Abstract
The simultaneous sorption behavior and characteristics of cadmium (Cd) and sulfamethoxazole (SMX) on rice straw biochar were investigated. Isotherms of Cd and SMX were well modeled by the Langmuir equation (R 2>0.95). The calculated maximum adsorption parameter (Q) of Cd was similar in single and binary systems (34 129.69 and 35 919.54 mg/kg, respectively). However, the Q of SMX in a binary system (9 182.74 mg/kg) was much higher than that in a single system (1 827.82 mg/kg). The presence of Cd significantly promoted the sorption of SMX on rice straw biochar. When the pH ranged from 3 to 7.5, the sorption of Cd had the characteristics of a parabola pattern with maximum adsorption at pH 5, while the adsorption quantity of SMX decreased with increasing pH, with maximum adsorption at pH 3. The amount of SMX adsorbed on biochar was positively correlated with the surface area of the biochar, and the maximum adsorption occurred with d 250 biochar (biochar with a diameter of 150–250 μm). Scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) showed that the removal of Cd and SMX by rice straw biochar may be attributed to precipitation and the formation of surface complexes between Cd or SMX and carboxyl or hydroxyl groups. The results of this study indicate that rice straw biochar has the potential for simultaneous removal of Cd and SMX from co-contaminated water.
Keywords: Biochar, Rice straw, Simultaneous sorption, Cadmium (Cd), Sulfamethoxazole (SMX)
1. Introduction
Cadmium (Cd) is an extremely toxic metal with unknown biological functions. Excessive Cd released into the environment by mining, industrial, and agricultural activities (Kannan and Rengasamy, 2005) causes high ecological risks to ground water and soil biota, thus threatening human health through the food chain (Loganathan et al., 2012). Veterinary antibiotics are commonly used to treat disease or promote growth of animals. However, their abuse has caused growing concerns about antibiotic-resistant organisms which have the potential to become superbugs that are immune to antibiotics (Martinez, 2008; Allen et al., 2010). Sulfamethoxazole (SMX) is a widely used sulfonamide antibiotic. Previous studies reported that it was detected in soils in higher concentrations than other antibiotics (Stoob et al., 2007). A lower removal rate of SMX compared to macrolides, trimethoprim and other sulfonamides was reported in final effluents of wastewater treatment facilities (Luo et al., 2011). The coexistence of heavy metals and antibiotics in the environment has recently been reported (Máthé et al., 2012; Matyar, 2012). However, techniques for the simultaneous removal of Cd and SMX from wastewater have rarely been published.
Numerous methods have been developed for the removal or in situ stabilization of pollutants, including precipitation, flotation, ion exchange, membrane-related processes, electrochemical techniques, biological processes, and adsorption (Febrianto et al., 2009). Among these, adsorption of pollutants by sorbents has been proven to be an efficient technique for the stabilization of pollutants (Yadanaparthi et al., 2009). However, the large-scale application of sorbents may be cost-prohibitive (Tong et al., 2011). Biochar is pyrolyzed from biomass under conditions of limited oxygen, and is considered to be an alternative low-cost sorbent. Biochar has a high stability against decay and a superior ability to retain nutrients, which may be beneficial in decreasing the bioavailability of pollutants, thus mitigating their ecological toxicities (Lehmann, 2007). For example, broiler litter-derived char formed under a low pyrolysis temperature (350 °C) improved the immobilization of Cu(II), Cd(II), and Ni(II) in soils (Uchimiya et al., 2010). The ability of oak bark char to remove Pb(II) and Cd(II) from water, represented by the amount of metal adsorbed per unit surface area (SA) (0.5157 mg/m2 for Pb(II) and 0.213 mg/m2 for Cd(II)), was much greater than that of high-priced commercial activated carbon (Mohan et al., 2007).
Biochars made from agricultural wastes, including fruit peels (Chen and Chen, 2009), pine needles (Chen et al., 2008), grass (Bornemann et al., 2007), hardwood (Chen et al., 2011), and crop straw (Chun et al., 2004) have been widely investigated. According to the agricultural crop database of China, 0.2 billion tons of rice is produced in China every year (http://www.zzys.gov.cn/nongqing.aspx). However, most rice straw is discarded or burned inappropriately, leading to serious air pollution caused by emission of carbon monoxide (CO), non-methane hydrocarbons (NMHCs), nitrogen oxides (NOx), particulate matter with an aerodynamic diameter of 10 μm or less (PM10), and sulfur dioxide (SO2) (Qu et al., 2012), and resource losses. Previous studies reported that rice straw biochar could be applied to enhance the immobilization of organic pollutants (Nag et al., 2011) or heavy metals (Chen et al., 2011; Jiang et al., 2012), but the potential for abatement of multiple contaminants was not fully explored.
The main purposes of this study were to investigate: (1) the simultaneous sorption behavior and characteristics of Cd and SMX on rice straw biochar; (2) the interactions between Cd and SMX in their simultaneous sorption process; (3) the effects of solution pH and biochar diameter on sorption of Cd and SMX on biochar.
