Abstract
Background
Herpes virus infections may have a significant role in chronic lymphocytic leukaemia (CLL) due to their ability to modulate the host’s immune system.
Materials and methods
We examined the seroprevalence of four herpes viruses [Cytomegalovirus (CMV), Epstein–Barr Virus (EBV), human herpes virus (HHV)-6 and -7] in a cohort of European CLL patients (cohort 1, n = 100) in relation to the immunoglobulin variable heavy (IGHV) chain gene use and compared serological results with those obtained from age- and gender-matched healthy adults (n = 100).
Results
CMV-seroprevalence was significantly higher in CLL cohort 1 (79%) than in the control cohort (57%, P = 0·001); the seroprevalence of EBV (89% vs. 94%), HHV-6 (73% vs. 60%), or HHV-7 (35% vs. 35%) was not. In CLL cohort 1, use of IGHV3-30 was more prevalent among CMV-seropositive and of IGHV3-21 among HHV-7-seronegative cases. To investigate the generalizability of these findings, we investigated the herpes virus seroprevalence in a second cohort of age-matched CLL patients from a different geographical area (USA, n = 100, cohort 2). In cohort 2, CMV-seroprevalence was comparable with that of the control cohort (53%). Seroprevalence of EBV, HHV-6 and HHV-7 were 85%, 88% and 73% respectively. In CLL cohort 2, use of IGHV3-30 or IGHV3-21 was not associated with any of the herpes viruses investigated.
Conclusions
CMV-seropositivity is associated with CLL in selected patient cohorts. However, the considerable variation in herpes virus-specific seropositivity between geographically distinct CLL cohorts indicates that seropositivity for any of the four human herpes viruses investigated is not generally associated with CLL.
Keywords: Chronic lymphocytic leukaemia, herpes viruses, human cytomegalovirus, IGHV gene use, serology
Introduction
Chronic lymphocytic leukaemia (CLL) accounts for approximately 30% of all leukaemias and is the most common one among older adults in Western countries [1]. Infectious diseases are one of the major causes of morbidity and mortality in CLL patients. The 5-year risk for severe infection was 26% in one cohort of 125 patients analysed over a period of 10 years [2].
Infectious pathogens may also possibly be involved in the genesis of CLL. An increasing body of evidence suggests that CLL evolves from repetitive activation of particular B-cell clones through the B-cell receptor (BCR) by conventional antigens such as latent viruses or commensal bacteria [1,3,4]. Epidemiological studies on large patient numbers suggested that clinically diagnosed episodes of pneumonia are associated with development of CLL 1–5 years later [5]. Chronic or recurrent infectious diseases were found to be associated with poor risk features, including unmutated IGHV status [6]. In addition, evidence was found for the involvement of cytomegalovirus (CMV) in the ontogeny of CD4+ T-large granular lymphocyte lymphocytosis, a lymphoproliferative disorder also believed to be driven by a common antigen [7].
Herpes virus infections exhibit multiple characteristics that are compatible with theoretical prerequisites for a significant role in CLL. Herpes viruses have in common a strict species specificity, worldwide distribution and frequent reactivations from latency with periods of viraemia [8-10]. Particularly four herpes viruses, CMV, Epstein–Barr virus (EBV), human herpes virus (HHV)-6 and -7, have multiple mechanisms for modulating the host’s immune system, allowing them to establish latency in cells of the immune system (reviewed in [11]).
Furthermore, herpes virus infections are common in the general adult population – for example, average seroprevalence of CMV is 60% [12,13] and of EBV > 90% [14-16]. Nevertheless, seroprevalence of herpes viruses may vary considerably between different groups of patients according to lifestyle. Almost 100% of homosexual men and intravenous drug users are seropositive for CMV-specific antibodies [17,18], whereas seroprevalence is not higher in HIV-infected haemophiliac patients than in the general population [19]. Seroprevalence of herpes virus infections in CLL patients has not yet been investigated in a case–control study.
The aim of this study was to evaluate the seroprevalence of CMV, EBV, HHV-6 and -7 in CLL patients and to compare it with the use of IGHV genes as a first step to evaluate a possible association between herpes virus infections and CLL. For this purpose, we examined the prevalence of IgG-antibodies to each of these viruses in a cohort of CLL patients and a cohort of healthy age-matched adults. Subsequently, we tested the generalizability of our findings in a second cohort of CLL patients from a different geographical region that were matched by age with the first cohort of CLL patients.
