (Boehmeria nivea Gaud) using thidiazuron. Bot Stud. 2007;93:173–180. [Google Scholar]
- 45.Watas AA, Ben JJ, Tal E, Solomon H. In vitro propagation of Grevillea species. Acta Hort. 1992;316:51–54. [Google Scholar]
- 46.Xu Z, Um YC, Kim CH, Lu G, Guo DP, Liu HL, Bah AA, Mao A. Effect of plant growth regulators, temperature and sucrose on shoot proliferation from the stem disc of Chinese jiaotou (Allium chinense) and in vitro bulblet formation. Acta Physiol Plant. 2008;30(4):521–528. doi: 10.1007/s11738-008-0150-x. [DOI] [Google Scholar]
- 47.Yamada Y, Sekiya J, Koshimizu K. Cytokinin-induced shoot formation. Phytochemistry. 1972;11(3):1019–1021. doi: 10.1016/S0031-9422(00)88447-2. [DOI] [Google Scholar]
- 48.Zaizuhana S, Puteri J, Noor MB, Noral'ashikin Y, Muhammad H, Rohana AB, Zakiah I. The in vivo rodent micronucleus assay of Kacip Fatimah (Labisia pumila) extract. Trop Biomed. 2006;23(2):214–219. [PubMed] [Google Scholar]
- 49.Zolman BK, Martinez N, Millius A, Adham AR, Bartel B. Identification and characterization of arabidopsis indole-3-butyric acid response mutants detective in novel peroxisomal enzymes. Genetics. 2008;180(1):237–251. doi: 10.1534/genetics.108.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]