2. Materials and methods
2.1. Biochar and chemicals
Rice straw biochar made at 400 °C was provided by the Institute of Urban Environment, Chinese Academy of Sciences. Biochar with different diameters was prepared according to the method of Oleszczuk et al. (2012) with some modification. The rice straw biochar was firstly sieved through a 150-μm sieve, then a 250-μm sieve, and finally a 425-μm sieve to obtain biochar subsamples with different diameters: <150, 150–250, and 250–425 μm. The surface parameters of biochar were determined using an SA and porosity analyzer (Micromeritics, Tristar 3020, USA), and the elemental compositions of carbon (C), hydrogen (H), and nitrogen (N) were detected using an elemental analyzer (Thermo Finnigan, Flash EA1112, USA). The content of oxygen (O) was calculated as the difference between C, H, N and ash. Fourier transform infrared spectroscopy (FTIR) spectra were collected in the range of 600–4 000 cm−1 using a Nicolet FTIR spectrophotometer (Themo Fisher Scientific LLC, Nicolet 6700, USA). A scanning electron microscopy (SEM; CorlzeisD, Utral 55, Germany) was used to distinguish the sorption characteristics of Cd and SMX on biochar in a single Cd or SMX system and in a binary system. SMX (99.9% purity) was purchased from Sigma-Aldrich Chemical Co. Sodium salts and Cd(NO3)2·4H2O were of analytical grade or better.
2.2. Batch experiments
Batch experiments were conducted to investigate the adsorption isotherms of Cd and SMX in single and binary systems. A stock solution of Cd (1 000 mg/L) was prepared by dissolving Cd(NO3)2·4H2O in a background solution consisting of 0.01 mol/L NaNO3 to maintain a constant ionic strength and 200 mg/L NaN3 to inhibit biological activity. SMX was firstly dissolved in methanol, and then diluted with background solution to 500 mg/L as a stock solution. In single systems, the series of initial concentrations were 5, 10, 25, 50, and 100 mg/L for Cd and 5, 10, 20, 40, 80, and 200 mg/L for SMX. In binary systems, SMX was added into Cd single systems as above, and the initial concentration of SMX was 20 mg/L. Cd was mixed with SMX single systems as above, and the initial concentration of Cd was 200 mg/L. Batch experiments were performed by adding 20 ml of single (Cd/SMX) or binary (Cd+SMX) solutions to 0.05 g biochar samples in 50 ml vials equipped with Teflon-lined screw caps. The vials were kept in the dark and rotated vertically on a rotator at 150 r/min for 24 h at room temperature ((25±2) °C) and then equilibrated for another 16 h (Lertpaitoonpan et al., 2009). After equilibration, the vials were centrifuged. The supernatant was filtered through a 0.45-μm filter and then stored at 4 °C prior to analyses of Cd and SMX. The biochar used in this experiment was d 250 (biochar with a diameter of 150–250 μm), and all treatments were performed in triplicate.
Sorption edge experiments were conducted to investigate the effects of solution pH and biochar diameter on the simultaneous removal of Cd and SMX in single and binary systems. In single systems, the initial concentration of Cd was 200 mg/L and of SMX was 20 mg/L. In the binary system, the initial concentrations of Cd and SMX were the same as in the corresponding single system. The initial pH of the solution was adjusted to 3, 4, 5, 6, or 7.5 using 0.01 mol/L HCl or NaOH. The biochar used in experiments to determine the effects of pH was d 250 biochar. Biochars with different diameters (<150, 150–250, and 250–425 μm) were added to both single systems and the binary system at pH 6. The remaining procedures were the same as those used in the adsorption isotherm experiments. All treatments were performed in triplicate.
2.3. Analytical methods
SMX concentrations in the supernatants were determined using high-performance liquid chromatography (HPLC; Agilent 1200, USA) with a reversed-phase XDB-C18 column (5 μm, 4.6 mm×150 mm) and an ultraviolet (UV) detector at 265 nm. Chromatography was performed at 25 °C with acetonitrile/water (40/60, v/v) containing 0.1% acetic acid as the mobile phase at a flow rate of 1 ml/min. The equilibrium concentrations of Cd were determined by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500a, USA).
2.4. Statistical analysis
All data were analyzed using SPSS 16.0, and Student’s t-test was applied to test for significant differences between the means.
3. Results
3.1. Characterization of biochar
Surface elemental compositions (C, H, O, N), specific SA, and pore volume parameters of the rice straw biochar are shown in Table 1. The SA of the biochar was not significantly related to its diameter. The d 250 biochar had the largest SA (3.58 m2/g), followed by the d 150 (1.25 m2/g) and d 425 (0.25 m2/g) biochars. However, the d 425 biochar had the largest micropore area and volume among the three biochars.
Table 1.