Materials and methods
Samples
We collected serum samples from a total of 100 consecutive CLL patients within a prospective cohort study of patients who fulfilled the diagnostic and immunophenotypic criteria for CLL [20] (Collection period: 1 January 2004–31 September 2005) and presented for evaluation at the referral centre of the Medical University of Vienna (CLL cohort 1, Table 1). Majority of these CLL patients was included in the cohort at an early clinical stage of CLL – 79% of patients were at Rai stage 0 or 1, 14% were at stage 2 and 7% were at stage 3 or 4. Mean age at the time of sample collection was 66 years (range, 26–90 years; SD, 11 years) and 44% of patients were female. Of these 100 CLL patients, 73% were previously untreated at the time of sample collection. During follow-up, 21 of the 73 previously untreated CLL patients were treated. Median time between diagnosis and treatment was 43 months in the 48 CLL patients treated. All patients of CLL cohort 1 were Caucasian.
Table 1.
Selected characteristics of the patients of CLL cohort 1 (n = 100)
| CLL cohort 1 |
CLL cohort 2 |
|
|---|---|---|
| Rai-stage at diagnosis, no. of patients | ||
| 0 | 53 | 25 |
| 1 | 26 | 42 |
| 2 | 14 | 13 |
| 3 | 2 | 5 |
| 4 | 5 | 14 |
| Therapy before sample collection | ||
| No, no. of patients | 73 | 79 |
| Time since diagnosis [months], median | 8·7 | 21·1 |
| Median time of follow-up [months] | 47 | NA |
| Yes, no. of patients | 27 | 21 |
| Time since diagnosis [months], median | 95 | 41·4 |
| Time between diagnosis and treatment [months], median |
46 | 11 |
| Steroids only | 3 | 0 |
| Fludarabin, no. of patients | 10 | 10 |
| Fludarabin + cyclophosphamide | 2 | 4 |
| Chlorambucil | 15 | 5 |
| Alemtuzumab | 1 | 2 |
NA, data not available.
To test for generalizability of findings obtained in CLL cohort 1, we collected also serum samples from a cohort of CLL patients evaluated at the University of California, San Diego, USA (n = 100, CLL cohort 2). These patients were matched by age and gender with the patients of CLL cohort 1. Of these patients, 92% were Caucasian. Patients of both CLL cohorts were treated when they developed symptomatic and/or progressive disease, as per National Cancer Institute (NCI, Bethesda, MD, USA) working group criteria [21,22].
Blood was collected only from consenting patients, and institutional review board approval and informed consent were obtained in all cases, in accordance with the Declaration of Helsinki. Serum samples were cryopreserved at −70 °C and thawed only once for this investigation. All peripheral-blood mononuclear cell samples contained more than 90% CLL cells.
Additionally, 100 healthy adults (control cohort) who came to the Clinical Institute of Virology, Vienna, Austria, for determination of virus-specific immunity after vaccination were matched case-by-case for age (±5 years) and gender with the respective patients of cohort 1 (control cohort). All healthy controls as well as CLL patients were from the same ethnical group (Caucasian). Serum samples collected from these individuals were tested in parallel with the samples from the CLL patients for the presence of different herpes virus-specific IgG-antibodies. Institutional review board approval for retrospective testing of cryopreserved samples from these individuals was obtained in all cases.
Serological investigation and IGHV mutation status
Serum samples were tested by commercially available antibody assays according to the manufacturers’ instructions. All serum samples from CLL cohorts 1 and 2, as well as from healthy adults were tested with the same assays and at the same institution (Institute of Virology, Medical University of Vienna). CMV- and EBV-specific IgG-antibodies were detected using semi-quantitative enzyme-linked immunosorbent assays (ELISA) (Medac Diagnostika, Hamburg, Germany). IgG-antibodies specific for human herpes virus 6 (HHV-6) and HHV-7 were detected by qualitative immunofluorescence assays (IFT; PanBio Diagnostics, Sinnamon Park, Qld, Australia). Sequence analysis of expressed IGHV was performed as described previously elsewhere [23].
Statistical analysis
Comparison of two groups was carried out using the Mann–Whitney U-test for quantitative parameters and Fisher’s exact test for qualitative parameters. Logistic regression analysis was used to analyse the relationship between test results obtained by ELISA and age of the patients. A P value of < 0·05 was considered statistically significant.
Results
Herpes virus-specific seroprevalence
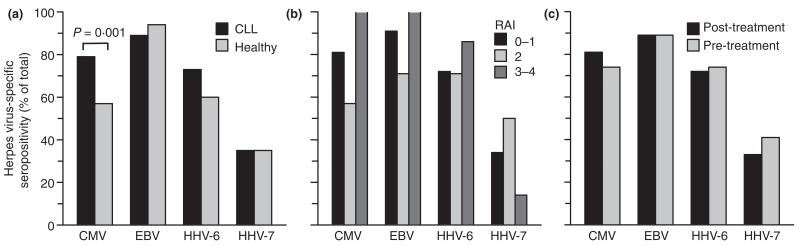
CMV-specific IgG-antibodies were detected more frequently in the patients of CLL cohort 1 than in that of healthy, age-matched adults [79% vs. 57%, P = 0·001 (Fisher’s Exact), Fig. 1a]. We detected a trend for a higher prevalence of HHV-6-specific IgG-antibodies in the test cohort compared to that of the control cohort [73% vs. 60%, P = 0·072 (Fisher’s Exact)]. Seroprevalence of EBV and HHV-7 was similarly high in both cohorts [89% vs. 94%, P = 0·311; 35% vs. 35%, P = 0·118 (Fisher’s Exact)]. Median levels of CMV- and EBV-specific IgG-antibodies were 134 and 77·5 U mL−1, respectively, and were well above test-specific cut-off values for a negative result (16 U mL−1). CMV-seroprevalence was not associated with the age of the CLL patients. The median age of CMV-seropositive patients was 65 years, whereas the median age of CMV-seronegative patients was 66 years [P = 0·459 (logistic regression)].
