Abstract
African swine fever virus (ASFV) causes an acute haemorrhagic disease of domestic pigs against which there is no effective vaccine. The attenuated ASFV strain OUR T88/3 has been shown previously to protect vaccinated pigs against challenge with some virulent strains including OUR T88/1. Two genes, DP71L and DP96R were deleted from the OUR T88/3 genome to create recombinant virus OUR T88/3ΔDP2. Deletion of these genes from virulent viruses has previously been shown to reduce ASFV virulence in domestic pigs. Groups of 6 pigs were immunised with deletion virus OUR T88/3ΔDP2 or parental virus OUR T88/3 and challenged with virulent OUR T88/1 virus. Four pigs (66%) were protected by inoculation with the deletion virus OUR T88/3ΔDP2 compared to 100% protection with the parental virus OUR T88/3. Thus the deletion of the two genes DP71L and DP96R from OUR T88/3 strain reduced its ability to protect pigs against challenge with virulent virus.
Keywords: African swine fever virus, Attenuation, Virulence
Highlights
-
•
Attenuated strain OUR T88/3 protects pigs against challenge with virulent strain OUR T88/1.
-
•
OUR T88/3 encodes virulence associated genes DP71L and DP96R.
-
•
Construction of recombinant virus OUR T88/3ΔDP2 with DP71L and DP96R genes deleted.
-
•
Growth of recombinant virus OUR T88/3ΔDP2 in vitro is similar to OUR T88/3.
-
•
Pigs inoculated with OUR T88/3ΔDP2 show reduced protection when challenged with OUR T88/1
Introduction
African swine fever is a devastating haemorrhagic disease of domestic pigs caused by a double-stranded DNA virus, African swine fever virus (ASFV). ASFV is the only member of the Asfarviridae family and replicates predominantly in the cytoplasm of cells (Dixon et al., 2000). The virus replicates in macrophages and those cells with markers characteristic of intermediate and late stages of differentiation which are susceptible to infection (McCullough et al., 1999).
Virulent strains of ASFV can kill domestic pigs within about 5–14 days of infection with a mortality rate approaching 100%. Domestic pigs infected with less virulent isolates can survive infection and recovered pigs can gain immunity to subsequent challenge with non-virulent and related virulent viruses (Detray, 1957; Boinas et al., 2004 and King et al., 2011).
ASFV can infect and replicate in warthogs (Phacochoerus sp.), bushpigs (Potamocherus sp.) and soft ticks of the Ornithodoros species, but in these species few if any clinical signs are observed and long term persistent infections can be established (Wilkinson, 1984). The disease is currently endemic in many sub-Saharan countries and in Europe in Sardinia. Following its introduction to Georgia in the Trans Caucasus region in 2007, ASFV has spread extensively through neighbouring countries including the Russian Federation and in 2012 the first outbreak was reported in Ukraine (Malogolovkin et al., 2012). Virus has been detected in wild boar in a number of different locations in both southern and western Russia. If ASFV becomes established in wild boar, the difficulty of disease eradication will be increased (Blome et al., 2012).
Although no vaccine is available for ASFV, it has been known for many years that pigs which recover from infection with less virulent isolates can be protected from challenge with related virulent viruses (Detray, 1957; Malmquist, 1963; Mebus and Dardiri, 1980). In addition pigs immunised with naturally attenuated ASFV or viruses attenuated by passage in tissue culture can also be protected from challenge with virulent viruses (Manso-Ribeiro et al., 1963; Leitao et al., 2001; Boinas et al., 2004 and King et al., 2011). For example Manso-Ribeiro et al., 1963 showed that virus attenuated by passage in porcine bone marrow culture when inoculated into pigs protected them against challenge with virulent virus. However, a substantial proportion of the vaccinated pigs developed unacceptable post-vaccination reactions including pneumonia, locomotor disturbances, skin ulcer, abortion and death.
Mechanisms of protection induced by attenuated viruses have been investigated. A role for antibodies in protection is suggested by studies showing that passive transfer of antibodies from immunised pigs to naïve pigs could confer protection against virulent challenge (Wardley et al., 1985; Onisk et al., 1994). Evidence suggests that neutralising antibodies are not fully effective but other protective roles for antibodies are possible (Zsak et al., 1993; Ruiz-Gonzalvo et al., 1983; Gómez-Puertas and Escribano, 1997). CD8+ T cells appear to have an essential role in protection induced by the OUR T88/3 isolate since depletion of this cell subset abrogated protection against challenge with virulent OUR T88/1 isolate (Oura et al., 2005). Further evidence for a role of IFNγ producing lymphocytes in protection comes from experiments showing that cross-protection induced by the OUR T88/3 isolate, against challenge with virulent isolates from different genotypes, was correlated with the ability of those isolates to specifically stimulate IFNγ producing lymphocytes from the immunised pigs (King et al., 2011).
Inoculation of recombinant viruses generated by the deletion of genes from virulent viruses, including 9GL or DP71L, has been shown to reduce virus virulence and protect pigs against challenge with the parental virulent virus (Zsak et al., 1996, 1998; Lewis et al., 2000). Deletion of other genes has been shown to reduce virus virulence but the effect of their deletion on induction of protective immunity has not yet been studied (Tulman et al., 2009).