Characteristics of rice straw biochars
| Biochar | Content (%) |
Surface area (m2/g) | Micropore area (m2/g) | Micropore volume (cm3/g) | ||||
| Ash | N | C | O | H | ||||
| d 150 | 29.45 | 2.28 | 38.39 | 27.90 | 1.98 | 1.25 | 8.43 | 0.0037 |
| d 250 | 3.58 | 5.42 | 0.0025 | |||||
| d 425 | 0.25 | 11.61 | 0.0045 | |||||
d 150, d 250, and d 425 represent biochars with diameters <150, 150–250, and 250–425 μm, respectively
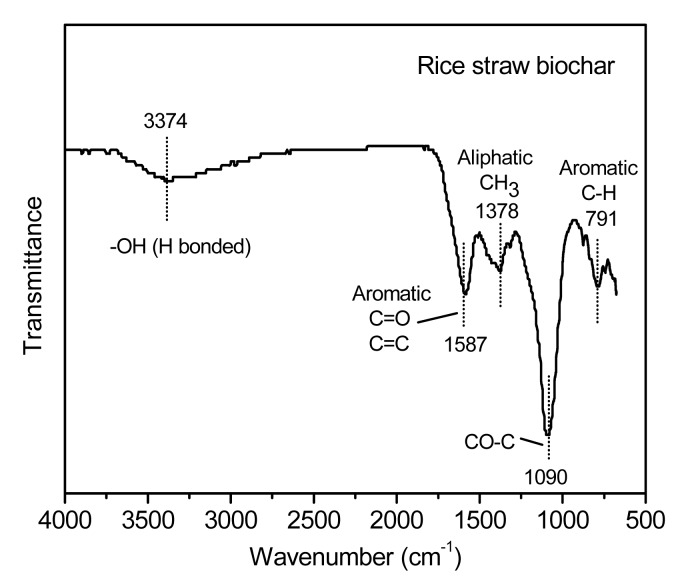
The FTIR spectrum revealed the principle surface functionalities of rice straw biochar (Fig. 1). The spectrum of biochar was characterized by five bands at wave numbers 3 374, 1 587, 1 378, 1 090, and 791 cm−1. The bands at 3 374, 1 090, 791, and 1 587 cm−1 are attributed to hydroxyl (–OH) stretching, CO–C stretching of secondary hydroxyl, aliphatic CH2 deformation, and aromatic C=C ring or COO– group stretching, respectively (Fu et al., 2009; Harvey et al., 2011; Kumar et al., 2012). At 1 378 cm−1, bands are attributed to aliphatic CH3 deformation (Harvey et al., 2011) or O–H/C–H bending of hydroxyl, acid, phenol and methyl (Fu et al., 2009).
Fig. 1.
FTIR image of a rice straw biochar
3.2. Adsorption isotherms of Cd
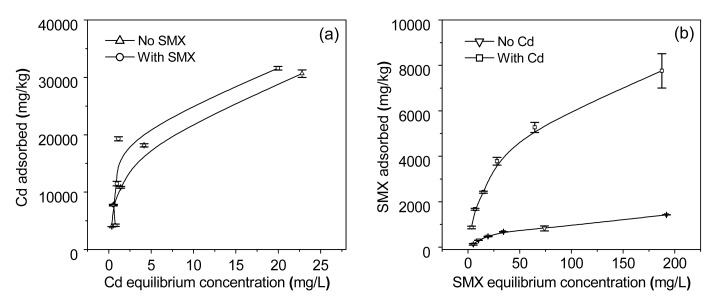
Cd adsorption capacity increased with increasing Cd concentration in the equilibrium solution (Fig. 2). The amount of Cd adsorbed on biochar was similar in solutions with or without SMX. The Langmuir and Freundlich models were used to simulate the sorption characterization in this study. The equations of the two models are given as follows:
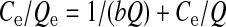 , ,
|
(1) |
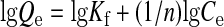 , ,
|
(2) |
where C e is the concentration of adsorbate in the equilibrium solution (mg/L), Q e is the amount of the metal adsorbed per unit weight of biochar (mg/kg), Q is the maximum adsorption capacity (mg/kg), b is the bonding energy coefficient, n is a sorption intensity constant, and K f is the Freundlich constant. The calculated parameters are listed in Table 2. The Langmuir model was more suitable for Cd adsorption (R 2=0.96 for Cd with SMX, and 0.99 without SMX) than the Freundlich model (R 2=0.61 for Cd with SMX, and 0.94 without SMX). The sorption behavior of Cd on biochar in the presence of SMX as indicated by Q and b (Q=35 919.54 mg/kg, b=0.370) was not significantly different from that in the system without SMX (Q=34 129.69 mg/kg, b=0.360).
Fig. 2.
Adsorption isotherms of Cd (a) and SMX (b) on rice straw biochar as affected by their concentrations in the equilibrium solution
Table 2.
