Abstract
Genomic technology has completely changed the way in which we are able to diagnose human genetic mutations. Genomic techniques such as the polymerase chain reaction, linkage analysis, Sanger sequencing, and most recently, massively parallel sequencing, have allowed researchers and clinicians to identify mutations for patients with Pendred syndrome and DFNB4 non-syndromic hearing loss. While thus far most of the mutations have been in the SLC26A4 gene coding for the pendrin protein, other genetic mutations may contribute to these phenotypes as well. Furthermore, mouse models for deafness have been invaluable to help determine the mechanisms for SLC26A4-associated deafness. Further work in these areas of research will help define genotype-phenotype correlations and develop methods for therapy in the future.
Key Words: SLC26A4, Enlarged vestibular aqueduct, Massively parallel sequencing, Hearing loss, Otoconia
Introduction
Human mutations in SLC26A4 lead to the most common hereditary cause of syndromic deafness known as Pendred's syndrome (PS) [1]. Several SLC26A4 mutations have also been linked to a non-syndromic form of deafness DFNB4, where the ear appears to be affected exclusively. A prominent clinical characteristic of the inner ear in SLC26A4-related deafness is the enlarged vestibular aqueduct (EVA). In addition, PS patients show enlargement of the thyroid glands (goiter) that in some cases are associated with hypothyroidism [2]. SLC26A4, together with SLC26A3 and SLC26A6, belong to a subgroup of SLC26 proteins that function as coupled Cl–/ HCO3– exchangers [3]. However, SLC26A4 participates in transcellular I− transport as well [4]. SLC26A4, also known as pendrin, is expressed in different tissues, including the inner ear [5], the luminal membrane of follicular cells in the thyroid [6], the renal cortical collecting duct [7, 8] the salivary gland ducts [4] and the respiratory tract ciliary epithelium [9]. The apparent enlargement of the thyroid gland in some of the PS individuals is a result of impaired I− organification in the thyroid [10]. This function is probably mediated by HCO3−/I− and Cl−/I−exchange and subsequently by active secretion of I− into the thyroid follicular space. In the inner ear, the work on Slc26a4 null mice reveals the role of pendrin in regulating the pH of endolymphatic fluids through secretion of HCO3−ions [11].
Functional assays have shown that different mutations within the SLC26A4 sequence have different effects on pendrin transport activity [12]. Furthermore, whereas the number of pendrin transmembrane domains is still ambiguous in the literature [13], it is clear that some of the mutations affect pendrin sub-cellular localization so that pendrin fails to reach the plasma membrane [14]. Thus, the variable effect of each mutation on pendrin function, expression and localization can explain some of the clinical heterogeneity observed among affected individuals. Over the years, a long list of SLC26A4 mutations related to human deafness has been published in the literature [15]. Despite the wide efforts to predict the clinical outcome of all mutations spanning the SLC26A4 sequence, a genotype-phenotype correlation is not complete. The complex structure of pendrin as a transmembrane protein limits the ability to faithfully predict the effect of a single mutation. A resolved crystal structure of pendrin in the future may provide the possible link between specific genetic insult to the severity of the phenotype.
The aim of this review is to summarize the contribution of the cross-talk between human and mouse genetics in the study of SLC26A4-related deafness. By utilizing state-of-the-art sequencing technologies, the known list of pendrin mutations is expected to be enriched with novel mutations that were out of the range of conventional sequencing methods. Finally, the contribution of different mouse models enhances the ability to understand pendrin function as part of the complex network of the auditory system.
SLC26A4 (PDS) deafness mutations
Current estimates demonstrate that SLC26A4 mutations are involved in 4% −10% of hereditary hearing loss [15, 16], with close to 200 mutations involved in both Pendred syndrome (PS; MIM#274600) and non-syndromic hearing loss (NSHL) DFNB4 (MIM#600791) (http://www.healthcare.uiowa.edu/labs/pendredandbor/slcMutations.htm). Most mutations are associated with temporal bone abnormalities ranging from isolated enlarged vestibular aqueduct (EVA) to Mondini dysplasia [16]. The mutations are located throughout the coding region and include missense, nonsense, splice site and frameshift mutations [15]. The hearing impairment associated with mutations in SLC26A4 is primarily sensorineural, variable in severity; bilateral or unilateral; congenital, prelingual or perilingual onset; frequently beginning at high frequencies, and sometimes has a fluctuating and/or progressive course [17]. In many cases, there is a conductive component, although the middle ear remains intact. It has been suggested that the large vestibular aqueduct functions as a third mobile window in the inner ear, resulting in an air-bone gap at low frequencies [18].