The DP71L gene is present in genomes of ASFV isolates encoding either a long form, 184 amino acids, or short form, 70–72 amino acids (Sussman et al., 1992; Vydelingum et al., 1993; Goatley et al., 1999). A conserved carboxy-terminal domain of 56 amino acids is present in both forms of DP71L and in the Herpes simplex virus neurovirulence factor ICP34.5 and host genes GADD34 and MyD116 (Goatley et al., 1999). These genes share the function of dephosphorylating eukaryotic translation initiation factor 2 alpha (eIF-2α) by recruiting protein phosphatase 1 catalytic subunit (PP1c) to dephosphorylate eIF-2α (Rivera et al., 2007; Zhang et al., 2010). However in cells infected with DP71L gene deletion mutants in the ASFV E70 and Malawi LIL20/1 strains, phosphorylation of eIF-2α was still inhibited, suggesting that the virus has other mechanism to inhibit this pathway. In eukaryotic cells, control of the availability of active non-phosphorylated eIF-2α by reversible phosphorylation is the key and rate-limiting step regulating global protein synthesis (Wek et al., 2006). eIF-2α can be phosphorylated by a number of different kinases, including the double-stranded RNA activated PKR and stress activated PERK kinases, and viruses have evolved specific mechanisms to inhibit these pathways and hence reduce the inhibition of global protein synthesis (Mulvey et al., 2007; Poppers et al., 2000 and Sharp et al., 1998).
Inoculation of pigs with a deletion mutant of the virulent E70 strain of ASF lacking the short form of DP71L resulted in reduced virus virulence and all convalescent animals survived challenge with the virulent parental E70 strain (Zsak et al., 1996). Conversely, Afonso et al., 1998 showed that the deletion of the long form of DP71L gene from the virulent strains Malawi 20/1 and Pr4 did not reduce virus virulence and that inoculation with the recombinant deletion viruses caused 100% mortality. Deletion of the DP71L gene from all three strains E70, Malawi LIL20/1 and Pr4 did not alter the ability of the recombinant virus to replicate in macrophages in vitro. The attenuated E70ΔDP71L virus was restored to virulence by insertion of a 20 kb fragment of ASFV DNA including three members of multigene family (MGF) 360 and three members of MGF 530 into its genome. These results suggest that other genes may compensate for the loss of DP71L in some isolates (Neilan et al., 2002).
The DP96R gene is located at the right hand of the genome immediately downstream of the DP71L gene and is highly conserved sharing 92–96% amino acid identity among most strains of ASFV. The exceptions are; DP96R is absent from the virulent strain Malawi LIL20/1 and a longer form of the gene (156 amino acids) is encoded by virulent Haiti strain. DP96R has no obvious similarity with any genes in public databases. Deletion of the DP96R gene from the virulent strain E70 did not reduce replication within macrophages in vitro compared to parental virus but all pigs inoculated with the E70ΔDP96L recombinant virus survived infection and had a significant 100 to 1000-fold reduction in viraemia (Zsak et al., 1998).
The naturally attenuated OUR T88/3 isolate has been shown to induce good levels of protection against lethal challenge with related virulent viruses (Boinas et al., 2004; Oura et al., 2005; King et al., 2011). However adverse reactions including fever and joint swelling are observed in some pigs post-inoculation. In one report (King et al., 2011) 5 out of 12 pigs immunised with OUR T88/3 developed a transient pyrexia and in another experiment one pig (1 out of 7) inoculated with OUR T88/3 developed continued pyrexia and was euthanised. In the current study we investigated the effect of deleting additional genes DP71L and DP96R from the OUR T88/3 genome to determine the effects on induction of clinical signs and protective immunity in pigs. Although replication of this virus deletion mutant in macrophages in vitro was not reduced, inoculation of pigs with the deletion virus, OUR T88/3ΔDP2, reduced protection against challenge with virulent virus OUR T88/1 compared to the parental virus OUR T88/3.
Results and discussion
Isolation of recombinant virus OUR T88/3ΔDP2
The DP71L and DP96R genes were deleted from the ASFV OURT88/3 isolate and replaced with the GUS gene under control of the ASFV p72 promoter. This was achieved by homologous recombination between plasmid pΔDP2loxPGUS and the virus genome (see Materials and methods and Fig. 1). Recombinant viruses were identified by expression of the GUS gene and purified by infection at limiting dilution. Recombinant, virus OUR T88/3ΔDP2, was selected for further characterisation. Genomic DNA was isolated from wild type OUR T88/3 and OUR T88/3ΔDP2 and analysed by PCR to test for the insertion of the GUS marker gene and deletion of the DP71L and DP96R genes (Fig. 2). Primers 18RSEQ and 19RSEQ were designed to anneal within the MGF360 18R gene, and the non-coding region upstream of the MGF360 19R gene, that flank the insertion site. PCR using these primers amplified a 851 bp fragment using wild type virus OUR T88/3 as template (Fig. 2 lane 1. The size of this band is consistent with that of a PCR product containing both the DP71L and DP96R genes and the expected size of flanking regions. A PCR using the same primers and genomic DNA from OUR T88/3ΔDP2 amplified a 2.25 kb fragment that is consistent with the size of the GUS marker gene cassette inserted to replace the DP71L and DP96R genes (Fig. 2 lane 2. To confirm that OUR T88/3ΔDP2 contains the GUS gene, a PCR was carried using an internal GUS gene primer, RGUS, and primer 18RSEQ. This PCR amplified a fragment consistent with the expected 1.6 kb size (Fig. 2 lane 4 when DNA from OUR T88/3ΔDP2 was used as template. As expected no PCR fragment was detected using these primers with wild type OUR T88/3 DNA as template (Fig. 2 lane 3. To confirm that the GUS marker gene had been inserted at the correct site within the genome of recombinant OUR T88/3ΔDP2, a PCR reaction was carried out using primers RGUS and EXT18R, that anneals within the MGF360 18R gene but is 5′ and external to the sequence of the left flanking region of pΔDP2loxPGUS. PCR with these primers using OUR T88/3 as template generated no products (Fig. 2 lane 5, whereas OUR T88/3ΔDP2 template generated a 2.1 kb fragment (Fig. 2 lane 6. Taken together the PCR data showed that OUR T88/3ΔDP2 contains the GUS gene in place of the DP71L and DP96R genes.
