Abstract
Pendrin (SLC26A4), a Cl−/anion exchanger encoded by the gene PDS, is highly expressed in the kidney, thyroid and inner ear epithelia and is essential for bicarbonate secretion/chloride reabsorption, iodide accumulation and endolymph ion balance, respectively. The molecular mechanisms controlling pendrin activity in renal, thyroid and inner ear epithelia have been the subject of recent studies. The effects of ambient pH, the hormone aldosterone and the peptide uroguanylin (UGN; the “intestinal natriuretic hormone”), known modulators of electrolyte balance, on transcription of the pendrin gene, have been investigated. Luciferase reporter plasmids containing different length fragments of the human PDS (hPDS) promoter were transfected into renal HEK293, thyroid LA2, and inner ear VOT36 epithelial cells. Acidic pH decreased and alkaline pH increased hPDS promoter activity in transfected HEK293 and VOT36, but not in LA2 cells. Aldosterone reduced hPDS promoter activity in HEK293 but had no effect in LA2 and VOT36 cells. These pH and aldosterone-induced effects on the hPDS promoter occurred within 96-bp and 89-bp regions, respectively, which likely contain distinct response elements to these modulators. Injection of UGN into mice resulted in decreased pendrin mRNA and protein expression in the kidney. Exposure of transfected HEK293 to UGN decreased hPDS promoter activity. The findings provided evidence for the presence of a UGN response element within the 96-bp region overlapping with the pH response element on the hPDSpromoter. Pendrin is also expressed in airway epithelium. The cytokins interleukin 4 (IL-4) and interleukin-13 (IL-13), known regulators of airway surface function, have been shown to increase hPDS promoter activity by a STAT6-dependent mechanism. In conclusion, systemic pH, the hormone aldosterone, and the peptide UGN influence renal tubular pendrin gene expression and, perhaps, pendrin-mediated Cl−/HCO3− exchange at the transcriptional level. Pendrin-driven anion transport in the endolymph and at the airway surface may be regulated transcriptionally by systemic pH and IL-3/IL-4, respectively. The distinct response elements and the corresponding transcription factors mediating the effect of these modulators on the PDS promoter remain to be identified and characterized.
Key Words: Anion exchange, Acid-base transport, Aldosterone, Uroguanylin, Promoter, Renal tubule, Thyroid, Inner ear
Introduction
The Pendrin Gene (PDS/SLC26A4) and its Product, Pendrin/SLC26A4 Protein
Everett et al. [1] identified the PDS gene as the locus of mutations causing Pendred syndrome, an autosomal recessive disorder characterized by sensorineural hearing loss and enlargement of the thyroid gland [2, 3]. The 21 exon PDS gene resides immediately 3’ to the closely homologous DRA/SLC26A3 gene on chromosome 7q31.1 [4], suggesting an ancient gene duplication [5]. The ~5 kb PDS transcript is most highly expressed in thyroid, kidney and inner ear [1, 6, 7], but is detected also in respiratory epithelial cells [8], trophoblasts [9], endometrium [10], testis [11], vas deferens [12], and mammary gland [13]. The PDS transcript encodes the 780 amino acid pendrin protein, sharing with other SLC26 superfamily proteins the predicted topographic disposition of a short N terminal cytoplasmic domain followed by ~12 transmembrane spanning regions and a C-terminal cytoplasmic STAS domain [1, 14, 15, 16]. However, the exact number of transmembrane spanning regions of pendrin is controversial [16]. Functional studies in heterologous expression systems have revealed that pendrin functions as an electroneutral plasmalemmal anion exchanger [17]. Pendrin functions in the renal cortical collecting duct (CCD) in Cl−/HCO3− [18, 19] and Cl−/I− exchange [20]. Pendrin is believed also to mediate Cl−/HCO3− exchange in the inner ear and Cl−/I− exchange in the thyroid [21, 22].
Pendred Syndrome
Pendred syndrome is the most common form of syndromic deafness, and accounts for up to 10% of hereditary deafness [23, 24, 25]. The profound hearing loss in Pendred syndrome is particularly pronounced at high frequency, and occasionally accompanied by impaired vestibular function [23]. It is associated with radiologically detectable enlargement of the vestibular aqueduct (EVA) and with temporal bone abnormalities [2, 26]. Pendred syndrome is further characterized by a thyroidal iodide organification defect variably accompanied by euthyroid goiter [2, 3]. Pendrin mutations without thyroid dysfunction are linked to recessive nonsyndromic deafness (DFNB4) [27] with EVA [28]. Among the more than 200 different PDS mutations identified in deaf patients, we with our collaborators have reported 14 PDS variants novel at time of publication, and functionally characterized those encoding nontruncated missense mutants [28, 29, 30].
Expression and Function of Pendrin
Pendrin in the kidney
The CCD plays a vital role in acid-base homeostasis and electrolyte balance [31, 32], and includes principal cells and intercalated cells (types A, B, and non-A non-B [31, 32]). Pendrin protein is located at or near the apical membrane of type B- and non-A non-B intercalated cells (IC) of the CCD [32]. Pendrin contributes to acid–base balance by secreting HCO3− into the tubular lumen in exchange for luminal Cl− [18], and to regulation of blood pressure and systemic fluid balance via the same Cl−reabsorption [19, 33, 34]. In contrast to the impaired inner ear and thyroid function of Pendred syndrome, no overt acid-base disturbances such as metabolic alkalosis or electrolyte abnormalities have been detected in these patients under otherwise unstressed conditions, but at least two Pendred syndrome patients with intercurrent illnesses exhibited unusually severe metabolic alkalosis [35, 36].
Pendrin in the inner ear
Pendrin knockout mice develop profound deafness, show signs of vestibular disease and have marked dilatation of inner ear structures [37]. Pendrin is expressed in several locations of the cochlea and the vestibule of the mouse inner ear [38, 39]. This expression pattern involves regions important for endolymphatic fluid resorption, consistent with pendrin's essential role in the normal development and proper function of the inner ear [38].
Immunohistochemical analysis demonstrating coexpression of pendrin, vacuolar H+-ATPase and cytosolic carbonic anhydrase II in epithelial cells of the murine endolymphatic sac [39] suggests that pendrin may exert its action on the endolymph by controlling acid-base balance in this fluid compartment, which is essential for normal hearing. The pendrin-positive cells in the endolymphatic duct and sac resemble morphologically the pendrin-expressing non-A non-B intercalated cells of the renal CCD [39]. The role of pendrin in maintaining acid base balance in the inner ear is further supported by the recent demonstration [21, 40] of markedly lower pH, higher [Ca2+], and complete loss of endocochlear potential in the endolymph of slc26a4−/− mice relative to those of wild-type and heterozygous mice. However, pendrin's transport substrates in situ, and its exact role in inner ear function and in determining the ionic composition of the endolymph, remain incompletely defined.