In many populations, mutations in the SLC26A4 gene are the major genetic cause of temporal bone inner ear malformations, accounting for up to 90% of the typical PS population, 80% of individuals with EVA and the same rate among patients with Mondini dysplasia [19, 20, 21]. It is inconclusive whether the presence or absence of cochlear malformations is related to the severity of HL or whether or not there is a genotype-phenotype correlation. Pryor et al. [22] summarized that two mutant SLC26A4 alleles are involved in all PS patients, whereas the NS EVA patients had either one or no SLC26A4 mutations, suggesting that PS and NS EVA are distinct clinical and genetic phenomena. An additional study among French Caucasian families with NSHL and EVA [16] reported that patients with biallelic mutations had more severe deafness, an earlier age of onset, and a more fluctuating course of hearing levels than patients in whom no mutation was identified. In another study [23], a significant difference was found in the distribution of SLC26A4 mutations in PS versus non-PS (EVA–Mondini) patients, with PS patients more likely to have two mutations comparing to one or none in non-PS. Although heterogeneity was observed in PS patients, it was lower than observed in NS EVA–Mondini. Further analysis of EVA-Mondini patients also showed that two mutations are more likely associated with Mondini dysplasia compared to one or zero associated with EVA [23].
While these studies report genotype-phenotype correlations, most studies do not find any kind of correlation with respect to hearing severity, fluctuation, progression, vertigo, or goiter, as the same combination of mutations have been described that result in variable phenotypic expression. These phenotypes range from isolated NSHL to NS EVA to Mondini dysplasia to PS, suggesting that the same etiology underlies all conditions [21, 24]. Furthermore, phenotypes are variable, even with the same mutations. T416P [25], L445W [26], H723R [21, 27] and the IVS7-2A>G [28] are involved in either PS or NSHL. Moreover, even intrafamilial phenotypic variability was observed, e.g., the L445W mutation was identified in all affected individuals of a large family, either with PS or with NSHL [26]. Among another family with three offspring with compound R409H/1561_1571CTTGGAATGGC PS mutations, extreme variability was observed, both for the degree of impairment and the age of onset [29]. These findings are supported by localization assays, where retention of pendrin in the endoplasmic reticulum (ER) was observed, rather than targeting to the plasma membrane, for mutations involved in PS, including L236P, T416P, G384E and V239D [30, 31] as well as for mutations associated with NSHL, including T410M, Q446R and c.1458_1459insT [14, 32].
The lack of genotype-phenotype correlation and the excess of NSHL cases, with or without EVA, with only one or zero SLC26A4 mutation suggest that NSHL/EVA/ PS is a complex disease involving other genetic factors. This assumption led to the detection of digenic heterozygosity of SLC26A4/FOXI1 and SLC26A4/ KCNJ10 mutations [33, 34]. FOXI1, a transcriptional regulating factor of SLC26A4, and KCNJ10, have also been implicated in the development of inner ear pathology. Interestingly, Kcnj10 expression is downregulated in Slc26a4-depleted mice, which contributes to the failure of endocochlear potential generation [35]. FOXI1 mutations were observed in PS and non-syndromic EVA patients and an additional NS EVA patient was found to be double heterozygous for SLC26A4/FOXI1 [34]. Similarly, KCNJ10 mutations were found in NS EVA patients as well as in a double heterozygosity state with a SLC26A4 mutation, SLC26A4/KCNJ10 [33]. Thus, FOXI1 and KCNJ10 are two genes that may contribute to the understanding of EVA heterogeneity. There are most likely many more genes and other factors to be identified, including modifier genes, or/and nutritional factors including iodine uptake [14, 21], which may determine the thyroid phenotype and the differences between and within PS and NSHL.
The high prevalence of SLC26A4 mutations involved in hereditary HL and its involvement in disease phenotypes emphasizes the importance of the molecular characterization of the SLC26A4 gene, as well as the identification of additional interactors/modifiers in the diagnosis of deafness. To date, routine clinical diagnostic tests for deafness have consisted of screening for the relevant mutations in a certain population. Comprehensive testing for the entire gene is not done routinely due to high costs. Implementing advanced sequencing technologies for clinical use might overcome this limitation and lead to the identification of additional mutations in a larger SLC26A4-associated-deafness group, enabling further genotype-phenotype analysis in larger cohorts. Recently, such an approach has been employed by applying DNA capture and massively parallel sequencing to identify inherited mutations involved in HL, including 246 genes involved in both human and mouse HL [36]. This technique also has the ability to speed up the discovery of genes that might interact and influence each other, as all variants in all genes associated with deafness may be identified in one experiment. Whole exome or genome sequencing might be an even better tool for interactors/modifiers detection, but the challenge of analyzing the data and determining which variants are the causative ones still remains.