Fig. 1.
Schematic diagram showing generation of recombinant ASF virus OUR T88/3ΔDP2 with the deletion of the DP71L and DP96R genes. Recombinant virus OUR T88/3ΔDP2 was created by homologous recombination between the MGF360 18R and MGF360 19R genes on the wild type OUR T88/3 genome and transfer vector plasmid pΔDP2loxPGUS resulting in the deletion of the DP71L and DP96R genes and the insertion of vp72GUS gene.
Fig. 2.
Analysis of genomic viral DNA gene deletions and insertions by PCR. Viral DNA was extracted from wild type OUR T88/3 virus and the recombinant virus OUR T88/3∆DP2. Specific fragments were amplified by PCR and the products were analysed by electrophoresis on 1% agarose gels. The following primer sets were used in the lanes 1+2 (18RSEQ and 19RSEQ), lanes 3+4 (18RSEQ and RGUS), lanes 5+6 (EXT18R and RGUS). The following viral genomic DNAs were used as templates in the lanes 1, 3 and 5 (OUR T88/3) and lanes 2, 4 and 6 (OUR T88/3ΔDP2).
Growth characteristics of recombinant virus OUR T88/3ΔDP2
To investigate whether deletion of DP71L and DP96R genes affected virus replication, the growth of OUR T88/3ΔDP2 was compared to parental OURT88/3 virus in macrophages. Cells were infected at high (10 TCID50/cell) or low (0.1 TCID50/cell) multiplicity of infection and total virus was harvested from supernatants at different times post-infection. Fig. 3 shows that there were no significant differences between the titres of wild OUR T88/3 and those of OUR T88/3ΔDP2 viruses recovered at any of the time points measured, or at either multiplicity of infection. This suggested that deletion of DP71L and DP96R did not significantly affect the replication of OUR T88/3 in primary porcine macrophages.
Fig. 3.
Replication kinetics of OUR T88/3 and recombinant OUR T88/3ΔDP2 viruses. Pig bone marrow macrophages were infected at a high multiplicity of infection (m.o.i.) of 10 or low m.o.i. of 0.1 with parental OUR T88/3 strain or recombinant virus OUR T88/3ΔDP2. At various hours post-infection, as indicated on the x axis, total virus was harvested and infectious virus titrated on 96 well plates by analysis of infection on cultures of pig bone marrow macrophages. The virus titre (TCID50/ml) is the mean of three individual observations. Titres obtained following infection with viruses OUR T88/3 high m.o.i.  and low m.o.i.
and low m.o.i.  ; OUR T88/3ΔDP2 high m.o.i.
; OUR T88/3ΔDP2 high m.o.i. and low m.o.i.
and low m.o.i.  are indicated.
are indicated.
Challenge of pigs with virulent OUR T88/1 immunized with OUR T88/3 or OUR T88/3ΔDP2
Two groups of six pigs were immunized with either OUR T88/3 (Group 1) or OUR T88/3ΔDP2 (Group 2), three weeks later both groups of pigs were challenged with virulent OUR T88/1. After the initial immunisations with OUR T88/3 and OUR T88/3ΔDP2, both groups of pigs showed similar average body temperatures and clinical scores (data not shown). Individually, three pigs from group 1 (pig numbers 2, 5 and 6) and three pigs from group 2 (pig numbers 13, 15 and 18) showed adverse reactions in the form of swollen joints. The clinical scores observed post-challenge of the pigs with OUR T88/1 are shown in Fig. 4. At three days post-challenge two pigs (numbers 14 and 18) from group 2 exhibited clinical scores greater than 7 rising to above 15 by day four post-challenge. At day six post-challenge these two pigs were terminated for ethical reasons within the remit of the Home Office Licence. In group 3 (the control, unvaccinated pigs), all three animals (numbers 19, 20 and 21) exhibited clinical scores between 1 and 2 at day three post-challenge, rising to between 8 and 9 at day five. These pigs were all terminated at day six post-challenge. The main difference in clinical signs observed in pigs 14 and 18 on day 3 post-challenge, compared to the control pigs which were not immunised with OUR T88/3 were in the more severe levels of recumbancy and inappetance observed for pigs 14 and 18. None of the animals in group 1 had clinical scores above 4 up to day six post-challenge. Interestingly, pigs 14 and 18 from group 2 exhibited higher clinical scores at days three and four post-challenge than the non-immunized pigs from group 3. However, the clinical scores of all pigs in group 3 continued to rise from day four to five post-challenge, whereas those of pigs 14 and 18 decreased from score 15 to score 8 between days four and five. All of the pigs in group 1 and four of the pigs from group 2 had clinical scores below 4 during the 15 days they were kept post-challenge. Thus 66% of the pigs immunized with the deletion recombinant OUR T88/3ΔDP2 survived subsequent challenge with virulent OUR T88/1 whereas 100% of the animals immunised with wild type OUR T88/3 were protected. Furthermore, there were no clear differences in the adverse reactions observed after the initial immunisation with the two viruses.
Fig. 4.
Clinical scores post-challenge with OUR T88/1. Clinical scores (y-axis) of individual pigs from the three separate groups at different days post-challenge (x-axis). Pigs were challenged at day 0 (C-0am). Clinical scoring system as designed by King et al. (2011).