Pendrin in the thyroid gland
Pendrin resides on the thyrocyte apical membrane, where its activity as a Cl−/I− exchanger is essential for cellular iodide efflux into the follicular lumen [22, 23]. Pendred syndrome-associated loss-of-function mutations in the pendrin protein impair the ability of the thyroid gland to accumulate iodide in the follicular lumen which leads to insufficient thyroid hormone synthesis, and compensatory goiter in Pendred syndrome patients [2]. Since other transporters are able to transport I− into the follicular lumen, patients with Pendred syndrome are usually euthyroid. Occasional mutant pendrin alleles unique to patients with DFNB4 and EVA may maintain residual anion exchange activity which is sufficient to eliminate or postpone the onset of goiter, but not of inner ear disease [41].
Epithelial cell-specific expression of the pendrin gene in the kidney, inner ear, and thyroid
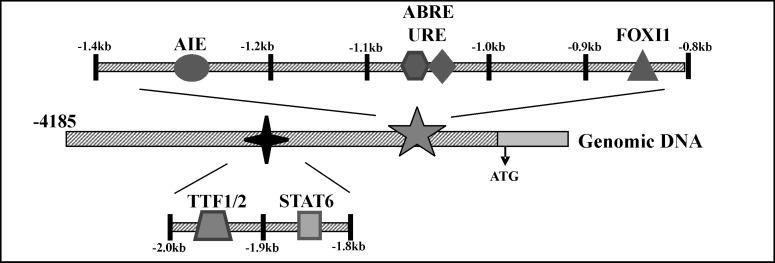
Cell type-specific promoter activity was analyzed by luciferase expression in transiently transfected epithelial and nonepithelial cells driven by all or part of 4.2kb of human PDS (hPDS) 5'-flanking sequence [42]. Distinct differences in expression/activity of deduced positive/negative regulatory elements (PREs/NREs) within the hPDS promoter between thyroid LA2, renal HEK293 and inner ear VOT36 cells were demonstrated with only basal activity in fibroblast NIH3T3 cells [42]. These studies showed putative PREs between −1433bp and −1342bp in renal HEK293 cells, between −1952bp and −1838bp in thyroid LA2 cells, and between −2060bp and −1952bp in VOT36 inner ear cells. The PRE at −1433bp to −1342bp contains consensus binding sites for the transcription factors hepatocyte nuclear factor 3 (HNF-3) and Wilms tumor protein (WT1). The PRE at −1952bp to −1838bp has thyroid transcription factors 1 and 2 (TTF-1 and TTF-2) sites. The TTF-1 and/or TTF-2 binding sites located between −1946bp and −1938bp and between −1942bp and −1933bp, respectively, on the hPDS promoter are essential for the activity of this promoter in thyroid LA2 cells [42] (Fig. 1). The inner-ear PRE at −2060bp to −1952bp lacks known consensus binding sites.
Fig. 1.
A schematic model of the 5’ flanking region of the human pendrin gene depicting the 600bp and the 200bp DNA regions between −0.8kb and −1.4kb and between −1.8kb and −2.0kb, respectively, on the hPDS promoter. These regions contain functionally significant elements that appear to play a role in the regulation of this gene by various modulators and transcription factors. ABRE, acid-base response element, AIE, aldosterone-induced element, URE, uroguanylin response element, FOXI1, forkhead box transcription factor I1, STAT6, signal transducer and activator of transcription protein 6, TTF1/2, thyroid transcription factors 1 and 2. See text for details.
Pendrin in the airway epithelium
Pendrin is highly expressed at the apical membrane of bronchial epithelial cells following exposure to interleukin 4 (IL-4) or interleukin-13 (IL-13) (see later) or antigen exposure, or in models of asthma or chronic obstructive pulmonary disease [8, 43, 44]. Pendrin functions as a CI−/HCO3− exchanger in airway epithelial cells [45]. It has been demonstrated that pendrin regulates airway surface liquid (ASL) thickness via its function as an anion exchanger [43]. This action of pendrin has been attributed to the movement of Cl− into the epithelial cell in exchange for secreted HCO3−, which leads to osmotic flow of water from lumen to the interstitium, thereby decreasing the thickness of the ASL and increasing viscosity of the luminal mucus [43]. In line with this action of pendrin, this anion exchanger has been shown to mediate mucus production in bronchial asthma and chronic obstructive pulmonary disease [44], and to contribute to asthma exacerbation induced by viral infections and allergens [43]. Pendrin also functions in the bronchial epithelial cell as a SCN−/Cl− exchanger [8]. Since SCN− is an anion with antioxidant/antimicrobial properties, the pendrin-mediated secretion of SCN− into the lumen (in exchange for Cl− entering the cell) may contribute to the innate defense of the mucosal surface. Interestingly, however, there is no evidence of respiratory disease in individuals affected by mutations in the pendrin gene.
Modulation of Pendrin Activity
Modulation of pendrin activity in the kidney
Several factors are known to control acid-base transport in various nephron segments including the CCD. These factors include systemic pH, body K+ and Cl− stores, extracellular fluid volume, as well as hormones such as aldosterone, parathyroid hormone, thyroid hormone, and angiotensin II [32, 46, 47]. Whereas the modulation of renal acid-base transport has been thoroughly investigated at the tubular and cellular levels, the molecular mechanisms of this modulation remain to be established. Systemic pH, aldosterone, angiotensin II, and Cl− levels regulate the activity of several ion transport processes involved in acid-base transport in the CCD, including pendrin activity [18, 19, 33, 34, 48, 49, 50].
Effect of pH
Immunolocalization studies [48, 49, 51] in acid-loaded rats and mice revealed decreased levels of pendrin protein expression in the apical membrane of IC accompanied by a shift of immunoreactive protein from membrane to cytosolic regions as well as decreased expression of PDS mRNA. In HCO3− -loaded animals, pendrin protein was exclusively expressed in the IC apical membrane [51]. In addition, acid-loading decreased, whereas alkali-loading increased, the proportional number of pendrin-positive cells in the CCD [51]. Pendrin was absent from the kidneys of slc26a4 -/- mice, and tubules from alkali-loaded null mice failed to secrete HCO3− [18]. These studies have suggested that pendrin plays an important role in acid base regulation and that this anion exchanger is essential for HCO3− secretion following systemic HCO3− loading.
Effect of aldosterone
Aldosterone stimulates proton secretion in the CCD by activating several transport processes, including epithelial Na+ channels in the apical membrane of principal cells as well as H+-ATPase and H+/K+-ATPase activity in the apical membrane and Cl−/HCO3− exchanger activity in the basolateral membrane of α-intercalated cells [46, 47]. These actions of aldosterone, which result in net excretion of acid in the distal tubule, are mediated by a variety of genomic mechanisms involving the action of aldosterone-induced regulatory proteins, as well as by nongenomic pathways [52, 53, 54]. Verlander and colleagues [19] demonstrated upregulation of pendrin mRNA and protein in mouse kidney in response to administration of the aldosterone analog, deoxycorticosterone.