Figure 1.
Relative rates of seropositivity for the four herpes viruses investigated. (a) Comparison of seropositivity between patients of CLL cohort 1 (n = 100) and healthy adults of the control cohort (n = 100); (b) clinical stage of CLL in relation to seropositivity (Rai 0–1, n = 79; Rai 2, n = 14; Rai 3–4, n = 7); (c) samples collected pre-treatment from CLL patients (n = 73) vs. those collected post-treatment (n = 27).
Advanced stages of CLL are frequently associated with low total IgG-levels, which may possibly cause false-negative results in the different herpes virus-specific antibody assays. We therefore evaluated retrospectively results obtained by the CMV-specific assay in relation to total IgG-levels measured in serum at the time of sample collection. Total IgG-levels ranged between < 195 and 4400 mg dL−1 (median, 748 mg dL−1; SD, 595 mg dL−1, n = 93) and were not significantly different between CMV-seropositive and -seronegative CLL patients [median, 750 mg dL−1 vs. 681 mg dL−1, P = 0·973 (Mann–Whitney U-test)].
Association of herpes virus seroprevalence with CLL prognostic factors and disease progression
Herpes virus seroprevalence did not have a statistically significant correlation with a more advanced clinical stage of CLL at the time of diagnosis (Fig. 1b). Rates of seropositivity were almost identical in patients who were treated before sample collection and those previously untreated (Fig. 1c), although all patients with Rai stage 3 or 4 (n = 7) at the time of diagnosis were positive for CMV- and EBV-specific IgG-antibodies. Seropositivity for each of the herpes virus infections investigated was comparable between patients with expression of an unmutated (n = 33) and mutated (n = 39) IGHV gene [CMV, 79% vs. 82%, P = 0·772 (Fisher Exact); EBV, 82% vs. 92%, P = 0·285 (Fisher Exact); HHV-6, 79% vs. 74%, P = 0·783 (Fisher Exact); HHV-7, 46% vs. 31%, P = 229 (Fisher Exact)].
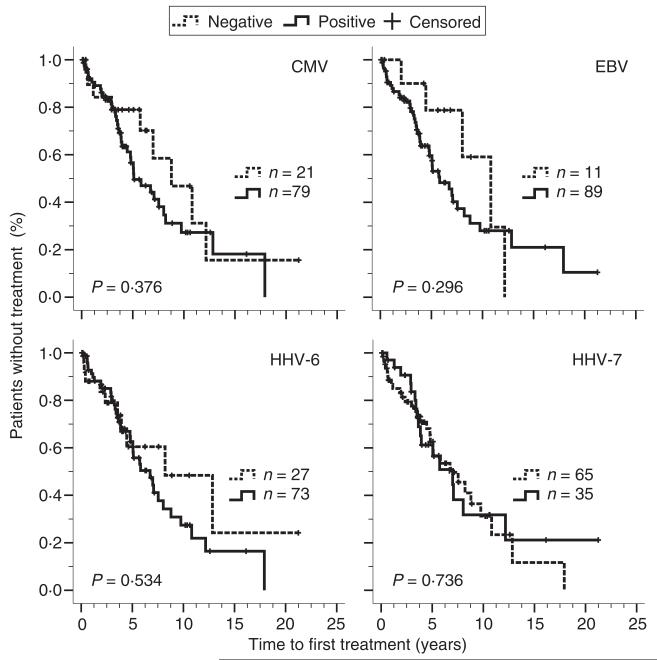
A total of 48 CLL patients received treatment for CLL during follow-up, including 21 patients who received treatment after collection of serum samples. The 52 untreated CLL patients were followed for a median time of 47 months after diagnosis. The time to first treatment after diagnosis was similar in patients with and without herpes virus infection (Fig. 2).
Figure 2.
Time between diagnosis of CLL and commencement of therapy in relation to seropositivity for herpes viruses. Kaplan–Meier curves depict the proportion of untreated patients with CLL according to the time since diagnosis. The patients are grouped according to test results for selected herpes virus-specific IgG-antibodies in serum. Patients not treated during follow-up period were included in this analysis as censored cases.