Pendrin: crosstalk between human and mouse
A wide array of organisms, including zebrafish, chick and mouse, has complemented the genetics of deafness field with an in-depth understanding of protein function. Among the models, the striking similarity between the human and mouse inner ear structure and function has defined the mouse as a prominent animal model for human deafness [37]. The tools of gene overexpression, depletion and targeted mutagenesis has enabled researchers to create reliable animal models for genetic forms of hearing loss in order to mimic the corresponding mutation in humans. Once a novel human deafness gene is discovered, the generation of an animal model is optimal for studying its function.
Two major approaches are commonly used for cloning and studying unknown genes. The reverse genetics approach (genotype-driven) begins with a candidate gene chosen by bioinformatics tools e.g. homology to other known genes. The candidate gene is ‘knocked out’ in a conditional/permanent manner or mutated in an animal model for further investigation [38]. The resulting knockout phenotype may be one out of the three following scenarios: compatible with the working hypothesis, an unexpected or no phenotype. In contrast, with the forward genetics approach (phenotypic-driven), no prior assumption about any of the mutated genes is made. Instead, by ‘shooting in the dark,’ mutations are randomly generated and the phenotype is screened. The phenotype of interest is chosen and the journey to identify the gene begins. The chemically-induced N-ethyl-N-nitrosourea (ENU) mutagenesis is such an approach [39].
Following the positional cloning of SLC26A4 [1], a knock-out mouse model for PS was the first to be established [40]. The work on the Slc26a4 null mice illuminated the physiological and functional role of pendrin in various systems, including the inner ear (reviewed in this issue by Wangemann et. al.). The generation of additional Slc26a4 knockout mouse was reported in a kidney study which focused on pendrin function in the urinary system [41]. To further understand the clinical variability observed in humans carrying different mutations of pendrin, three additional mouse models were investigated. The Slc26a4loop mouse, generated by ENU mutagenesis, led to the discovery of new inner ear pathology that has complemented the work on the Slc26a4 null mice with its novel phenotypic variation [42]. A spontaneous mutation in Slc26a4 was found in the Slc26a4pdsm mouse model (http://mousemutant.jax.org/articles/mmrmutantpdsm.html). The reported inner ear characteristics of Slc26a4pdsm appear to be similar to the previously studied mouse strains. Finally, the attempt to expand the mouse mutations spectrum had led to the generation of the Slc26a4tm1Dontuh knock-in mouse that carries a common East Asian mutation [43]. In the following section we highlight the similarity and differences between the available Slc26a4 mouse models, with an emphasis on the inner ear.
Common auditory dysfunction of Slc26a4 mouse models
The inner ear is a remarkable organ that contains both the auditory and vestibular systems responsible for two prominent functions. Due to the close anatomical proximity, these two systems share many genetic, physiological and functional elements in common. As a result, many genetic mutations that affect the auditory system have consequences on the vestibular system in a direct or indirect manner. The general structure of the ear (Fig. 1) can be divided functionally into the conductive portion, comprised of the outer and middle ear, and the sensitive sensory portion of the inner ear (Fig. 1A). The inner ear is quite vulnerable to damage, as is evident by the long list of known human mutations that leads to hearing impairment [44]. The cochlea is a snail shape structure that contains the sensory organ of the ear known as the organ of Corti [45]. This sensory epithelium is associated with an endolymph field compartment called the scala media (Fig. 1B). The sensory cells of the auditory system, known as hair cells, are stimulated by mechanical energy originating from acoustic stimuli (Fig. 1C). Specialized actin rich protrusions at the apical suface of the cell form an organized structure of a hair bundle that is immersed in endolymph (Fig. 1D). Upon deflection of the hair bundle an influx of ions depolarize the cells, propagated via channels that are located between the tips of neighboring stereocilia [46, 47]. The unique chemical composition of the endolymph, with its high potassium (K+) and low sodium (Na+) and calcium (Ca+2) concentrations, is essential for hair cell depolarization and normal auditory function [48]. Hence, it should come as no surprise that many of the known genetic insults that lead to deafness affect channels and transporters that maintains the delicate homeostasis of the inner ear fluids [49]. Among this list of proteins, pendrin stands out in its prominent role of maintaining the acid-base balance of the endolymph and plays a cardinal function in the normal hearing mechanism.