It has been proposed that in addition to the role of pendrin in acid-base balance, mediated by HCO3− secretion into the tubular lumen [18, 51], this anion exchanger may also play an important role in blood pressure regulation by reabsorbing Cl− in exchange for secreted HCO3− [19, 33, 34]. Verlander et al. [19] found that Pds(-/-) mice failed to show weight gain and hypertension in response to chronic mineralocorticoid (DOC) administration, responses characteristic of wildtype mice. The same investigators showed that angiotensin II increased transcellular Cl− reabsorption in the isolated perfused mouse CCD through a pendrin-dependent process [50, 55]. These studies indicate that pendrin-mediated Cl− transport may be important in the pathogenesis of hypertension induced by mineralocorticoid and/or angiotensin II. Recently, Kim et al [56] showed that epithelial Na+ channel (ENaC) function and abundance were lower in pendrin null mice than in wild type mice leading to reduced blood pressure in the former mice. Pendrin was shown to modulate ENaC function by changing luminal HCO3− concentration [57]. These studies further support the important role of pendrin in blood pressure control.
Effect of Cl−
Systemic and tubule lumen Cl−concentrations affect pendrin protein levels and activity [33, 34, 50, 58]. Quentin et al. [33] showed decreased pendrin protein abundance in the rat renal cortex in response to high Cl− intake, and increased pendrin protein abundance in response to Cl− depletion. Verlander et al. [58] described pendrin protein upregulation in response to NaCl restriction and downregulation in response to NaCl load in β intercalated cells of the mouse kidney. These data reveal pendrin's importance to transcellular chloride reabsorption in the connecting tubule and CCD, and suggest a major role for pendrin in electrolyte homeostasis and blood pressure regulation.
Modulation of pendrin activity in the thyroid and inner ear
Thyroid function and Na+/I− symporter activity are known to be regulated by several factors including thyroid-stimulating hormone (TSH), I−, and thyroglobulin (Tg) [59]. Northern blot analysis [60] has demonstrated induction of PDS expression by low concentration of Tg, but not by TSH, insulin NaI or interferon γ (IFN-γ) in the rat thyroid cell line FRTL-5. In contrast, in the more differentiated rat thyroid PC Cl3 cell line, TSH increased PDS mRNA expression, and pendrin expression and localization were regulated by insulin and influenced by PKC-ε-dependent intracellular pathway [61]. In addition, the transcription factor TTF-1, a regulator of thyroid differentiation in vivo, was found also to regulate the expression of pendrin in rat FRTL-5 cells [62]. There is evidence that the transcription factor forkhead box I1 (Foxi1), which is necessary for pendrin expression in type B IC of the mouse CCD [63], is also an upstream regulator of the pendrin gene during mouse inner ear development [64]. Moreover, mutations in the Foxi1 binding sites, located between −889bp and −873bp on the hPDS promoter (Fig. 1), abolish Foxi1-mediated transcriptional activation of hPDS, and may result in enlarged vestibular aqueduct syndrome [28, 65]. However, the cellular and molecular mechanisms controlling pendrin activity in the thyroid and inner ear are essentially unknown.
Modulation of pendrin in the airway epithelium
Interleukin-4 (IL-4) and interleukin-13 (IL-13), cytokines produced by Th2 cells, act directly on airway epithelial cells to induce mucus production, increased viscosity of the ASL, and airway hyperreactivity [66]. IL-4 and IL-13 alter the expression of several genes in bronchial epithelial cells including the pendrin gene [8, 43, 44, 67]. Both cytokines have been shown to stimulate PDS mRNA levels in human and mouse airway epithelial cells [44, 68]. Pendrin knockout mice had reduced allergen-induced airway hyperactivity and inflammation compared with control mice, and IL-13 stimulation of tracheal epithelial cells of the pendrin-deficient mice caused an increase in ASL thickness compared with wildtype mice [43]. Also, IL-4 induced strong upregulation of pendrin-mediated SCN−/Cl− exchange in the human bronchial epithelium [8]. Finally, γ INF, a Th1 cytokine induced during viral infection, has been implicated in the increased PDS mRNA expression in human bronchial epithelial cells in the setting of virus-induced exacerbations of asthma [43].
Regulation of PDS promoter Activity
Effect of Ambient pH on hPDS Promoter Activity
Ambient pH modulates pendrin mRNA and protein expression in the CCD [48, 49, 51]. Also, pendrin plays an important role in controlling the acid-base status of the endolymphatic sac [6, 39, 69]. To explore further the molecular mechanisms of these pendrin regulatory influences, we examined the direct effect of ambient pH on activity of the hPDS promoter [42]. For this purpose, renal HEK293, thyroid LA2 and inner ear VOT36 cells, were transiently transfected with reporter vectors expressing luciferase under the upstream control of hPDS promoter fragments of decreasing length. Transfected cells were exposed to acidic pH (7.0-7.1), normal pH (7.35-7.45) or alkaline pH (7.6-7.7). Ambient acid pH decreased and alkaline pH increased hPDS promoter activity in renal and inner ear cells but not in thyroid cells.
These results suggest that ambient pH likely plays a role in modulating pendrin activity at the transcriptional level in kidney and inner ear, but is without effect in the thyroid. In addition, the findings provided evidence for the presence of a pH-regulated cis-acting element(s) on the hPDS promoter within the 389bp between −1433bp and −1044bp upstream to the hPDS translation start site.
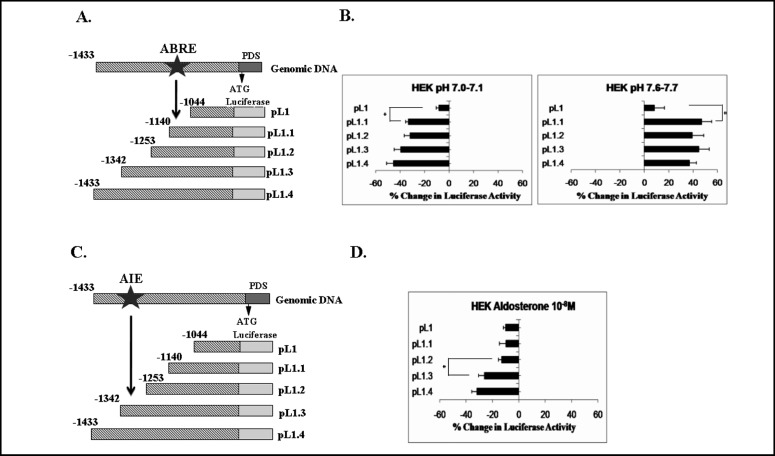
To further define this putative pH-response element located within the 389bp protein region, fine deletion analysis of this region was performed. For this purpose, the plasmids pL1, pL1.1, pL1.2, pL1.3 and pL1,4 (Fig. 2A) were transfected into HEK293 and VOT36 cells which were exposed to acidic, normal and alkaline pH, as above. A similar pattern of reporter gene activity was evident in HEK293 cells (Fig. 2B) and in VOT36 cells (data not shown). While transfection with pL1.4 (1.4kb), pL1.3 (1.3kb), pL1.2 (1.2kb), and pL1.1 (1.1kb) resulted in similar magnitude of acidic pH-induced inhibition and alkaline pH-induced stimulation of luciferase activity in HEK293 and VOT36 cell lines, further truncation of the fragment to 1.0kb (pL1) caused a marked reduction in acidic pH- and alkaline pH-induced effects in both cell lines.