Calculated Langmuir and Freundlich parameters of Cd and SMX adsorption isotherms
| Treatment | Freundlich model |
Langmuir model |
||||
| K f (L/kg) | 1/n | R 2 | Q (mg/kg) | b | R 2 | |
| Cd | 8 477.20 | 0.45 | 0.94 | 34 129.69 | 0.360 | 0.99 |
| Cd+SMX | 9 808.12 | 0.42 | 0.61 | 35 919.54 | 0.370 | 0.96 |
| SMX | 62.40 | 0.62 | 0.94 | 1 827.82 | 0.016 | 0.96 |
| SMX+Cd | 542.25 | 0.54 | 0.97 | 9 182.74 | 0.026 | 0.99 |
3.3. Adsorption isotherms of SMX
The quantity of SMX adsorbed on biochar increased with increasing SMX concentration in the equilibrium solution (Fig. 2). The Langmuir and Freundlich models were again used to simulate the sorption characteristics (Table 2). The Langmuir model (R 2=0.99 for SMX with Cd, and 0.96 without Cd) fitted the data better than the Freundlich model (R 2=0.97 for SMX with Cd, and 0.94 without Cd). The Langmuir parameters (Q and b) indicated that the adsorption of SMX on biochar was significantly enhanced by the addition of Cd.
3.4. Effect of biochar diameter
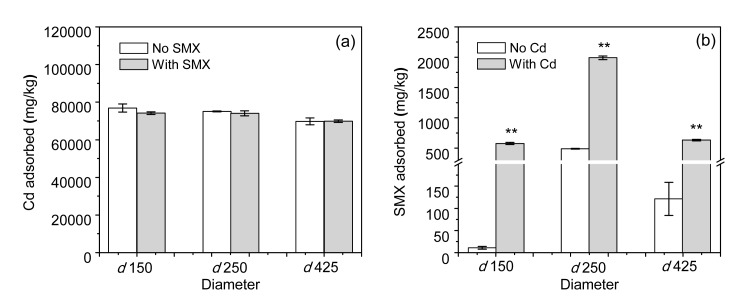
Differences in the diameter of the biochar showed no significant influence on the sorption of Cd, regardless of the presence of SMX (Fig. 3). However, d 250 biochar adsorbed a significantly larger amount of SMX than did the other two biochars in both the single and binary systems.
Fig. 3.
Effect of biochar diameter on adsorption of Cd (a) and SMX (b) on rice straw biochar
d 150, d 250, and d 425 represent biochars with diameters <150, 150–250, and 250–425 μm, respectively. * P<0.05, ** P<0.01: significant differences between SMX solely and in combination with Cd at each diameter
3.5. Effect of solution pH
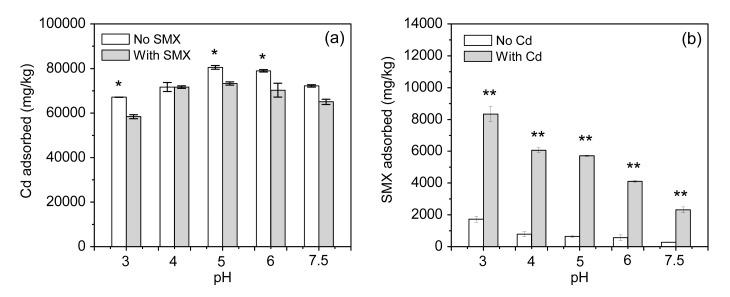
The solution pH significantly influenced the sorption of Cd and SMX onto biochar (Fig. 4). The amount of Cd adsorbed kept on increasing with increasing pH, until the pH plateaued at pH 5, in both single and binary systems. Then, the amount of Cd adsorbed decreased with the increasing pH value. The trend of SMX sorption was negatively correlated with the pH values (Fig. 4). The highest adsorption of SMX occurred at pH 3 in both single and binary systems.
Fig. 4.
Effect of pH on the adsorption of Cd (a) and SMX (b) on rice straw biochar
* P<0.05, ** P<0.01: significant differences between SMX solely and in combination with Cd at each pH
4. Discussion
4.1. Adsorption capability of rice straw biochar for Cd and SMX
A few studies have evaluated the capacity of some carbonaceous materials to adsorb Cd (Kannan and Rengasamy, 2005; Mohan et al., 2007; Wang et al., 2010). Adsorbents produced from different agricultural or industrial materials varied in their sorption characteristics (Yadanaparthi et al., 2009). Carbon adsorbents with an initial Cd concentration of 2.25 mg/L at a dose of 5 g/L of 12–20 meshes carbon achieved an adsorption capacity of 600 mg/kg. According to the Langmuir isotherm, bamboo charcoal had a maximum Cd adsorption capacity of 12 080 mg/kg under the conditions of 3.33 g/L of 200 meshes bamboo charcoal and an initial Cd concentration of 20–100 mg/L (Wang et al., 2010). When the amount and particle size of carbon and the initial concentration of Cd were 10 g/L, 9 μm, and 10–66 mg/L, respectively, straw-activated carbon had the greatest adsorption capacity for Cd2+ (4 140 mg/kg) among various activated carbons based on Langmuir isotherm data (Kannan and Rengasamy, 2005). Based on the maximum adsorption capacity parameter Q of the Langmuir isotherm in this study, rice straw biochar showed a strong ability to adsorb Cd in both single (34 129.69 mg/kg) and binary systems (35 919.54 mg/kg), and the adsorption capacity was much higher than that of previously reported adsorbents. In conclusion, rice straw biochars are efficient in the adsorption of Cd.