Herpes virus seroprevalence in a second CLL cohort from a different geographical region
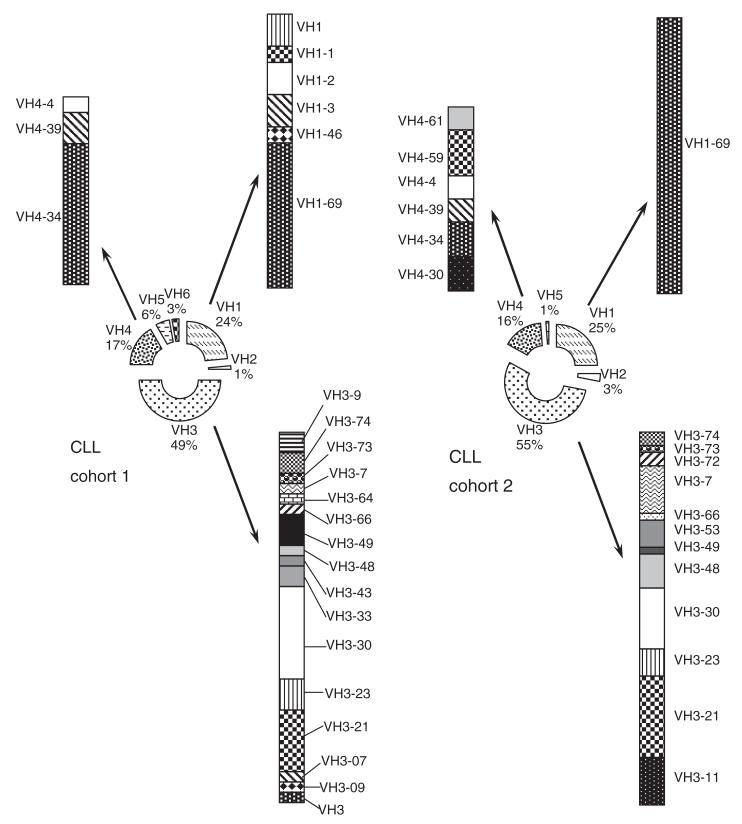
To evaluate the generalizability of the noted differences in herpes virus seroprevalence between the European CLL cohort 1 and the healthy adult cohort, we also examined the herpes virus seroprevalence in a second cohort of CLL patients from the US (CLL cohort 2, n = 100). Patients of cohort 2 were matched by age and gender with those of CLL cohort 1 and were from a geographical region that had the same overall CMV-seroprevalence in the healthy adult population (60%) [12]. The two CLL cohorts were comparable with respect to IGHV mutational status [mutated IGHV gene, 54% vs. 57%, P = 0·757 (Fisher Exact)] and relative frequency distribution of IGHV subgroups (Fig. 3). The most frequently observed IGHV-subgroups in both CLL cohorts were IGHV 1, 3 and 4. The use of IGHV4-34-gene was more common in patients of CLL cohort 1 than in those of CLL cohort 2 [nine of 72 (13%) vs. three of 100 (3%), P = 0·03 (Fisher Exact)].
Figure 3.
Comparison of IGHV gene use between CLL cohorts 1 and 2. The relative number of CLL IGHV sequences using genes of each IGHV subgroup is shown in the centre donuts, and the distribution of sequences using particular IGHV 1, 3 and 4 genes is depicted peripherally.
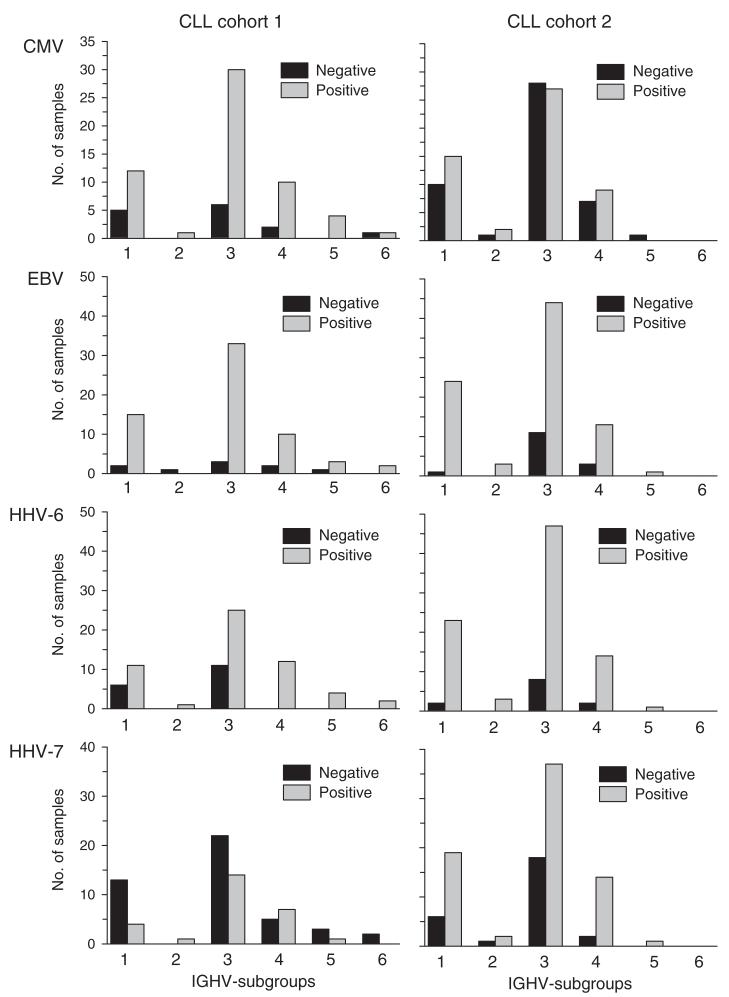
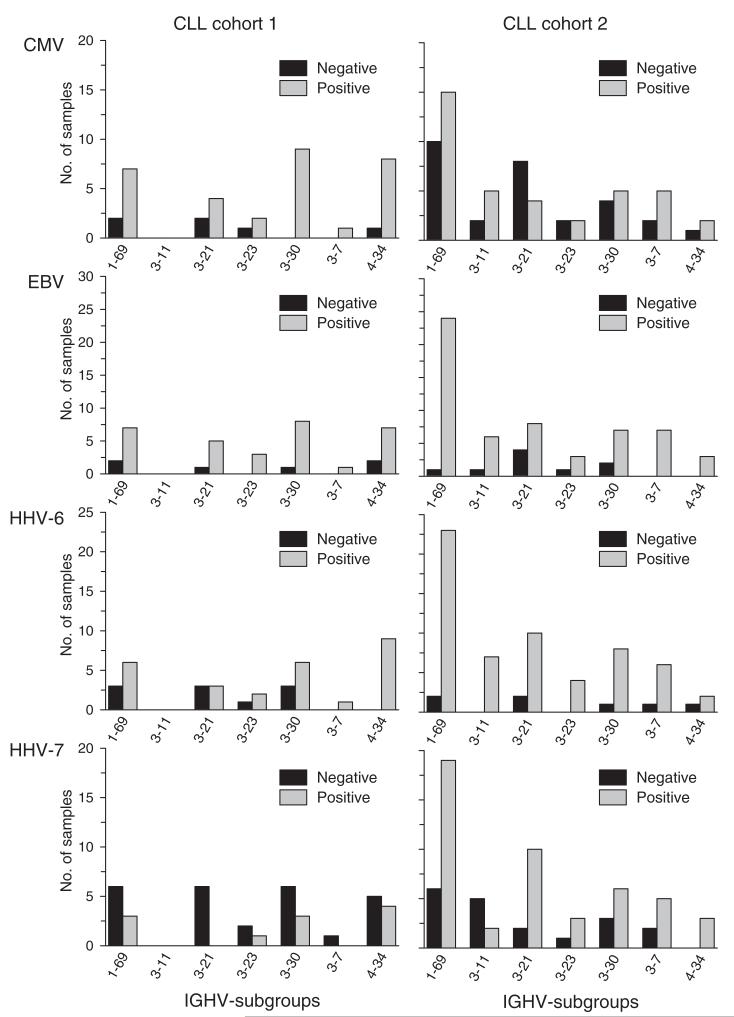