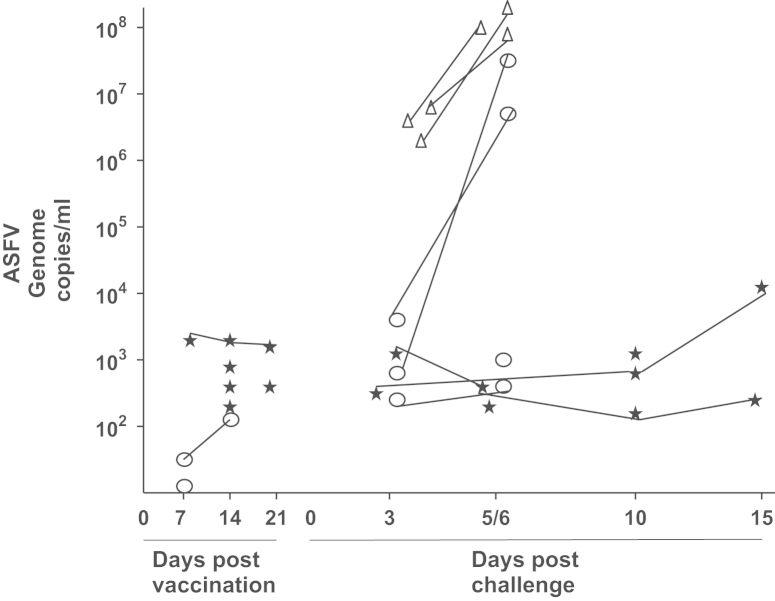
Virus titres obtained from blood from immunised and control pigs before and after virulent virus challenge
EDTA blood samples were obtained from all animals in groups 1 and 2 at days 7, 14 and 21 post-immunisation. After challenge with virulent virus OUR T88/1, blood samples were taken from pigs in all three groups and tissues were also taken at post mortem. The samples were tested for the presence and titres of ASFV genomic DNA using qPCR. The results (see Fig. 5) show that blood from five pigs from group 1 and three pigs from group 2 contained low levels of ASFV genomic DNA post-immunisation with OUR T88/3. The level of ASFV DNA detected was less than 104 copies/ml. On days 3 and 6 post-challenge with OUR T88/1, all three pigs from the non-immunised group 3 had high levels of ASFV DNA which was greater than 106 copies/ml at day 3 and rose to greater than 5×107 copies/ml at day 6. The two pigs from group 2 (numbers 14 and 18), which were not protected, had low levels of ASFV genomic DNA in blood on day 3 (less than 6×103 copies/ml) but by day 6 this had increased to greater than 5×106 copies/ml. Apart from the pigs, that were terminated at day 6, only four of the six pigs from group 1 (numbers 2, 3, 4 and 6) and two pigs from group 2 (numbers 13 and 15) had any detectable levels of ASFV genomic DNA at day 3 post-challenge. All of these pigs had levels of virus DNA below 104 copies per ml, with the exception of pig 12 which had a copy number of 8×104 copies per ml at day 3 post-challenge. The pigs with low copy numbers of ASFV genomic DNA had either low or no clinical scores and all were protected against virulent ASFV challenge. At 15 days post-challenge one pig from group 1 (number 6) had levels of ASFV DNA of approximately 104 copies/ml but did not show high clinical scores and was still fully protected against ASFV. Two pigs from group 1 and two from group 2 (1, 5, 16 and 17) had no detectable ASFV genomic DNA in the blood either post-immunisation with OUR T88/3 or post-challenge with OUR T88/1 at the time points examined and had low or no clinical scores.
Fig. 5.
Viraemia estimated by qPCR for individual pigs post-inoculation and post-challenge. Viraemia estimated by qPCR and expressed as ASFV genome copy per ml of blood (y-axis) for individual pigs at different days post-inoculation and post-challenge (x-axis). Group 1 OUR T88/3  , Group 2 OUR T88/3ΔDP2
, Group 2 OUR T88/3ΔDP2  and Group 3 unvaccinated
and Group 3 unvaccinated  . Five pigs had no detectable ASFV DNA in blood post-immunisation (pig 1—Group 1, pigs 14, 15, 17 and 18—Group 2). Four pigs had no detectable levels of ASFV DNA in blood post-challenge (pigs 1 and 5—Group1, pigs 16 and 17—Group 2).
. Five pigs had no detectable ASFV DNA in blood post-immunisation (pig 1—Group 1, pigs 14, 15, 17 and 18—Group 2). Four pigs had no detectable levels of ASFV DNA in blood post-challenge (pigs 1 and 5—Group1, pigs 16 and 17—Group 2).
Virus titres in the spleen
The spleens of all 15 pigs were tested for the presence of ASFV genomic DNA. DNA was detected only in the five pigs (numbers 14 and 18 from group 2 and all three pigs from group 3), which had high clinical scores and were terminated on day 6 post-challenge. The levels of DNA detected varied from 5×103 to 3×104 ASFV genome copies per mg of tissue (data not shown).
Discussion
In this study we investigated whether deletion of genes from the ASFV low virulence isolate OUR T88/3 would reduce adverse clinical reactions post-immunisation but maintain induction of high levels of protection post-challenge with virulent isolate OUR T88/1. We took advantage of the adjacent genomic location of virulence associated genes DP71L and DP96R to delete both genes from OUR T88/3. These genes have not previously both been deleted from the same virus. The results showed that deletion of DP71L and DP96R from OUR T88/3 did not reduce the replication of the deletion mutant OUR T88/3ΔDP2 in primary porcine macrophages in vitro compared to parental OUR T88/3. However in vivo immunisation of pigs showed that two out of six pigs inoculated with the deletion virus OUR T88/3ΔDP2 were not protected against challenge with virulent virus OUR T88/1 whereas all six pigs inoculated with the parental strain OUR T88/3 were protected.