Fig. 2.
Effect of ambient pH (A and B) and of aldosterone (C and D) on the hPDS promoter in HEK293 cells. A and C: schematic representation of the DNA constructs of hPDS promoter in the luciferase reporter vector, pGL3-basic, used in these experiments. ABRE, putative acid-base response element; AIE, putative aldosterone-induced element. B and D: cells were transfected with pGL3-basic or pGL3-basic containing segments of decreasing size corresponding to the 5'-flanking region of hPDS (pL1.4, pL1.3, pL1.2, pL1.1, and pL1). Cells were exposed to acidic pH (7.0-7.1), normal pH (7.35-7.45), or alkaline pH (7.6-7.7) (B) or to 10-8 M aldosterone (D) for 24 h. Subsequently, luciferase activity was measured. To control for transfection efficiency, cells were cotransfected with the LacZ- containing vector, pCH110, and luciferase activity was normalized to β-galactosidase activity. Data represent the %change in luciferase activity in cells exposed to experimental media (with acidic pH, alkaline pH, or aldosterone) relative to cells exposed to control medium (pH 7.35-7.45 without aldosterone). Values are means ±SE of 3-5 independent experiments, each performed in quadruplicate. Acidic pH-induced inhibition and alkaline pH-induced stimulation of luciferase activity were evident in HEK293 cells, which markedly decreased when the fragment size was shortened from 1.1 kb to 1 kb (B). Aldosterone-induced inhibition of luciferase activity was demonstrated in HEK293 cells, which markedly diminished when the fragment size was truncated from 1.3 kb to 1.2 kb (D). *P < 0.01 (modified from reference [42]).
These findings provide evidence for the presence of a putative acid-base response element (ABRE) within the 96bp between −1140bp and −1044bp on the hPDS promoter in the kidney and the inner ear (Figs. 1, 2B). In silico analysis of this region [70, 71] detected no transcription factor binding sites or regulatory elements known to be influenced by pH changes.
The findings are in line with the modulatory effects of acidosis and alkalosis on pendrin protein activity observed in the rodent kidney at the tubular and cellular levels [19, 48, 49, 50, 51] and demonstrate, for the first time, that systemic pH may directly regulate pendrin-mediated, renal tubular HCO3− secretion and Cl− reabsorption at the transcriptional level. These data further suggest that systemic pH may modulate endolymph pH by directly regulating pendrin gene transcription. The molecular mechanisms by which this pH-induced effect on the PDS promoter is achieved remain to be explored.
Li and colleagues [72] have shown that the proline-rich tyrosine kinase 2 (Pyk2), a member of the focal adhesion kinase family, is directly activated by acidic pH and that Pyk2 activation is required for acid activation of c-Src kinase and the proximal tubular Na+/H+ exchanger 3 (NHE3). The authors suggest that Pyk2 may serve as a pH sensor that, via phosphorylation of c-Src, provides subsequent activation of the MAPK and JNK signaling pathways, in turn increasing transcription of the NHE3 gene [72, 73]. The proximal tubular basolateral Na+-HCO3−cotransporter, NBCe1, may be regulated by a similar pathway [70]. Similar pH-sensing mechanisms in the CCD and the endolymphatic sac of the inner ear [39] might modulate pendrin gene promoter activity.
The 96-bp 5‘- flanking region of PDS between _1044 and _1140 harbors a putative pH response element that is active in transfected renal and inner ear cells (Figs. 1, 2B). In silico analysis [70, 71] of this DNA region revealed putative binding sites for transcription factors known to be regulated by Mitogen-activated protein kinase (MAPK) signaling pathways, including Elk-1, c-Jun, activating transcription factor-2 (ATF-2), cAMP response element-binding (CREB), nuclear factor of activated T-cells −4 (NFAT-4), c-Myc and N-Myc, among others. Thus, the MAPK pathway may be involved in pH-induced modulation of the pendrin gene promoter, just as it has been implicated in regulation of other H+ and HCO3−transporting pathways [72, 73, 74]. Alternatively, a direct effect of ambient pH-mediated alterations in intracellular pH on this pH-response element residing on the PDS promoter cannot be excluded. Further studies are needed to elucidate the exact location and the molecular nature of this novel pH-response element.
Our findings on the effects of ambient pH on the hPDS promoter in transfected renal cells were further supported by our experiments demonstrating acid-induced inhibition and alkali-induced stimulation of endogenous PDS mRNA levels in renal HEK293 cells [42].
Effect of Aldosterone on hPDS Promoter Activity
Aldosterone modulates pendrin protein and mRNA expression in the CCD [19]. To explore the molecular mechanisms of this aldosterone-induced effect on pendrin activity, we examined the direct effect of aldosterone on the hPDS promoter [42]. To this end, HEK293 as well as LA2 and VOT36 cells were transiently transfected with different length fragments of this promoter, as above, and exposed to 10-8 M aldosterone. Aldosterone caused a marked decrease in hPDS promoter activity in HEK293 cells without alterating pH of the experimental medium (7.35-7.45). Aldosterone had no effect on PDS promoter activity in transfected LA2 and VOT36 cells. Taken together, these results suggest that aldosterone may directly affect the hPDS promoter to transcriptionally modulate pendrin activity in the kidney, but not in thyroid and inner ear. The findings provide evidence that the hPDS promoter likely contains an aldosterone induced element (AIE) located in the 389 bp region between −1433 and −1044 of the hPDS gene upstream region [42].
Further analysis of the aldosterone-induced element residing within this 389bp promoter region, required fine deletion analysis. To this end, HEK293 cells transfected with plasmids pL1, pL1.1, pL1.2, pL1.2, pL1.3 and pL1.4 (Fig. 2C) were exposed to 10-8M aldosterone. As seen in Fig. 2D, the aldosterone-induced 35% inhibition in luciferase activity driven by the 1.4kb fragment (pL1.4) remained essentially unchanged when the fragment was shortened to 1.3kb (pL1.3). However, truncation to 1.2kb (pL1.2) markedly reduced aldosterone-dependent inhibition of reporter gene activity. No additional reduction in reporter activity was noted upon further truncation to 1.1kb (pL1.1) or 1kb (pL1) [42].
These data indicate that a putative aldosterone-response element likely resides within the 89bp between −1342 and −1253 on the hPDS promoter in the kidney (Figs. 1, 2C). In silico analysis [70, 71] revealed a single mineralocorticoid receptor (MR) binding site within this region between −1293 and −1287. Additional aldosterone-sensitive sites within this 89 bp region remain undefined.
RT-PCR analysis detected MR mRNA in aldosterone-responsive HEK293 cells, but not in the LA2 and VOT36 cells that showed no response to this hormone [42]. The association of aldosterone-regulated promoter activity with the presence of MR in renal HEK293 cells suggests that aldosterone influences pendrin gene activity directly via the genomic pathway, and this may reflect the biology of the intact kidney.