Previous studies on adsorption of SMX focused mainly on carbon nanotubes (Ji et al., 2009; Wu et al., 2012). Carbon nanotubes are considered an effective adsorbent for removing antibiotics from aqueous solution, and the K f value, which represents the sorption affinity of SMX in carbon nanotubes, is about 500 L/kg (Ji et al., 2009; Zhang et al., 2010b). Although in our study the Langmuir model gave a better data fit than the Freundlich model, previous studies used the Freundlich parameter to describe the sorption of SMX, so K f was adopted for comparison. The K f values of SMX were 60.40 L/kg in the single systems and 542.25 L/kg in the binary systems. This result was consistent with prediction by the Langmuir parameter Q. Apparently, the SMX adsorption capacity of rice straw biochar in solution was significantly enhanced by the addition of Cd, and was equivalent to that of carbon nanotubes. As reported, acid-treated wheat-residue-derived black carbon showed a higher capacity to adsorb SMX than did raw carbon or maize-residue derived carbon. The K f of acid wheat carbon was 570 L/kg, higher than that of maize (200 L/kg) (Ji et al., 2011), which was higher than that of the rice straw biochar in the single system, but comparable to that in the binary condition in this study. This suggests that rice straw biochar could be an effective alternative adsorbent for SMX, and further treatment of rice straw biochar may increase this ability.
4.2. Sorption mechanism of Cd on rice straw biochar
The good fit of the Langmuir adsorption isotherm was based on monolayer coverage of the adsorbate on the surface of the adsorbent, indicating that the sorption of Cd on rice straw biochar is monolayer sorption on a fixed number of surface sites on the biochar (Wang et al., 2010). Several studies have reported that a larger SA of biochar can enhance sorption of heavy metals such as As, Pb, Cu, Zn, and Cd, by providing more surface sites (Mohan et al., 2007; Chen et al., 2011). For example, corn straw char produced at 600 °C has a larger SA than hardwood char produced at 450 °C, leading to greater adsorption capacities of corn straw char for Cu and Zn (Chen et al., 2011). However, in our study, different diameters showed no significant effect on the sorption of Cd on biochar, regardless of the presence of SMX (Fig. 3). Xu et al. (2012) reported that sorption would not be expected to depend on the SA if the adsorbent had a low SA (5.61 m2/g). In our study, the SA of three biochars were comparatively low (Table 1), and hence, an effect of biochar diameter on Cd sorption was not obvious. The maximum adsorption capacity of rice straw biochar for Cd calculated by the Langmuir model was similar to the result of the study by Xu et al. (2013), which attributed the large adsorption capacity of dairy manure-derived biochar for Cd (32 036.85 mg/kg) to the formation of metal-phosphate and carbonate precipitates on the surface of biochar. Echeverría et al. (1998) also reported the importance of precipitation in the sorption of Cd by adsorbents. In our study, the amount of adsorption was also extraordinarily large, and the SEM images (Fig. 5) of rice straw biochar after Cd adsorption showed visible precipitates on the surface of the biochar, indicating that precipitation was involved in the process.
Fig. 5.
SEM images of rice straw biochar before and after equilibration with solutions of Cd and SMX
Harvey et al. (2011) reported that Cd sorption occurred via cation-exchange on biochars produced at <350 °C, but predominantly via two distinct cation-π bonding mechanisms on biochars produced at ≥350 °C. In our study, the rice straw biochar was produced at 400 °C, which suggests that Cd2+-π bonding may have been involved. Hydrogen-bonding interaction could also contribute to the sorption of Cd because of the presence of hydroxyl (–OH) in biochar, indicated by the band at 3 374 cm−1 (Fig. 1) (Chen et al., 2011). Besides, electrostatic interaction and ion exchange could also contribute to Cd sorption on rice straw biochar.
In this study, the amount of Cd adsorption increased with increasing pH and trended to be stable after pH 5 in both single and binary systems (Fig. 4). Similar results were reported by Chen et al. (2011) and Liu and Zhang (2009), regardless of the original material of the biochar. As the pHzpc (zero point of charge) of biochar is between 2.0 and 3.5 (Mukherjee et al., 2011), the surface charge of biochar in the solution was mainly negative in the pH range (3, 4, 5, 6 and 7.5) in our study. Negative charge on the surface of the biochar increased with the increase in solution pH from 3 to 5. Thus, the enhanced electrostatic attraction could promote adsorption of cationic Cd onto the negatively charged surface of the biochar. There is also competition between protons and metal cations for sorption sites on the surface of the biochar (Martins et al., 2004). When the pH exceeded 5, the decreasing adsorption trend was likely caused by the formation of hydroxide complexes (Chen et al., 2011).
4.3. Sorption mechanism of SMX on rice straw biochar
Although biochar diameter had no significant effect on sorption of Cd, it had a significant effect on sorption of SMX. The d 250 biochar with the largest SA showed the maximum amount of adsorption of SMX in both single and binary systems, which confirmed that the SA is of great importance to the sorption of organic molecules on adsorbents (Shinogi and Kanri, 2003).