CMV-seroprevalence was 53% in CLL cohort 2, which was significantly lower than that found in CLL cohort 1, but comparable with that noted in the general population. EBV-seroprevalence in CLL cohort 2 was similar to that found in CLL cohort 1 (85%). HHV-6- and HHV-7-seroprevalence were clearly higher in patients of CLL cohort 2 than in the cohort of healthy adults (88% and 73% respectively). Rates of herpes virus-specific seropositivity with respect to the IGHV-subgroups reflected overall herpes virus seroprevalence within the two CLL cohorts (Fig. 4a).
Figure 4.
Comparison of IGHV gene use between CLL cohorts 1 and 2 with respect to herpes virus-specific seropositivity. (a) Comparison of IGHV subgroups in relation to herpes virus-specific serology. (b) Comparison of the most frequently used IGHV genes in relation to herpes virus-specific serology.
IGHV gene use was analysed also in relation to herpes virus-specific seropositivity for the most commonly used IGHV genes (> 4% of samples from both CLL cohorts, Fig. 4b). In CLL cohort 1, all patients with CLL cells who used the IGHV3-20 gene were CMV-seropositive, whereas those with CLL cells that used the IGHV3-21 gene were HHV-7-seronegative. These associations were not apparent in the second cohort of CLL patients. Conversely, all patients in CLL cohort 2 who had CLL cells that used IGHV3-11 or IGHV3-23 were found to be HHV-6-seropositive, an association that was not apparent in CLL cohort 1.