Verlander and colleagues [19] demonstrated upregulation of pendrin mRNA and protein in mouse kidney in response to systemic administration of the aldosterone analog, deoxycorticosterone. To explain this effect of aldosterone, consistent with NaCl retention but not with the acid-secreting properties of this hormone, the authors proposed that in their in vivo model, pendrin attenuated aldosterone-induced metabolic alkalosis by augmenting HCO3− secretion. However, the direct effect of aldosterone on the PDS gene was not investigated in these studies. Using the model of transfected cell lines, we have isolated the effect of aldosterone on the pendrin gene promoter, and we demonstrate that this hormone directly inhibits PDS promoter activity in renal cells (Fig. 2D). This finding demonstrates, for the first time, that aldosterone may regulate pendrin-mediated HCO3−secretion at the transcriptional level. Moreover, our finding of isolated, aldosterone-induced direct inhibition of the pendrin gene promoter [as opposed to the complex aldosterone/alkalosis-induced stimulation of pendrin activity demonstrated in vivo [19]] is in concert with the net H+-secretion and HCO3− reabsorption associated with aldosterone action [46, 47]. The PDS promoter-inhibiting action of aldosterone involved the 89-bp region between −1342 and −1253 of the PDS 5‘ - flanking region (Fig. 2D), and was not secondary to pH changes in the medium (see above).
In line with this kidney-specific inhibitory effect of aldosterone on hPDS promoter activity was our finding demonstrating an aldosterone-induced decrease in endogenous pendrin mRNA levels in renal HEK293 cells [42]. Our findings on the direct effect of aldosterone on pendrin gene transcription and a possible role of Cl− on transcription of this gene (currently under investigation) may be of major biological importance and may improve our understanding of blood pressure control at the level of the gene.
The modulators of acid-base balance used in our studies, namely, ambient pH and aldosterone, had no effect on the hPDS promoter in the thyroid cell line LA2. This finding is not surprising considering that acid-base conditions are not known to regulate thyroid hormone metabolism and that the thyroid gland does not contain aldosterone-responsive epithelial cells. Pendrin functions in both thyroid gland and kidney as an exchanger of Cl−and I−. Hence, future studies examining the modulatory effect of these two anions on pendrin activity and transcription in thyrocytes and collecting duct intercalated cells will be of great importance.
Effect of uroguanylin on the hPDS promoter
In preliminary experiments, we have investigated the effect of uroguanylin (UGN) on the hPDS promoter. UGN is a low molecular weight peptide hormone similar in structure and activity to the secretary diarrhea-causing E. coli heat-stable enterotoxin (STa) and produced primarily in intestine [75, 76, 77]. Salt ingestion induces secretion of UGN into the intestinal lumen which results in intestinal secretion of electrolytes and water [76, 77]. In addition, UGN plays a major role in the regulatory link between the intestine and the kidney by increasing urinary NaCl and water excretion in response to dietary NaCl intake (but not intravenous administration), thereby serving as an “intestinal natriuretic factor” [78, 79, 80, 81]. UGN activates guanylate cyclase C in proximal tubular epithelial cells [77, 78], but the cellular and molecular pathways responsible for UGN action in the CCD remain poorly understood.
Real time-PCR analysis of human embryonic kidney (HEK293) cells exposed to UGN (1 µM) for 24 hrs showed a decrease in endogenous pendrin mRNA levels compared to control cells. Adult mice received UGN (20U) or vehicle intravenously. Twenty four hours later, kidneys were harvested for pendrin mRNA quantification (real time PCR) and pendrin protein visualization (immunofluorescence). UGN- injected animals displayed a decrease in pendrin mRNA and protein expression in renal tubules. The effect of UGN on the human PDS (hPDS) promoter was examined. Luciferase reporter plasmids containing different length fragments of this promoter were transfected into HEK293 cells. Exposure of cells to UGN (1 µM) decreased hPDS promoter activity compared to activity in control cells. Furthermore, the findings provided evidence for the presence of a hypothetical UGN response element within the 96bp region between −1140bp and −1044bp on the hPDS promoter (Fig. 1). This region overlaps with the previously demonstrated hypothetical pH response element, but is distinct from the proposed aldosterone response element. Characterization of the role of UGN transcriptional reglation in extracellular fluid homeostasis and blood-pressure regulation awaits future studies.
Effect of IL-4 and IL-13 on the hPDS promoter
Upregulation of pendrin expression by IL- 4 and IL- 13 results in increased production of mucus and increased viscosity of the ASL [43, 44]. To investigate the molecular mechanisms responsible for this effect of IL-4 and IL-13 on pendrin activity, Nofziger et al [67] examined the effect of these cytokines on the hPDS promoter. To this end, hPDS promoter fragments of decreasing size, cloned into luciferase reporter vectors, were transfected into HEK-Blue cells. Stimulation of these cells with IL-4 and IL-13 induced markedly increased hPDS promoter activity that was maximal with a construct containing 1894 bp of the hPDS 5’ flanking region.
IL-4 and IL-13 regulate gene transcription through activation of the transcription factor STAT6 (signal transducer and activator of transcription protein 6; also known as IL- 4 nuclear activated factor, IL- 4NAF). STAT6 exerts its activity by binding to the N4GAS motif (consisting of 5’ TTC(N4)GAA 3’ where N is any nucleotide) on the promoter of the target gene [82, 83]. Having identified this STAT6 DNA binding sequence at positions −1812 to −1803 on the hPDS promoter, Nofziger et al [67] examined the involvement of this cis- acting element in the IL-4/IL-13 - induced effect on the promoter. The authors first demonstrated loss of IL-4/IL-13 activity on the hPDS promoter in STAT6-deficient HEK293 Phoenix cells. Mutation of the STAT6 binding site of the hPDS promoter rendered the promoter insensitive to IL-4/IL-13. In addition, fusion of the STAT6 binding site with an IL-4/IL-13-unresponsive promoter sequence conferred cytokine-sensitivity onto the unresponsive promoter.
Taken together, these studies provide strong evidence for the importance of the STAT6 pathway in the transcriptional regulation of the pendrin gene by IL-4 and IL-13.
In summary, acidic pH, aldosterone, and UGN decrease, and alkaline pH increases, hPDS promoter activity by acting on distinct response elements within this promoter (Fig. 1). The findings summarized in this review provide the first direct evidence that pendrinmediated Cl−/HCO3− exchange in the renal tubule, iodide accumulation in the thyroid, and endolymph ion balance in the inner ear may be differentially regulated at the transcriptional level by systemic pH and aldosterone. In addition, evidence suggests that IL-4/IL-13-induced effects on airway surface liquid composition and/or viscosity may be achieved by STAT6-mediated transcriptional regulation of the pendrin gene (Fig. 1). Future studies will investigate the molecular nature of the distinct response elements within the hPDS promoter mediating the effects of these modulators on pendrin gene transcription.