At days 3 and 5 post-challenge the two pigs from group 2 which were not protected (numbers 14 and 18) showed very high clinical scores (score 8 rising to score 16) which were significantly higher than the clinical scores (score 2 rising to score 8) for the non-immunised control pigs (numbers 19, 20 and 21). Although the clinical signs of pigs 14 and 18 were enhanced at days 3 and 5 post-challenge the levels of virus replication were lower by approximately 3log10 at day 3 and 1log10 at day 5 compared to control unvaccinated pigs. Thus, inoculation of OUR T88/3ΔDP2 in pigs 14 and 18 seemed to reduce virus replication post-challenge but also reduced protection and resulted in an increase in the clinical score at days 3 and 5 post-challenge compared to the non-immunised pigs.
The earlier and increased clinical scores observed post-challenge in the two pigs (14 and 18) from group 2 indicate an immune enhancement of disease may have occurred. Immune enhancement of disease has been documented for other virus infections. Severe disease induced by Dengue virus (DENV) is correlated with a second DENV infection and can be mediated by two mutually exclusive mechanisms. One of these, antibody enhancement of infection, occurs when cross-reactive or non-neutralizing antibodies bind to virus particles and form complexes which enhance virus infection of Fc receptor bearing cells, leading to increased virus load (Halstead and O'Rourke, 1977). The second mechanism involves cross-reactive memory T cells (Rothman and Ennis 1999; Mongkolsapaya et al., 2003). Vaccination with RSV can also lead to enhanced disease (Chin et al., 1969). Although RSV titres in the lung can be reduced after RSV virus challenge disease can be enhanced (Prince et al., 2001). A Th2-like immune response to RSV plays a major role in the enhancement of respiratory disease in the lungs (Graham et al., 1993). Inoculation of an inactivated RSV vaccine in mice does elicit RSV-specific antibodies but they have a limited affinity for neutralising epitopes on the RSV fusion protein due to a lack of affinity maturation (Delgado et al., 2009). The basis for the enhancement of clinical scores we observed in two of the vaccinated and challenged pigs requires further investigation. Antibody enhanced uptake of ASFV into macrophages has not been reported previously and has not been observed in our experiments. In one previous study treatment of macrophages with ASFV antibody complexes did not enhance virus replication (Alcamí and Viñuela, 1991).
In our experiment the higher clinical scores observed in pigs 14 and 18 did not correlate with increased viraemia. Thus antibody enhanced virus uptake and replication in cells is unlikely. Instead a possible cause for the increased clinical scores may be the induction of an acute inflammatory response which initiates a cytokine storm (Tisoncik et al., 2012).
Attempts to enhance safety and immunogenicity of vaccinia virus (VACV) vaccine strains by targeted gene deletions have met with some success. Deletion of either E3L, a double-stranded RNA virus protein, B15R (soluble receptor for IL-1β) or A41 (chemokine binding protein) from VACV were shown to increase T cell responses with no reduction in virus replication efficiency (Clark et al., 2006; Cottingham et al., 2008; Jentarra et al., 2008). However, immunogenicity was only maintained or increased when higher doses of inoculating virus compared to wild type virus were administered.
Although deletion of DP71L and DP96R genes from the OUR T88/3 strain did not reduce replication in vitro in pig macrophages, compared to parental virus, possibly virus replication is reduced in vivo and reduced antigen load could explain the reduced protection induced by immunisation with OUR T88/3ΔDP2. However from our current study it is not possible to draw this conclusion since samples were collected at 7 day intervals post-immunisation and transient viraemia may not have been detected.
In previous studies immunisation of pigs with virulent viruses from which DP71L or DP96R genes were deleted did result in reduced virus replication in pigs. No reduction in replication was observed in macrophages in vitro but reduced viraemia (between 100 and 100 fold) and clinical signs were observed following immunisation of pigs with E70 virus lacking DP71L gene. In contrast deletion of DP71L from virulent Malawi LIL20/1 or Pr4 did not reduce virulence or virus replication in vivo (Zsak et al., 1996; Afonso et al., 1998). Similarly deletion of the DP96R gene from the virulent virus E70 did not affect the growth characteristics of the virus in macrophage cell cultures in vitro but levels of viraemia in immunised pigs were reduced between 100 and 1000 fold in pigs (Zsak et al., 1996, 1998; Afonso et al., 1998). Based on these previous studies we would expect that deletion of both DP71L and DP96R genes could reduce virus replication in vivo. The reduced protection we observed following immunisation with OUR T88/3ΔDP2 compared to parental OUR T88/3 may have resulted from a failure of the virus to prime an appropriate protective immune response. Oura et al. (2005) demonstrated that pigs inoculated with OUR T88/3 and depleted of CD8+ lymphocytes were no longer fully protected against challenge with OUR T88/1. This indicates that CD8+ lymphocytes play an important role in the protective immune response to ASFV infection. Deletion of the two genes DP71L and DP96R which may encode for protective CD8+ epitopes may be a contributing factor of towards why the deletion virus had a reduced ability to protect against virulent challenge. Further knowledge of the functions of DP71L and DP96R genes are required to investigate this hypothesis.
Although deletion of DP71L and DP96R was unsuccessful at improving OUR T88/3 as a vaccine, deletion of different ASFV genes may give rise to an improved vaccine. An alternative vaccine approach may be to delete ASFV genes from a parental virulent strain of ASFV which may result in an attenuated recombinant virus which does not give rise to adverse clinical reactions but induces effective protection.
Immunisation with OUR T88/3ΔDP2 did not appear to significantly reduce the adverse clinical reactions observed in pigs compared to parental OUR T88/3 since two pigs in each of groups 1 and 2 had transient joint swelling. Although it is possible that increasing the dose of OUR T88/3ΔDP2 may improve protection levels by increasing antigen load, this may also increase adverse clinical reactions.