Solution pH also significantly influenced the sorption of SMX on biochar. The sorption of SMX at selected pH values reflected the sorption behavior of different SMX species. SMX has two pKa (acid dissociation constant) values, 1.7 and 5.7 (Lucida et al., 2000). The cationic, neutral, and anionic species of SMX dominate at pH values of <1.7, around 3.7, and >5.7, respectively (Zhang et al., 2010a). Therefore, in this study, the majority of SMX in solution was in neutral form at pH 3. Neutral species would have decreased with increasing pH, whereas anionic species would have increased, and the electrostatic repulsion between the negatively charged biochar surface and anionic SMX species would have become stronger. This is one possible reason why the amount of SMX sorption was negatively correlated with the pH values. The solubility of SMX changed with solution pH. The minimum solubility of SMX was 281 mg/L at pH 3.22 (25 °C); however, its solubility can increase to 17 900 mg/L (pH=7.5) or 560 mg/L (pH=1.7) (Dahlan et al., 1987). Apparently, the amount of SMX adsorption on rice straw biochar was negatively related to solubility. When the pH increased from 3 to 7.5, the increasing amount of soluble SMX would result in less hydrophobic partitioning between SMX and the rice straw biochar, indicating that hydrophobic partitioning is an important mechanism of SMX sorption on rice straw biochar.
SMX is a strong π-acceptor compound because of amino functional groups and N-heteroaromatic rings (Zhang et al., 2010a). The π-π electron donor-acceptor (EDA) (Zhu et al., 2004) is a mechanism controlling the strong interaction between nitroaromatics and black carbon (char) (Zhu and Pignatello, 2005). In the FTIR spectrum (Fig. 1), the COO– groups (1 587 cm−1) in rice straw biochar may facilitate the π-π EDA interaction between SMX and biochar. The π-acceptor ability would increase with protonation, which would mean that a higher pH would lead to weaker interaction of π-π EDA between SMX and biochar in this study. In summary, the largest amount of adsorption at pH 3 was the result of various sorption mechanisms of SMX on rice straw biochar.
4.4. Effect of Cd on SMX sorption on rice straw biochar
Previous studies reported that the interaction between heavy metals and organic compounds could be either antagonistic (Wang et al., 2010) or synergistic (Wu et al., 2009). A notable promotion of SMX sorption by Cd was observed (Figs. 2–4) in our study. In the co-contaminated solution, Cd2+ would affect the sorption of SMX by modifying the biochar surface. Cd ions could first be adsorbed on the surface of the biochar, and the negative charge on the surface of the biochar would be mitigated (Wu et al., 2009). Then, the electrostatic repulsion between the biochar surface and anionic SMX could be moderated, thereby increasing the adsorption of SMX. The Cd first adsorbed on the surface of the biochar could also act as a Cd bridge, similar to those of Ca2+ and Mg2+ (Wan et al., 2010), facilitating the adsorption of SMX. Cd2+ could decrease the competition between SMX and water for sorption sites by decreasing the hydrophobicity of the local region (Chen et al., 2007). The presence of Cd not only modified the surface of the biochar, but produced a Cd-SMX complex (Wu et al., 2012) with a higher sorption affinity on biochar than SMX, based on the metal complexes (Wang et al., 2008). In conclusion, the increase in the amount of SMX adsorption caused by Cd could be the result of all these positive effects.
5. Conclusions
This study investigated the ability of rice straw biochar to remove Cd and SMX from aqueous solution, and described the sorption characteristics of Cd and SMX on the biochar. The following conclusions can be drawn based on the experimental results: (1) rice straw biochar had a strong ability to remove Cd and SMX from aqueous solution; (2) the presence of SMX had no significant impact on the sorption of Cd, whereas the presence of Cd significantly promoted sorption of SMX; (3) the solution pH significantly influenced the sorption of both Cd and SMX, with maximum sorption occurring at pH 5 for Cd and at pH 3 for SMX; (4) biochar diameter significantly affected the sorption of SMX, with maximum adsorption occurring with d 250. This study demonstrated that rice straw biochar is an effective sorbent for removing Cd and SMX from solution.