Regulated expression of other SLC26 genes
Transcriptional regulation of other SLC26 genes has been reported. The basal activity of the SLC26A3 promoter in Caco-2 and LS174T colon carcinoma cells is sustained by nuclear factor HNF-4 [84]. Na butyrate stimulates this activity by increasing binding of Yin Yang 1 (YY1) and GATA factors to defined cis-elements, whereas interferon gamma (IFN-γ) reduces transcriptional activity [84]. The effect of IFN-γ is mediated through an IFN-γ response element containing a critical STAT-1 binding site at −933 to-925, mutation of which abrogated the inhibitory activity of IFN-γ [85]. An IFN-γ inhibitory response element active in Caco-2 cells was also found in the SLC26A6 promoter between −318 and −300. This is consistent with inflammatory suppression of both intestinal Cl− reabsorption and HCO3− secretion. Lactobacilli exert an anti-inflammatory action on enterocytes. Raheja et al demonstrated that exposure of Caco-2 cells or intact mice to lactobacilli increased SLC26A3 promoter activity in parallel with increased surface expression of the protein [86]. The transcriptional elements involved remain undescribed.
Transcriptional regulation of the pendrin gene by IFN-γ in respiratory tract has not been reported. However, Like the SLC26A4/pendrin gene, the outer hair cell gene encoding the mechanotransducer prestin/SLC26A5 is induced during development by thyroxine. Thyroxine activates SLC26A5 gene transcription through the TRβ receptor [87]. SLC26A3/DRA mRNA abundance in Caco-2 colon carcinoma cells is also increased by thyroxine by an unknown mechanism [88].
The SLC26A2/DTDST sulfate/anion exchanger is induced during differentiation of C3H10T1/2 chondrocytes by bone morphogenetic protein-2 (BMP-2). The SLC26A2 promoter contains a TATA box preceded by a GC-rich region with two specificity protein-1 (SP-1) binding sites and a core binding factor alpha 1 (CFBA1) binding site. Basal transcriptional activity required the region between −309 and −275 containing a xenobiotic-responsive element (XRE). BMP-2 enhancement of transcriptional activity was associated with increased binding of undefined nuclear proteins to a fragment including this XRE [89].
Colonic expression of the SLC26A2/DTDST gene is also decreased in colon cancer, in parallel with decreased expression of sialyl 6-sulfo-Lewisx antigen. This transformation-associated downregulation, as well as engineered suppression of transcription, is associated with markedly increased growth rate. However, most cultured colon carcinoma cells exhibit increased SLC26A2 expression after treatment with inhibitors of histone deacetylase, consistent with epigenetic control of gene expression [90]. Direct transcriptional regulation of SLC26A2 has not been reported. Possible epigenetic regulation of pendrin transcription remains a topic of future investigation.
Acknowledgements
This review includes data derived during support by the following grants: I. Zelikovic was supported by the USA-Israel Binational Science Foundation, by the Rappaport Institute for Research in the Medical Sciences and by the Dr. Y. Rabinovitz Research Fund, Technion -Israel Institute of Technology; J. Rozenfeld received support from the Dora and Sydney Gabrel Fund, Rambam Medical Center, Haifa, Israel; SL Alper was supported by NIH grants DK43495 and DK34854 (Harvard Digestive Diseases Center) and by the USA-Israel Binational Science Foundation; SL Carrithers was supported, in part, by NIH grants DK070374 and DK089892, and by KSTC-184-512-08-046. We thank Mrs. Ora Bider for her expert secretarial assistance.
References
- 1.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 2.Campbell C, Cucci RA, Prasad S, Green GE, Edeal JB, Galer CE, Karniski LP, Sheffield VC, Smith RJ. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat. 2001;17:403–411. doi: 10.1002/humu.1116. [DOI] [PubMed] [Google Scholar]
- 3.Reardon W, Trembath RC. Pendred syndrome. J Med Genet. 1996;33:1037–1040. doi: 10.1136/jmg.33.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haila S, Höglund P, Scherer SW, Lee JR, Kristo P, Coyle B, Trembath R, Holmberg C, de la Chapelle A, Kere J. Genomic structure of the human congenital chloride diarrhea (CLD) gene. Gene. 1998;214:87–93. doi: 10.1016/s0378-1119(98)00261-3. [DOI] [PubMed] [Google Scholar]
- 5.Soleimani M, Xu J. SLC26 chloride/base exchangers in the kidney in health and disease. Sem. Nephrol. 2006;26:375–385. doi: 10.1016/j.semnephrol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Everett LA, Morsli H, Wu DK, Green ED. Expression pattern of the mouse ortholog of the Pendred's syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci USA. 1999;96:9727–9732. doi: 10.1073/pnas.96.17.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003;F229-F241 doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 8.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4- stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 9.Bidart JM, Lacroix L, Evain-Brion D, Caillou B, Lazar V, Frydman R, Bellet D, Filetti S, Schlumberger M. Expression of Na+/I- symporter and Pendred syndrome genes in trophoblast cells. J Clin Endocrin Metab. 2000;85:4367–4372. doi: 10.1210/jcem.85.11.6969. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Royaux IE, Everett LA, Mori-Aoki A, Suzuki S, Nakamura K, Sakai T, Katoh R, Toda S, Green ED, Kohn LD. Expression of PDS/Pds, the Pendred syndrome gene, in endometrium. J Clin Endocrin Metab. 2002;87:938–941. doi: 10.1210/jcem.87.2.8390. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix L, Michiels S, Mian C, Arturi F, Caillou B, Filetti S, Schlumberger M, Bidart JM. HEX, PAX-8 and TTF-1 gene expression in human thyroid tissues: a comparative analysis with other genes involved in iodide metabolism. Clin Endocrinol. 2006;64:398–404. doi: 10.1111/j.1365-2265.2006.02477.x. [DOI] [PubMed] [Google Scholar]
- 12.Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3-transporters. Am J Physiol Cell Physiol. 2006;290:C1560–1571. doi: 10.1152/ajpcell.00468.2005. [DOI] [PubMed] [Google Scholar]
- 13.Rillema JA, Hill MA. Prolactin regulation of the pendrin-iodide transporter in the mammary gland. Am J Physiol Endocrinol Metab. 2003;284:E25–28. doi: 10.1152/ajpendo.00383.2002. [DOI] [PubMed] [Google Scholar]
- 14.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 15.Shelden MC, Howitt SM, Price GD. Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol. 2010;27:12–23. doi: 10.3109/09687680903400120. [DOI] [PubMed] [Google Scholar]
- 16.Dossena S, Rodighiero S, Vezzoli V, Nofziger C, Salvioni E, Boccazzi M, Grabmayer E, Botta G, Meyer G, Fugazzola L, Peccoz PB, Paulmichl M. Functional characterization of wild-type and mutated pendrin (SLC26A4), the anion transporter involved in pendred syndrome. J Mol Endocrinol. 2009;43:93–103. doi: 10.1677/JME-08-0175. [DOI] [PubMed] [Google Scholar]