Discussion
In this study, we found that CMV-seroprevalence was significantly higher in one CLL cohort when compared with that in an age- and gender-matched control cohort from the general population. Nevertheless, this significant difference in CMV-seroprevalence was not present in a second cohort of CLL patients from a different geographical region. Consequently, latent CMV infection as indicated by positivity for CMV-specific IgG-antibodies may be associated with CLL in selected cohorts, but further virological or host-related factors are essential in the evolution or modification of the course of the disease.
In previous studies, CMV-seroprevalence was found to be 60% in the general population of industrialized countries with a slight increase in CMV-seroprevalence with increasing age of the individuals tested [12,13]. CLL is mainly a disease of older adults, with a median age at diagnosis of 70 years [24]. We included therefore an age-matched control cohort of healthy adults. CMV-seroprevalence found in the control cohort was identical to what may have been expected from previous, significantly larger studies [12,13], which indicates representative sampling of study participants. These features of our study design reduced the potential for bias. Nevertheless, the possibility of differences in the relative herpes virus seroprevalence arising by chance only could not be ruled out entirely, also due to the comparably small size of the cohorts studied. Consequently, definite conclusions cannot be drawn on a possible association of any of the herpes viruses investigated with CLL.
We found that the association of CMV-seropositivity with CLL noted in the European CLL cohort 1 cannot be generalized to all cases of CLL as such association was not apparent in the US CLL cohort 2. Variations in virological, host genetic and virus-host interactions between geographically distinct cohorts, however, also have to be considered when evaluating associations between relative herpes virus seroprevalence and CLL. For example, the geographical distribution of the various CMV-genotypes differs significantly between Europe and the US [25]. Furthermore, genetic differences between the two cohorts cannot be excluded entirely notwithstanding the fact that almost all patients of the two CLL cohorts were Caucasian. Geographical differences between IGHV-gene use are well-known and could allow genetic or infectious elements to shape both the normal and the ‘leukaemic’ repertoire differently [26]. Our findings demonstrate clearly the importance of using a second cohort from a different geographical region to evaluate the generalizability of noted associations between the relative seroprevalence of any one of the various human herpes viruses and CLL [27].
The HHV-6-seroprevalence found in CLL cohort 1 could be confirmed in CLL cohort 2 and was higher than in the healthy, age- and gender-matched healthy controls. Still, HHV-6- and -7-seroprevalence differed considerably between seroepidemiological studies (65–100% and 44–100% respectively) and was associated with geographical region surveyed [28-33]. Accordingly, the presently noted differences in HHV-6- and HHV-7-seroprevalence between CLL patients and healthy adults should be interpreted with caution.
Detection of CMV- and EBV-DNA in blood from CLL patients was linked with a subset of CLL patients who expressed stereotyped IGHV4-34 B-cell receptors [34]. Furthermore, we found in a previous study an association between chronic or recurrent infections and poor prognostic markers for CLL [6]. In this study, CMV- or EBV-seropositivity neither was an indicator of a faster progression of the disease nor was it associated with one of the more common IGHV genes, including IGHV4-34. Seropositivity for any of the various herpes viruses is a marker for latent infection only and does not allow drawing conclusions on frequency or level of herpes virus reactivation from latency before and after diagnosis of CLL. The differences between the various studies with regard to the virological and prognostic markers of the disease, however, further emphasize that frequency and level of CMV or EBV viraemia may vary significantly between adults latently infected with these herpes viruses [35].
Interestingly, the CMV-seroprevalence noted in the CLL patients and healthy adults in this study was similar or even higher to the relative rates of CMV reactivation and disease observed in patients treated with alemtuzumab (50%) [36]. Alemtuzumab (Campath ®), a humanized, anti-CD52 monoclonal antibody, is used as both first line-treatment and treatment for relapse/fludarabine-refractory CLL [37,38]. Infectious complications following treatment with alemtuzumab are common [36]. Multiple risk factors have been defined for the identification of patients at high risk for infectious complications [39-41], but this study appears to be the first to investigate herpes virus seropositivity. Routine assessment of CLL patients at the time of diagnosis should also include testing for herpes virus-specific seropositivity.
In conclusion, CMV-seroprevalence may be significantly higher in selected CLL cohorts than in age- and gender-matched healthy adults. This study, however, demonstrates also the importance of using a second cohort from a different geographical region to evaluate the generalizability of noted associations between the relative seroprevalence of any one of the various human herpes viruses and CLL. The detection of any of the herpes virus-specific IgG-antibodies is generally not associated with CLL, which points to further important influences in the evolution of CLL such as virus-host interactions.