Footnotes
Project supported by the National Key Technology R&D Program of China (No. 2012BAC17B04) and the Fundamental Research Funds for the Central Universities, China
Compliance with ethics guidelines: Xuan HAN, Cheng-feng LIANG, Ting-qiang LI, Kai WANG, Hua-gang HUANG, and Xiao-e YANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8(4):251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 2.Bornemann LC, Kookana RS, Welp G. Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere. 2007;67(5):1033–1042. doi: 10.1016/j.chemosphere.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 3.Chen BL, Chen ZM. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere. 2009;76(1):127–133. doi: 10.1016/j.chemosphere.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Chen BL, Zhou DD, Zhu LZ. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol. 2008;42(14):5137–5143. doi: 10.1021/es8002684. [DOI] [PubMed] [Google Scholar]
- 5.Chen JY, Zhu DQ, Sun C. Effect of heavy metals on the sorption of hydrophobic organic compounds to wood charcoal. Environ Sci Technol. 2007;41(7):2536–2541. doi: 10.1021/es062113+. [DOI] [PubMed] [Google Scholar]
- 6.Chen XC, Chen GC, Chen LG, Chen YX, Lehmann J, McBride MB, Hay AG. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour Technol. 2011;102(19):8877–8884. doi: 10.1016/j.biortech.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 7.Chun Y, Sheng GY, Chiou CT, Xing BS. Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol. 2004;38(17):4649–4655. doi: 10.1021/es035034w. [DOI] [PubMed] [Google Scholar]
- 8.Dahlan R, McDonald C, Sunderland VB. Solubilities and intrinsic dissolution rates of sulfamethoxazole and trimethoprim. J Pharm Pharmacol. 1987;39(4):246–251. doi: 10.1111/j.2042-7158.1987.tb06261.x. [DOI] [PubMed] [Google Scholar]
- 9.Echeverría JC, Morera MT, Mazkiarán C, Garrido JJ. Competitive sorption of heavy metal by soils. Isotherms and fractional factorial experiments. Environ Pollut. 1998;101(2):275–284. doi: 10.1016/S0269-7491(98)00038-4. [DOI] [PubMed] [Google Scholar]
- 10.Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater. 2009;162(2-3):616–645. doi: 10.1016/j.jhazmat.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Fu P, Hu S, Xiang J, Sun LS, Li PS, Zhang JY, Zheng CG. Pyrolysis of maize stalk on the characterization of chars formed under different devolatilization conditions. Energy Fuels. 2009;23(9):4605–4611. doi: 10.1021/ef900268y. [DOI] [Google Scholar]
- 12.Harvey OR, Herbert BE, Rhue RD, Kuo LJ. Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol. 2011;45(13):5550–5556. doi: 10.1021/es104401h. [DOI] [PubMed] [Google Scholar]
- 13.Ji LL, Chen W, Zheng SR, Xu ZY, Zhu DQ. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir. 2009;25(19):11608–11613. doi: 10.1021/la9015838. [DOI] [PubMed] [Google Scholar]
- 14.Ji LL, Wan YQ, Zheng SR, Zhu DQ. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: implication for the relative importance of black carbon to soil sorption. Environ Sci Technol. 2011;45(13):5580–5586. doi: 10.1021/es200483b. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Xu RK, Jiang TY, Li Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted ultisol. J Hazard Mater. 2012;229:145–150. doi: 10.1016/j.jhazmat.2012.05.086. [DOI] [PubMed] [Google Scholar]
- 16.Kannan N, Rengasamy G. Comparison of cadmium ion adsorption on various activated carbons. Water Air Soil Pollut. 2005;163(1-4):185–201. doi: 10.1007/s11270-005-0277-y. [DOI] [Google Scholar]
- 17.Kumar PS, Ramalingam S, Sathyaselvabala V, Kirupha SD, Murugesan A, Sivanesan S. Removal of cadmium(II) from aqueous solution by agricultural waste cashew nut shell. Korean J Chem Eng. 2012;29(6):756–768. doi: 10.1007/s11814-011-0259-2. [DOI] [Google Scholar]
- 18.Lehmann J. Bio-energy in the black. Front Ecol Environ. 2007;5(7):381–387. doi: 10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2. [DOI] [Google Scholar]
- 19.Lertpaitoonpan W, Ong SK, Moorman TB. Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere. 2009;76(4):558–564. doi: 10.1016/j.chemosphere.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZG, Zhang FS. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J Hazard Mater. 2009;167(1-3):933–939. doi: 10.1016/j.jhazmat.2009.01.085. [DOI] [PubMed] [Google Scholar]
- 21.Loganathan P, Vigneswaran S, Kandasamy J, Naidu R. Cadmium sorption and desorption in soils: a review. Crit Rev Environ Sci Technol. 2012;42(5):489–533. doi: 10.1080/10643389.2010.520234. [DOI] [Google Scholar]
- 22.Lucida H, Parkin JE, Sunderland VB. Kinetic study of the reaction of sulfamethoxazole and glucose under acidic conditions—I. Effect of pH and temperature. Int J Pharm. 2000;202(1-2):47–61. doi: 10.1016/S0378-5173(00)00413-0. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Xu L, Rysz M, Wang YQ, Zhang H, Alvarez PJJ. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol. 2011;45(5):1827–1833. doi: 10.1021/es104009s. [DOI] [PubMed] [Google Scholar]