- 17.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3-secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorti-costerone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, Wall SM. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol. 2009;297:F1069–1079. doi: 10.1152/ajprenal.90581.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol. 2007;292:F1314–1321. doi: 10.1152/ajprenal.00432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida A, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K. Mechanism of iodide/chloride exchange by pendrin. Endocrinology. 2004;145:4301–4308. doi: 10.1210/en.2004-0048. [DOI] [PubMed] [Google Scholar]
- 23.Kopp P. Pendred's syndrome and genetic defects in thyroid hormone synthesis. Rev Endocr Metabol Disord. 2000;1:109–121. doi: 10.1023/a:1010024722595. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Riazuddin S, Griffith AJ. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffield VC, Kraiem Z, Beck JC, Nishimura D, Stone EM, Salameh M, Sadeh O, Glaser B. Pendred syndrome maps to chromosome 7q21-34 and is caused by an intrinsic defect in thyroid iodine organification. Nat Genet. 1996;12:424–426. doi: 10.1038/ng0496-424. [DOI] [PubMed] [Google Scholar]
- 26.Johnsen T, Larsen C, Friis J, Hougaard-Jensen F. Pendred's syndrome. Acoustic, vestibular and radiological findings in 17 unrelated patients. J Laryngol Otol. 1987;101:1187–1192. doi: 10.1017/s0022215100103470. [DOI] [PubMed] [Google Scholar]
- 27.Anwar S, Riazuddin S, Ahmed ZM, Tasneem S, Ateeq Ul J, Khan SY, Griffith AJ, Friedman TB, Riazuddin S. SLC26A4 mutation spectrum associated with DFNB4 deafness and Pendred's syndrome in Pakistanis. J Hum Genet. 2009;54:266–270. doi: 10.1038/jhg.2009.21. [DOI] [PubMed] [Google Scholar]
- 28.Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, Kittles R, Eisenman D, Kim HJ, Niparko J, Thomsen J, Arnos KS, Nance WE, King KA, Zalewski CK, Brewer CC, Shawker T, Reynolds JC, Butman JA, Karniski LP, Alper SL, Griffith AJ. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat. 2009;30:599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi BY, Stewart AK, Nishimura KK, Cha WJ, Seong MW, Park SS, Kim SW, Chun YS, Chung JW, Park SN, Chang SO, Kim CS, Alper SL, Griffith AJ, Oh SH. Efficient molecular genetic diagnosis of enlarged vestibular aqueducts in East Asians. Genet Test Mol Biomarkers. 2009;13:679–687. doi: 10.1089/gtmb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai P, Stewart AK, Chebib F, Hsu A, Rozenfeld J, Huang D, Kang D, Lip V, Fang H, Shao H, Liu X, Shen Y, Yang W, Zelikovic I, Platt OS, Han D, Alper SL, Wu BL. Distinct and novel SLC26A4/ Pendrin mutations in Chinese and U.S. patients with non-syndromic hearing loss. Physiol Genomics. 2009;38:281–290. doi: 10.1152/physiolgenomics.00047.2009. [DOI] [PubMed] [Google Scholar]
- 31.Alpern RJ, Stone DK, Rector FC., Jr . Renal acidification mechanisms. In: Brenner BM, Rector FC Jr, editors. The Kidney. Philadelphia: Saunders; 2000. pp. 455–519. [Google Scholar]
- 32.Schwartz GJ. Plasticity of intercalated cell polarity: effect of metabolic acidosis. Nephron. 2001;87:304–313. doi: 10.1159/000045935. [DOI] [PubMed] [Google Scholar]
- 33.Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, Aronson PS, Eladari D. The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol. 2004;287:F1179–1188. doi: 10.1152/ajprenal.00211.2004. [DOI] [PubMed] [Google Scholar]
- 34.Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol. 2006;17:2153–2163. doi: 10.1681/ASN.2005101054. [DOI] [PubMed] [Google Scholar]
- 35.Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008;69:450–453. doi: 10.5414/cnp69450. [DOI] [PubMed] [Google Scholar]
- 36.Kandasamy N, Fugazzola L, Evans M, Chatterjee K, Karet F. Life-thereatening alkalosis in Pendred syndrome. Eur J Endocrinol. 2011;165:167–170. doi: 10.1530/EJE-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet. 2001;10:153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- 38.Choi B Y, Kim HM, Ito T, Lee KY, Li X, Monahan K, Wen Y, Wilson E, Kurima K, Saunders T, Petralia RS, Wangemann P, Friedman TB, Griffith A: Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J Clin Invest 2011;in press. [DOI] [PMC free article] [PubMed]
- 39.Dou H, Xu J, Wang Z, Smith AN, Soleimani M, Karet FE, Greinwald JH, Jr, Choo D. Co-expression of pendrin, vacuolar H+-ATPase alpha4-subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. J Histochem Cytochem. 2004;52:1377–1384. doi: 10.1177/002215540405201014. [DOI] [PubMed] [Google Scholar]
- 40.Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon JS, Park HJ, Yoo SY, Namkung W, Jo MJ, Koo SK, Park HY, Lee WS, Kim KH, Lee MG. Heterogeneity in the processing defect of SLC26A4 mutants. J Med Genet. 2008;45:411–419. doi: 10.1136/jmg.2007.054635. [DOI] [PubMed] [Google Scholar]
- 42.Adler L, Efrati E, Zelikovic I. Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol. 2008;294:C1261–1276. doi: 10.1152/ajpcell.00486.2007. [DOI] [PubMed] [Google Scholar]
- 43.Nakagami Y, Favoreto S, Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC, Erle DJ. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181:2203–2210. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED, Izuhara K. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008;180:6262–6269. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- 45.Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA: Novel role for pendrin in orchestrating bicarbonate secretion in CFTR-expressing airway serous cells. J Biol Chem 2011;in press. [DOI] [PMC free article] [PubMed]
- 46.Gennari FJ, Maddox DA. Renal regulation of acid-base homeostasis integrated response. In: Seldin DW, Giebisch GH, editors. The Kidney: Physiology and Pathophysiology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 2015–2053. [Google Scholar]
- 47.Hamm LL, Nakhoul NL. Renal acidification. In: Brenner BM, editor. Brenner & Rector's The Kidney. Philadelphia: Saunders; 2008. pp. 248–279. [Google Scholar]
- 48.Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol. 2003;284:F584–593. doi: 10.1152/ajprenal.00254.2002. [DOI] [PubMed] [Google Scholar]
- 49.Petrovic S, Wang Z, Ma L, Soleimani M. Regulation of the apical Cl-/HCO3-exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol. 2003;284:F103–112. doi: 10.1152/ajprenal.00205.2002. [DOI] [PubMed] [Google Scholar]
- 50.Pech V, Kim H Y, Weinstein MA, Everett AL, Pham DT, Wall MS. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]
- 51.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP. Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 2002;62:2109–2117. doi: 10.1046/j.1523-1755.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 52.Koppel H, Christ M, Yard BA, Bar PC, van der Woude FJ, Wehling M. Nongenomic effects of aldosterone on human renal cells. J Clin Endocrinol Metab. 2003;88:1297–1302. doi: 10.1210/jc.2002-020248. [DOI] [PubMed] [Google Scholar]