Therefore future research will focus on understanding the mechanisms involved in induction of the clinical reactions and testing different combinations of gene deletions from the ASFV genome to improve the safety and efficacy of attenuated viruses.
Materials and methods
Cells and viruses
Non-virulent, non-haemabsorbing ASFV isolate OUR T88/3 and virulent haemabsorbing isolate OUR T88/1 were both obtained from Ourique in Portugal and have been described previously (Boinas et al., 2004). Both OUR T88/3 and OUR T88/1 are p72 genotype I (Bastos et al., 2003; Chapman et al., 2008). Viruses were grown in primary macrophage cultures derived from bone marrow (Malmquist and Hay,1960). Titres of virus were determined as the amount of virus causing haemadsorption (for HAD isolates) or cytopathic effects (for non-HAD isolates) in 50% of infected cultures (HAD50/ml or TCID50/ml).
Construction of plasmid transfer vector p∆DP2loxPGUS
The plasmid transfer vector pΔDP2loxPGUS was constructed to facilitate the deletion of genes DP71L and DP96R from the genome of virus OUR T88/3. Using OUR T88/3 genomic DNA as template, a 445 bp fragment (Flank L) located at the 3′ terminus of the MGF360 18R gene at position 169125–169570 immediately upstream of the DP71L gene was amplified using the PCR primers DP71L 5(GTTTAAACTTAAGCTTTTGCGCGGCCTTGAGGTCAAG) and DP71L 3(CGCGGATCCATCGGTACCCGCTCGTGGGGTGAAAGAACGTCC). A 440 bp fragment (Flank R) located in the non-coding region upstream of the MGF 360 19R gene at position 170195–170635 and downstream of the DP96R gene to be deleted was amplified using the PCR primers DP96R 5(GGATCCAGCGGCCGCACGTACGTGTAAGTTTATAAACTATATAG) and DP96R 3(CCCTTCTAGACTCGAGAGATAACCATGGAAATTTTGTA). Flank L fragment was digested with Hind III and Kpn I and ligated into the vector pMGFloxPGUS vector (Abrams and Dixon, 2012) which was also digested with Hind III and Kpn I to create plasmid pFlankL-GUS. The pFlankL-GUS vector included the GUS gene under the control of the p72 promoter flanked by loxP sites. The flankR fragment was digested with Not I and Xho I and ligated into the vector pFlankL-GUS digested with the same enzymes to create the transfer vector pΔDP2loxPGUS. The plasmid pΔDP2loxPGUS contains a GUS marker gene flanked on the left hand side by the 3′ terminal section of the MGF360 18R gene which lies immediately upstream of the DP71L gene. To the right hand side of the GUS gene was located a fragment (440 bp) of the non-coding region upstream of the MGF360 19R gene which lies immediately downstream of the DP96R gene (Fig. 1).
Construction and isolation of recombinant virus OUR T88/3∆DP2
Primary pig bone marrow macrophages (35 mm dish, 106 cells) were infected with OUR T88/3 at a multiplicity of infection (m.o.i). of 10 and incubated at 37 °C for 5 h, and then washed with Earle's saline (10% porcine serum, penicillin/streptomycin 10,000 µmg/ml). A transfection mixture containing 250 µl Optimem (Gibco-Life Technologies), 5 µg p∆DP2loxPGUS and 7.5 µl TRANS-IT LT-1 (Mirus) transfection reagent was incubated at 20 °C for 20 min before adding it to the infected cells. Incubation was continued at 37 °C for 4 h before the addition of 1 ml Earle's saline and continued incubation at 37 °C. Virus was harvested from the infected and transfected cells 72 h post-infection and cell debris removed by centrifugation. Aliquots of virus containing supernatant were used to infect bone marrow macrophages on 96 well plates. At 90 h post-infection Earle's saline containing 100 µg/ml 5-bromo-4-chloro-1H-indol-3-yl β-d-glucopyranosiduronic acid (X-Gluc) was added and wells appearing ‘blue’ containing recombinant GUS expressing viruses were harvested. Infections at limiting dilution were further carried out on bone marrow macrophages containing X-Gluc until only one blue well per 96 well plate was observed to indicate infection with the recombinant deletion virus. No evidence of virus infection (cytopathic effect (cpe)) was observed in the other 95 wells. High titre stocks of recombinant virus OUR T88/3∆DP2 were grown up on pig bone marrow macrophages.
Purification and analysis of viral genomic DNA
Viral genomic DNA from OUR T88/3 or OUR T88/3∆DP2 virus harvests was purified from 300 µl of supernatant from infected cells using a GE Healthcare Illustra genomic Prep Mini Spin kit. Analysis of viral genomic DNA was carried out by PCR using the specific DNA primers 18RSEQ (GGGACTAGTCTCCGCCCCACTGCG), 19RSEQ (GTTTAGTGTGGTAGCAACACTATC, RGUS (CCTTCTCTGCCGTTTCCAAATCGCCGC) and EXT18R (CGCTCAGATGGGCAATCTGAGG).
Pig immunisation and challenge
Pigs used were cross-bred, large white×Landrace, of average weight 15 kg at the first inoculation. All pigs were maintained in high security SAPO4 facilities throughout and the experiment performed under Home Office licence PPL 70–6369. One group of six pigs were inoculated intramuscularly with 104TCID50 of low virulence isolate OUR T88/3 (Group 1) or deletion recombinant OUR T88/3∆DP2 (Group 2). Three weeks later both groups 1 and 2 and a third group (Group 3) containing three non-immunised pigs, were challenged intramuscularly with 104 HAD of virulent ASFV isolate OUR T88/1. ASFV-inoculated and challenged pigs were monitored for body temperature and other clinical signs and these were scored as reported by King et al. (2011). All pigs were examined by post-mortem at termination and spleen and lymph tissues were collected.