- 24.Martinez JL. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321(5887):365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 25.Martins RJE, Pardo R, Boaventura RAR. Cadmium(II) and zinc(II) adsorption by the aquatic moss Fontinalis antipyretica: effect of temperature, pH and water hardness. Water Res. 2004;38(3):693–699. doi: 10.1016/j.watres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Máthé I, Benedek T, Táncsics A, Palatinszky M, Lányi S, Márialigeti K. Diversity, activity, antibiotic and heavy metal resistance of bacteria from petroleum hydrocarbon contaminated soils located in Harghita County (Romania) Int Biodeter Biodegr. 2012;73:41–49. doi: 10.1016/j.ibiod.2012.05.018. [DOI] [Google Scholar]
- 27.Matyar F. Antibiotic and heavy metal resistance in bacteria isolated from the Eastern Mediterranean Sea Coast. Bull Environ Contam Toxicol. 2012;89(3):551–556. doi: 10.1007/s00128-012-0726-4. [DOI] [PubMed] [Google Scholar]
- 28.Mohan D, Pittman CU, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gomez-Serrano V, Gong H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci. 2007;310(1):57–73. doi: 10.1016/j.jcis.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee A, Zimmerman AR, Harris W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma. 2011;163(3-4):247–255. doi: 10.1016/j.geoderma.2011.04.021. [DOI] [Google Scholar]
- 30.Nag SK, Kookana R, Smith L, Krull E, Macdonald LM, Gill G. Poor efficacy of herbicides in biochar-amended soils as affected by their chemistry and mode of action. Chemosphere. 2011;84(11):1572–1577. doi: 10.1016/j.chemosphere.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 31.Oleszczuk P, Rycaj M, Lehmann J, Cornelissen G. Influence of activated carbon and biochar on phytotoxicity of air-dried sewage sludges to Lepidium sativum . Ecotoxicol Environ Saf. 2012;80:321–326. doi: 10.1016/j.ecoenv.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Qu CS, Li B, Wu HS, Giesy JP. Controlling air pollution from straw burning in china calls for efficient recycling. Environ Sci Technol. 2012;46(15):7934–7936. doi: 10.1021/es302666s. [DOI] [PubMed] [Google Scholar]
- 33.Shinogi Y, Kanri Y. Pyrolysis of plant, animal and human waste: physical and chemical characterization of the pyrolytic products. Bioresour Technol. 2003;90(3):241–247. doi: 10.1016/S0960-8524(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 34.Stoob K, Singer HP, Mueller SR, Schwarzenbach RP, Stamm CH. Dissipation and transport of veterinary sulfonamide antibiotics after manure application to grassland in a small catchment. Environ Sci Technol. 2007;41(21):7349–7355. doi: 10.1021/es070840e. [DOI] [PubMed] [Google Scholar]
- 35.Tong XJ, Li JY, Yuan JH, Xu RK. Adsorption of Cu(II) by biochars generated from three crop straws. Chem Eng J. 2011;172(2-3):828–834. doi: 10.1016/j.cej.2011.06.069. [DOI] [Google Scholar]
- 36.Uchimiya M, Lima IM, Klasson KT, Wartelle LH. Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere. 2010;80(8):935–940. doi: 10.1016/j.chemosphere.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Wan Y, Bao YY, Zhou QX. Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere. 2010;80(7):807–812. doi: 10.1016/j.chemosphere.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 38.Wang FY, Wang H, Ma JW. Adsorption of cadmium(II) ions from aqueous solution by a new low-cost adsorbent-bamboo charcoal. J Hazard Mater. 2010;177(1-3):300–306. doi: 10.1016/j.jhazmat.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Wang YJ, Jia DA, Sun RJ, Zhu HW, Zhou DM. Adsorption and cosorption of tetracycline and copper(II) on montmorillonite as affected by solution pH. Environ Sci Technol. 2008;42(9):3254–3259. doi: 10.1021/es702641a. [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Pan B, Wu M, Peng HB, Zhang D, Xing BS. Coadsorption of Cu and sulfamethoxazole on hydroxylized and graphitized carbon nanotubes. Sci Total Environ. 2012;427:247–252. doi: 10.1016/j.scitotenv.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 41.Wu WH, Wang HZ, Xu JM, Xie ZM. Adsorption characteristic of bensulfuron-methyl at variable added Pb2+ concentrations on paddy soils. J Environ Sci. 2009;21(8):1129–1134. doi: 10.1016/S1001-0742(08)62392-X. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res Int. 2013;20(1):358–368. doi: 10.1007/s11356-012-0873-5. [DOI] [PubMed] [Google Scholar]
- 43.Yadanaparthi SK, Graybill D, von Wandruszka R. Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. J Hazard Mater. 2009;171(1-3):1–15. doi: 10.1016/j.jhazmat.2009.05.103. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Pan B, Zhang H, Ning P, Xing BS. Contribution of different sulfamethoxazole species to their overall adsorption on functionalized carbon nanotubes. Environ Sci Technol. 2010;44(10):3806–3811. doi: 10.1021/es903851q. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Pan B, Yang K, Zhang D, Hou JA. Adsorption of sulfamethoxazole on different types of carbon nanotubes in comparison to other natural adsorbents. J Environ Sci Health A. 2010;45(12):1625–1634. doi: 10.1080/10934529.2010.506127. [DOI] [PubMed] [Google Scholar]
- 46.Zhu DQ, Pignatello JJ. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ Sci Technol. 2005;39(7):2033–2041. doi: 10.1021/es0491376. [DOI] [PubMed] [Google Scholar]
- 47.Zhu DQ, Hyun SH, Pignatello JJ, Lee LS. Evidence for pi-pi electron donor-acceptor interactions between pi-donor aromatic compounds and pi-acceptor sites in soil organic matter through pH effects on sorption. Environ Sci Technol. 2004;38(16):4361–4368. doi: 10.1021/es035379e. [DOI] [PubMed] [Google Scholar]