Acknowledgements
This study was funded in part by the Austrian Science Fund grant #J2750-B11, the Austrian Ministry of Education, Science and Culture through the project Gen-AU-Child (GZ 200·136/1 – VI/1/2005), a fellowship grant from the Austrian Society of Haematology and Oncology and the National Institutes of Health grant 5 PO1-CA081534 for the Chronic Lymphocytic Leukemia (CLL) Research Consortium. The manufacturer of the CMV- and EBV-ELISA (Medac, Hamburg, Germany) donated test kits.
Contributor Information
C. Steininger, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria; Moores Cancer Center, University of California, La Jolla, CA, USA
L. Z. Rassenti, Moores Cancer Center, University of California, La Jolla, CA, USA
K. Vanura, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria
K. Eigenberger, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria
U. Jäger, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria
T. J. Kipps, Moores Cancer Center, University of California, La Jolla, CA, USA
C. Mannhalter, Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna, Vienna, Austria
S. Stilgenbauer, Department of Internal Medicine III, University of Ulm, Ulm, Germany
T. Popow-Kraupp, Clinical Institute of Virology, Medical University of Vienna, Vienna, Austria
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78:374–7. [PubMed] [Google Scholar]
- 3.Widhopf GF, Brinson DC, Kipps TJ, Tighe H. Transgenic expression of a human polyreactive Ig expressed in chronic lymphocytic leukemia generates memory-type B cells that respond to nonspecific immune activation. J Immunol. 2004;172:2092–9. doi: 10.4049/jimmunol.172.4.2092. [DOI] [PubMed] [Google Scholar]
- 4.Widhopf GF, Goldberg CJ, Toy TL, Rassenti LZ, Wierda WG, Byrd JC, et al. Nonstochastic pairing of immunoglobulin heavy and light chains expressed by chronic lymphocytic leukemia B cells is predicated on the heavy chain CDR3. Blood. 2008;111:3137–44. doi: 10.1182/blood-2007-02-073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Rapkin JS, Caporaso NE, Mellemkjaer L, Gridley G, Goldin LR, et al. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanura K, Le T, Esterbauer H, Spath F, Porpaczy E, Shehata M, et al. Autoimmune conditions and chronic infections in chronic lymphocytic leukemia patients at diagnosis are associated with unmutated IgVH genes. Haematologica. 2008;93:1912–6. doi: 10.3324/haematol.12955. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Caballero A, Garcia-Montero AC, Barcena P, Almeida J, Ruiz-Cabello F, Tabernero MD, et al. Expanded cells in monoclonal TCR-alphabeta+/CD4+/NKa+/CD8−/+dim T-LGL lymphocytosis recognize hCMV antigens. Blood. 2008;112:4609–16. doi: 10.1182/blood-2008-03-146241. [DOI] [PubMed] [Google Scholar]
- 8.Britt WJ, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39:401–12. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 9.Ling PD, Lednicky JA, Keitel WA, Poston DG, White ZS, Peng R, et al. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14-month longitudinal study. J Infect Dis. 2003;187:1571–80. doi: 10.1086/374739. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during space-flight. J Infect Dis. 2000;182:1761–4. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 11.Hengel H, Brune W, Koszinowski UH. Immune evasion by cytomegalovirus–survival strategies of a highly adapted opportunist. Trends Microbiol. 1998;6:190–7. doi: 10.1016/s0966-842x(98)01255-4. [DOI] [PubMed] [Google Scholar]
- 12.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 13.Seale H, MacIntyre CR, Gidding HF, Backhouse JL, Dwyer DE, Gilbert L. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol. 2006;13:1181–4. doi: 10.1128/CVI.00203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi K, Tanaka-Taya K, Kazuyama Y, Ito YM, Hashimoto S, Fukayama M, et al. Prevalence of Epstein-Barr virus in Japan: trends and future prediction. Pathol Int. 2006;56:112–6. doi: 10.1111/j.1440-1827.2006.01936.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan A, Kilic SS, Kalkan A, Ozden M, Demirdag K, Ozdarendeli A. Seropositivity of Epstein-Barr virus in Eastern Anatolian Region of Turkey. Asian Pac J Allergy Immunol. 2003;21:49–53. [PubMed] [Google Scholar]
- 16.Morris MC, Edmunds WJ, Hesketh LM, Vyse AJ, Miller E, Morgan-Capner P, et al. Sero-epidemiological patterns of Epstein-Barr and herpes simplex (HSV-1 and HSV-2) viruses in England and Wales. J Med Virol. 2002;67:522–7. doi: 10.1002/jmv.10132. [DOI] [PubMed] [Google Scholar]
- 17.Collier AC, Meyers JD, Corey L, Murphy VL, Roberts PL, Handsfield HH. Cytomegalovirus infection in homosexual men. Relationship to sexual practices, antibody to human immunodeficiency virus, and cell-mediated immunity. Am J Med. 1987;82:593–601. doi: 10.1016/0002-9343(87)90105-7. [DOI] [PubMed] [Google Scholar]