Quantitative PCR analysis of virus copy number in blood and tissues
DNA was extracted from whole peripheral blood at different days post-inoculation and post-challenge or from tissues at post-mortem and analysed by quantitative PCR (qPCR) as described previously (King et al., 2003).
Acknowledgment
We acknowledge financial support from the BBSRC.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abrams C.C., Dixon L.K. Sequential deletion of genes from the African swine fever virus genome using the cre/loxP recombination system. Virology. 2012;10:142–148. doi: 10.1016/j.virol.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso C.L., Zsak L., C. Carrillo C., Borca M.V., Rock, D.L. M.V. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 1998;79:2543–2547. doi: 10.1099/0022-1317-79-10-2543. [DOI] [PubMed] [Google Scholar]
- Alcamí A., Viñuela E. Fc receptors do not mediate African swine fever virus replication in macrophages. Virology. 1991;181:756–759. doi: 10.1016/0042-6822(91)90912-u. [DOI] [PubMed] [Google Scholar]
- Bastos A.D., Penrith M.L., Crucière C., Edrich J.L., Hutchings G., Roger F., Couacy-Hymann E.R., Thomson G. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003;148:693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- Blome S., Gabriel C., Dietze K., Breithaupt A., Beer M. High virulence of African swine fever virus caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012;18:708. doi: 10.3201/eid1804.111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boinas F.S., Hutchings G.H., Dixon L.K., Wilkinson P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004;85:2177–2187. doi: 10.1099/vir.0.80058-0. [DOI] [PubMed] [Google Scholar]
- Chapman D.A.G., Tcherepanov V., Upton C., Dixon L.K. Comparison of the genome sequences of nonpathogenic and pathogenic African swine fever virus isolates. J. Gen. Virol. 2008;89:397–408. doi: 10.1099/vir.0.83343-0. [DOI] [PubMed] [Google Scholar]
- Chin J., Magoffin R.L., Shearer L.A., Schieble J.H., Lennette E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Clark R.H., Kenyon J.C., Bartlett N.W., Tscharke D.C., Smith G.L. Deletion of gene A41L enhances vaccinia virus immunogenicity and vaccine efficacy. J. Gen. Virol. 2006;87:29–38. doi: 10.1099/vir.0.81417-0. [DOI] [PubMed] [Google Scholar]
- Cottingham M.G., Andersen R.F., Spencer A.J., Saurya S., Furze J., Hill A.V., Gilbert S.C. Recombination-mediated genetic engineering of a bacterial artificial chromosome clone of modified vaccinia virus Ankara (MVA) PLoS ONE. 2008;3(2):e1638. doi: 10.1371/journal.pone.0001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P., Diaz L., Trento A., Chang H.Y., Mitzner W., Ravetch J., Melero J.A., Irusta P.M., Polack F.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detray D.E. Persistence of viremia and immunity in African swine fever. Am. J. Vet. Res. 1957;18:811–816. [PubMed] [Google Scholar]
- Dixon L.K., Costa J.V., Escribano J.M., Rock D.L., Vinuela E., Wilkinson P.J. In: Asfarviridae. Regenmortel M.H.V., Van F.C.M.B.D.H.L.O., editors. Academic Press; London: 2000. pp. 159–165. [Google Scholar]
- Goatley L.C., Marron M.B., Jacobs S.C., Hammond J.M., Miskin J.E., Abrams C.C., Smith G.L., Dixon L.K. Nuclear and nucleolar localization of an African swine fever virus protein, I14L, that is similar to the herpes simplex virus-encoded virulence factor ICP34.5. J. Gen. Virol. 1999;80:525–535. doi: 10.1099/0022-1317-80-3-525. [DOI] [PubMed] [Google Scholar]
- Gómez-Puertas P., Escribano J.M. Blocking antibodies inhibit complete African swine fever virus neutralization. Virus Res. 1997;49:115–122. doi: 10.1016/s0168-1702(97)01463-9. [DOI] [PubMed] [Google Scholar]
- Graham B.S., Henderson G.S., Tang Y.W., Lu X., Neuzil K.M., Colley D.G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- Halstead S.B., O'Rourke E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentarra G.M., Heck M.C., Youn J.W., Kibler K., Langland J.O., Baskin C.R., Ananieva O., Chang Y., Jacobs B.L. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine. 2008;26:2860–2872. doi: 10.1016/j.vaccine.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.P., Reid S.M., Hutchings G.H., Grierson S.S., Wilkinson P.J., Dixon L.K., Bastos A.D., Drew T.W. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods. 2003;107:53–61. doi: 10.1016/s0166-0934(02)00189-1. [DOI] [PubMed] [Google Scholar]
- King K., Chapman D., Argilaguet J.M., Fishbourne E., Hutet E., Cariolet R., Hutchings G., Oura C.A., Netherton C.L., Moffat K., Taylor G., Le Potier M.F., Dixon L.K., Takamatsu H.H. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine. 2011;29:4593–4600. doi: 10.1016/j.vaccine.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao A., Cartaxeiro C., Coelho R., Cruz B., Parkhouse R.M.E., Portugal F.C., Vigario J.D., Martins C.L.V. The nonhaemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol. 2001;(82):513–523. doi: 10.1099/0022-1317-82-3-513. [DOI] [PubMed] [Google Scholar]
- Lewis T., Zsak L., Burrage T.G., Lu Z., Kutish G.F., Neilan J.G., Rock D.L. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. J. Virol. 2000;74:1275–1285. doi: 10.1128/jvi.74.3.1275-1285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough K.C., Basta S., Knotig S., Gerber H., Schaffner R., Kim Y.B., Salmuller A. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98:203–212. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malogolovkin A., Yelsukova A., Gallardo C., Tsybanov S., Kolbasov D. Molecular characterization of African swine fever virus isolates originating from outbreaks in the Russian Federation between 2007 and 2011. Vet. Microbiol. 2012;158:415–419. doi: 10.1016/j.vetmic.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Malmquist W.A., Hay D. Hemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Am. J. Vet. Res. 1960;21:104–108. [PubMed] [Google Scholar]