- 53.Verrey F, Pearce D, Pfeiffer R, Spindler B, Mastroberardino L, Summa V, Zecevic M. Pleiotropic action of aldosterone in epithelia mediated by transcription and post-transcription mechanisms. Kidney Int. 2000;57:1277–1282. doi: 10.1046/j.1523-1755.2000.00962.x. [DOI] [PubMed] [Google Scholar]
- 54.Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Natl Acad Sci USA. 2004;101:2636–2641. doi: 10.1073/pnas.0307321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol. 2007;293:F1314–1324. doi: 10.1152/ajprenal.00155.2007. [DOI] [PubMed] [Google Scholar]
- 57.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3- J Am Soc Nephrol. 2010;21:1928–1941. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM. Dietary Cl- restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol. 2006;291:F833–839. doi: 10.1152/ajprenal.00474.2005. [DOI] [PubMed] [Google Scholar]
- 59.Dunn JT, Dunn AD. Update on intrathyroidal iodine metabolism. Thyroid. 2001;11:407–414. doi: 10.1089/105072501300176363. [DOI] [PubMed] [Google Scholar]
- 60.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 61.Muscella A, Verri SM, Urso L, Dimitri C, Botta G, Paulmichl M, Beck-Peccoz P, Fugazzola L, Storelli C. PKC-ε-Dependent cytosol-to-membrane translocation of pendrin in rat thyroid PC Cl3 cells. J Cell Physiol. 2008;217:103–112. doi: 10.1002/jcp.21478. [DOI] [PubMed] [Google Scholar]
- 62.Dentice M, Luongo C, Elefante A, Ambrosio R, Salzano S, Zannini M, Nitsch R, Di Lauro R, Rossi G, Fenzi G, Salvatore D. Pendrin is a novel in vivo downstream target gene of the TTF-1/Nkx-2.1 homeodomain transcription factor in differentiated thyroid cells. Mol Cell Biol. 2005;25:10171–10182. doi: 10.1128/MCB.25.22.10171-10182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blomquist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AEG, Bergstrom G, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113:1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerback S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development. 2003;130:2013–2025. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- 65.Yang T, Vidarsson H, Rodrigo-Blomqvist S, Rosengren SS, Enerback S, Smith RJ. Transcriptional control of SLC26A4 is involved in Pendred syndrome and nonsyndromic enlargement of vestibular aqueduct (DFNB4) Am J Hum Genet. 2007;80:1055–1063. doi: 10.1086/518314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 67.Nofziger C, Vezzoli V, Dossena S, Schonherr T, Studnicka J, Nofziger J, Vanoni S, Stephan S, Silva ME, Meyer G, Paulmichl M. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2011;90:399–405. doi: 10.1038/clpt.2011.128. [DOI] [PubMed] [Google Scholar]
- 68.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Griffith AJ, Wangemann P: Hearing loss associated with enlargement of the vestibular aqueduct: Mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 2011;in press. [DOI] [PMC free article] [PubMed]
- 70.Schug J, Overton GC. Modeling transcription factor binding sites with Gibbs Sampling and Minimum Description Length encoding. Proc Int Conf Intell Syst Mol Biol. 1997;5:268–271. [PubMed] [Google Scholar]
- 71.Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gluck SL. Acid sensing in renal epithelial cells. J Clin Invest. 2004;114:1696–1699. doi: 10.1172/JCI23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Espiritu DJ, Bernardo AA, Robey RB, Arruda JA. A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol. 2002;283:F663–F670. doi: 10.1152/ajprenal.00338.2001. [DOI] [PubMed] [Google Scholar]
- 75.Fonteles MC, Greenberg RN, Monteiro HS, Currie MG, Forte LR. Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol. 1998;275:F191–197. doi: 10.1152/ajprenal.1998.275.2.F191. [DOI] [PubMed] [Google Scholar]
- 76.Kita T, Kitamura K, Sakata J, Eto T. Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am J Physiol. 1999;277:G960–966. doi: 10.1152/ajpgi.1999.277.5.G960. [DOI] [PubMed] [Google Scholar]
- 77.Sindic A, Schlatter E. Cellular effects of guanylin and uroguanylin. J Am Soc Nephrol. 2006;17:607–616. doi: 10.1681/ASN.2005080818. [DOI] [PubMed] [Google Scholar]
- 78.Sindic A, Basoglu C, Cerci A, Hirsch JR, Potthast R, Kuhn M, Ghanekar Y, Visweswariah SS, Schlatter E. Guanylin, uroguanylin, and heat-stable euterotoxin activate guanylate cyclase C and/or a pertussis toxin-sensitive G protein in human proximal tubule cells. J Biol Chem. 2002;277:17758–17764. doi: 10.1074/jbc.M110627200. [DOI] [PubMed] [Google Scholar]
- 79.Potthast R, Ehler E, Scheving LA, Sindic A, Schlatter E, Kuhn M. High salt intake increases uroguanylin expression in mouse kidney. Endocrinology. 2001;142:3087–3097. doi: 10.1210/endo.142.7.8274. [DOI] [PubMed] [Google Scholar]
- 80.Forte LR. A novel role for uroguanylin in the regulation of sodium balance. J Clin Invest. 2003;112:1138–1341. doi: 10.1172/JCI20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, Shah RG, Sun C, Mann EA, Fonteles MC, Forte LR, Jackson BA, Giannella RA, Greenberg RN. Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int. 2004;65:40–53. doi: 10.1111/j.1523-1755.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 82.Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996;16:5811–5820. doi: 10.1128/mcb.16.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P. DNA Binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 84.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA) Am J Physiol Gastrointest Liver Physiol. 2007;293:G923–934. doi: 10.1152/ajpgi.00029.2007. [DOI] [PubMed] [Google Scholar]
- 85.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-{gamma} Am J Physiol Gastrointest Liver Physiol. 2010;298:G159–166. doi: 10.1152/ajpgi.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;298:G395–401. doi: 10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winter H, Braig C, Zimmermann U, Geisler HS, Fränzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, McGee J, Köpschall I, Rohbock K, Knipper M. Thyroid hormone receptors TRalpha1 and TRbeta differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci. 2006;119:2975–2984. doi: 10.1242/jcs.03013. [DOI] [PubMed] [Google Scholar]
- 88.Alrefai WA, Tyagi S, Mansour F, Saksena S, Syed I, Ramaswamy K, Dudeja PK. Sulfate and chloride transport in Caco-2 cells: differential regulation by thyroxine and the possible role of DRA gene. Am J Physiol Gastrointest Liver Physiol. 2001;280:G603–613. doi: 10.1152/ajpgi.2001.280.4.G603. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi T, Sugimoto T, Saijoh K, Fujii M, Chihara K. Cloning and characterization of the 5'-flanking region of the mouse diastrophic dysplasia sulfate transporter gene. Biochem Biophys Res Commun. 1997;238:738–743. doi: 10.1006/bbrc.1997.7380. [DOI] [PubMed] [Google Scholar]
- 90.Yusa A, Miyazaki K, Kimura N, Izawa M, Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70:4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]