- 18.Guinan ME, Thomas PA, Pinsky PF, Goodrich JT, Selik RM, Jaffe HW, et al. Heterosexual and homosexual patients with the acquired immunodeficiency syndrome. A comparison of surveillance, interview, and laboratory data. Ann Intern Med. 1984;100:213–8. doi: 10.7326/0003-4819-100-2-213. [DOI] [PubMed] [Google Scholar]
- 19.Jackson JB, Erice A, Englund JA, Edson JR, Balfour HH., Jr. Prevalence of cytomegalovirus antibody in hemophiliacs and homosexuals infected with human immunodeficiency virus type 1. Transfusion. 1988;28:187–9. doi: 10.1046/j.1537-2995.1988.28288179029.x. [DOI] [PubMed] [Google Scholar]
- 20.Kipps TJ. Chronic lymphocytic leukemia and related diseases. In: Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. 7th ed. Williams Hematology; McGraw-Hill; New York: 2006. pp. 1343–83. [Google Scholar]
- 21.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 22.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rassenti LZ, Kipps TJ. Clinical utility of assessing ZAP-70 and CD38 in chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2006;70:209–13. doi: 10.1002/cyto.b.20129. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez JA, Land KJ, McKenna RW. Leukemias, myeloma, and other lymphoreticular neoplasms. Cancer. 1995;75:381–94. doi: 10.1002/1097-0142(19950101)75:1+<381::aid-cncr2820751320>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Zipeto D, Hong C, Gerna G, Zavattoni M, Katzenstein D, Merigan TC, et al. Geographic and demographic differences in the frequency of human cytomegalovirus gB genotypes 1-4 in immunocompromised patients. AIDS Res Hum Retroviruses. 1998;14:533–6. doi: 10.1089/aid.1998.14.533. [DOI] [PubMed] [Google Scholar]
- 26.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stella S, Guida G, et al. Geographic patterns and pathogenetic implications of IGHV gene usage in chronic lymphocytic leukemia: the lesson of the IGHV3-21 gene. Blood. 2005;105:1678–85. doi: 10.1182/blood-2004-07-2606. [DOI] [PubMed] [Google Scholar]
- 27.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranger S, Patillaud S, Denis F, Himmich A, Sangare A, M’Boup S, et al. Seroepidemiology of human herpesvirus-6 in pregnant women from different parts of the world. J Med Virol. 1991;34:194–8. doi: 10.1002/jmv.1890340313. [DOI] [PubMed] [Google Scholar]
- 29.Clark DA, Alexander FE, McKinney PA, Roberts BE, O’Brien C, Jarrett RF, et al. The seroepidemiology of human herpesvirus-6 (HHV-6) from a case-control study of leukaemia and lymphoma. Int J Cancer. 1990;45:829–33. doi: 10.1002/ijc.2910450507. [DOI] [PubMed] [Google Scholar]
- 30.Stiller I, Pusztai R, Sombor E, Orosz L, Pal A, Tarodi B. Prevalence and avidity of human herpesvirus-6 specific IgG antibodies in pregnant women in Hungary. Acta Microbiol Immunol Hung. 2006;53:25–34. doi: 10.1556/AMicr.53.2006.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Krueger GR, Koch B, Leyssens N, Berneman Z, Rojo J, Horwitz C, et al. Comparison of seroprevalences of human herpesvirus-6 and -7 in healthy blood donors from nine countries. Vox Sang. 1998;75:193–7. [PubMed] [Google Scholar]
- 32.Cervera C, Marcos MA, Linares L, Roig E, Benito N, Pumarola T, et al. A prospective survey of human herpesvirus-6 primary infection in solid organ transplant recipients. Transplantation. 2006;82:979–82. doi: 10.1097/01.tp.0000229938.12722.ee. [DOI] [PubMed] [Google Scholar]
- 33.Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27:651–3. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostareli E, Hadzidimitriou A, Stavroyianni N, Darzentas N, Athanasiadou A, Gounari M, et al. Molecular evidence for EBV and CMV persistence in a subset of patients with chronic lymphocytic leukemia expressing stereotyped IGHV4-34 B-cell receptors. Leukemia. 2009 doi: 10.1038/leu.2008.379. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Steininger C, Kundi M, Kletzmayr J, Aberle SW, Popow-Kraupp T. Antibody maturation and viremia after primary cytomegalovirus infection, in immunocompetent patients and kidney-transplant patients. J Infect Dis. 2004;190:1908–12. doi: 10.1086/424677. [DOI] [PubMed] [Google Scholar]
- 36.Montillo M, Tedeschi A, Miqueleiz S, Veronese S, Cairoli R, Intropido L, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006;24:2337–42. doi: 10.1200/JCO.2005.04.6037. [DOI] [PubMed] [Google Scholar]
- 37.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 38.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 39.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–25. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 41.Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006107:885–91. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]