- Malmquist W.A. Serologic and immunologic studies with African swine fever virus. Am. J. Vet. Res. 1963;24:450–459. [PubMed] [Google Scholar]
- Manso-Ribeiro J., Nunes-Petisca J.L., Lopez-Frazao F., Sobral M. Vaccination against ASF. Bull. Off. Int. Epizoot. 1963;60:921–937. [Google Scholar]
- Mebus C.A., Dardiri A.H. Western hemisphere isolates of African swine fever virus: asymptomatic carriers and resistance to challenge inoculation. Am. J. Vet. Res. 1980;41:1867–1869. [PubMed] [Google Scholar]
- Mongkolsapaya J., Dejnirattisai W., Xu X.N., Vasanawathana S., Tangthawornchaikul N., Chairunsri A., Sawasdivorn S., Duangchinda T., Dong T., Rowland-Jones S., Yenchitsomanus P.T., McMichael A., Malasit P., Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Mulvey M., Arias C., Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J. Virol. 2007;81:3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilan J.G., Zsak L., Kutish G.F., Afonso C.L., Rock D.L. Novel swine virulence determinant in the left variable region of the African swine fever virus genome. J. Virol. 2002;76:3095–3104. doi: 10.1128/JVI.76.7.3095-3104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onisk D.V., Borca M.V., Kutish G., Kramer E., Irusta P., Rock D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology. 1994;198:350–354. doi: 10.1006/viro.1994.1040. [DOI] [PubMed] [Google Scholar]
- Oura C.A.L., Denyer M.S., Takamatsu H., Parkhouse R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005;86:2445–2450. doi: 10.1099/vir.0.81038-0. [DOI] [PubMed] [Google Scholar]
- Poppers J., Mulvey M., Khoo D., Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 2000;74:11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G.A., Curtis S.J., Yim K.C., Porter D.D. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 2001;82:2881–2888. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- Rothman A.L., Ennis F.A. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;25:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- Rivera J., Abrams C., Hernáez B., Alcázar A., Escribano J.M., Dixon L., Alonso C. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J. Virol. 2007;81:2923–2929. doi: 10.1128/JVI.02077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gonzalvo, F., Carnero, M.E., Bruyel, V., 1983. Immunological responses of pigs to partially attenuated African swine fever virus and their resistance to virulent homologous and heterologous viruses. In: African Swine Fever, EUR 8466 EN, Proceedings of CEC/FAO Research Seminar, Sardinia, Italy, September, 1981, pp.206–216. Edited by P.J. Wilkinson. Luxemburg, Belgium: Commission of the European Communities.
- Sharp T.V., Moonan F., Romashko A., Joshi B., Barber G.N., Jagus R. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology. 1998;250:302–315. doi: 10.1006/viro.1998.9365. [DOI] [PubMed] [Google Scholar]
- Sussman M.D., Lu Z., Kutish G., Afonso C.L., Roberts P., Rock D.L. Identification of an African swine fever virus gene with similarity to a myeloid differentiation primary response gene and a neurovirulence-associated gene of herpes simplex virus. J. Virol. 1992;66:5586–5589. doi: 10.1128/jvi.66.9.5586-5589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulman E.R., Delhon G.A., Ku B.K., Rock D.L. African swine fever virus. Curr. Top. Microbiol. Immunol. 2009;328:43–87. doi: 10.1007/978-3-540-68618-7_2. [DOI] [PubMed] [Google Scholar]
- Vydelingum S., Baylis S.A., Bristow C., Smith G.L., Dixon L.K. Duplicated genes within the variable right end of the genome of a pathogenic isolate of African swine fever virus. J. Gen. Virol. 1993;74:2125–2130. doi: 10.1099/0022-1317-74-10-2125. [DOI] [PubMed] [Google Scholar]
- Wardley R.C., Norley S.G., Wilkinson P.J., Williams S. The role of antibody in protection against African swine fever virus. Vet. Immunol. Immunopathol. 1985;9:201–212. doi: 10.1016/0165-2427(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wilkinson P.J. The persistence of African swine fever in Africa and the Mediterranean. Prev. Vet. Med. 1984;2:71–82. [Google Scholar]
- Zhang F., Moon A., Childs K., Goodbourn S., Dixon L.K. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J. Virol. 2010;84:10681–10689. doi: 10.1128/JVI.01027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Onisk D.V., Afonso C.L., Rock D.L. Virulent African swine fever virus isolates are neutralized by swine immune serum and by monoclonal antibodies recognizing a 72-kDa viral protein. Virology. 1993;196:596–602. doi: 10.1006/viro.1993.1515. [DOI] [PubMed] [Google Scholar]
- Zsak L., Lu Z., Kutish G.F., Neilan J.G., Rock D.L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J. Virol. 1996;70:8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Caler E., Lu Z., Kutish G.F., Neilan J.G., Rock. D.L. A nonessential African swine fever virus gene UK is a significant virulence determinant in domestic swine. J. Virol. 1998;72:1028–1035. doi: 10.1128/jvi.72.2.1028-1035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]