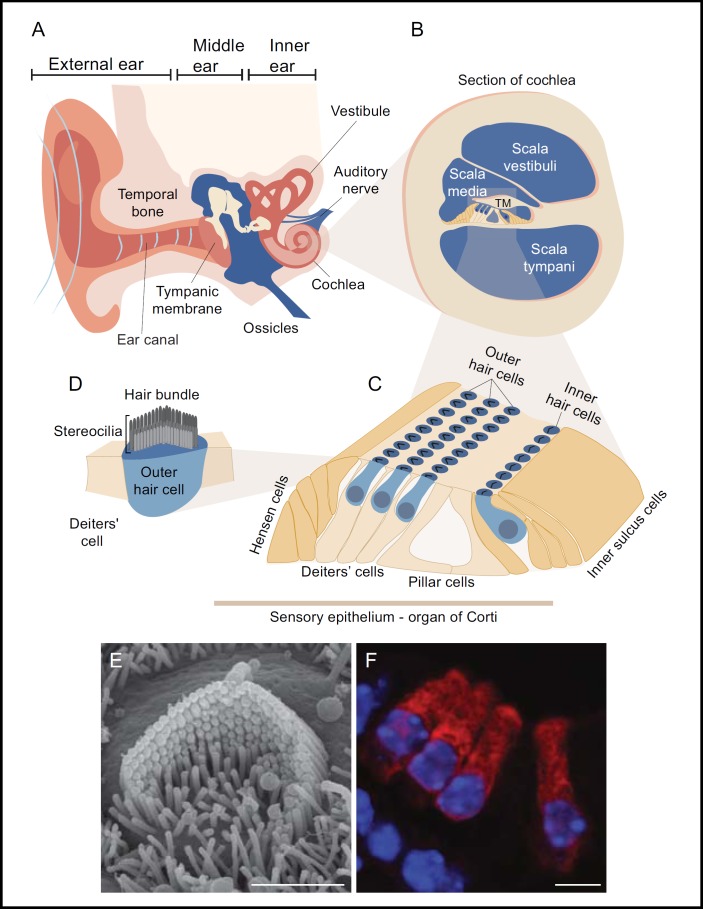
Fig. 1.
A general anatomical structure of the auditory system is demonstrated by schematic illustration. A: The ear is divided into three distinct anatomical compartments, the outer, the middle and the inner ear. Sound waves captured by the auricle of the external ear passes through the ear canal reaching the tympanic membrane leading to its vibrations. The middle ear conducts the mechanical energy of the sound vibration and transfers it to the inner ear. B: A cross section through the auditory portion of the inner ear (cochlea) reveals its associated fluid field compartments. C: A specialized sensory organ (organ of Corti) resides within the scala media along the coil shape of the cochlea from base to apex. The inner and outer hair cells are constantly elicited by mechanical forces driven by the acoustic stimulus. D: The apical surfaces of the hair cell contain hair bundle structures assembled by actin rich stereocilia with a typical staircase structure. Upon deflection of the hair bundle, mechanoelectrical channels position at the tip of two adjacent stereocilia are open and leads to hair cell depolarization. Complex innervations at the basal pole of the sensory cells propagate the electrical signal through the auditory nerve into the brain. E: A scanning electron micrograph showing hair bundle of an outer hair cell isolated from a newborn mouse. F: A cross section through the organ of Corti shows the arrangement of the inner hair cells with respect to three rows of outer hair cells. Florescent markers were applied for myosin VI (red) and nuclear DAPI staining (blue). Scale bars equal 2 μm in panel E, 4 μm in panel. Adapted from [37] with permission.
Generation of pendrin null mice has provided a tremendous tool in understanding the pathophysiology underlying this form of deafness observed in humans [40]. Initial characterization of the mouse clearly demonstrated the high similarity between the clinical pathogenesis of PS patients and the mouse phenotype. The auditory brainstem response (ABR) tests show that Slc26a4−/−mice are profoundly deaf, mimicking the early onset deafness in humans. Furthermore, Slc26a4−/− mice show a dramatic enlargement of the endolymphatic compartment with significant hydrops of the cochlea, as well as the endolymphatic sac and duct [40]. The changes in inner ear fluid compartments correlates with the typical enlargements of the vestibular aqueducts (EVA) of the affected individuals, as seen by computed tomography (CT) scan [28]. Subsequent work on the Slc26a4loop ([42] and Fig. 2) and the Slc26a4tm1Dontuh/tm1Dontuh [43] mice reported similar auditory characteristics, complementing the work on the Slc26a4−/− mice.
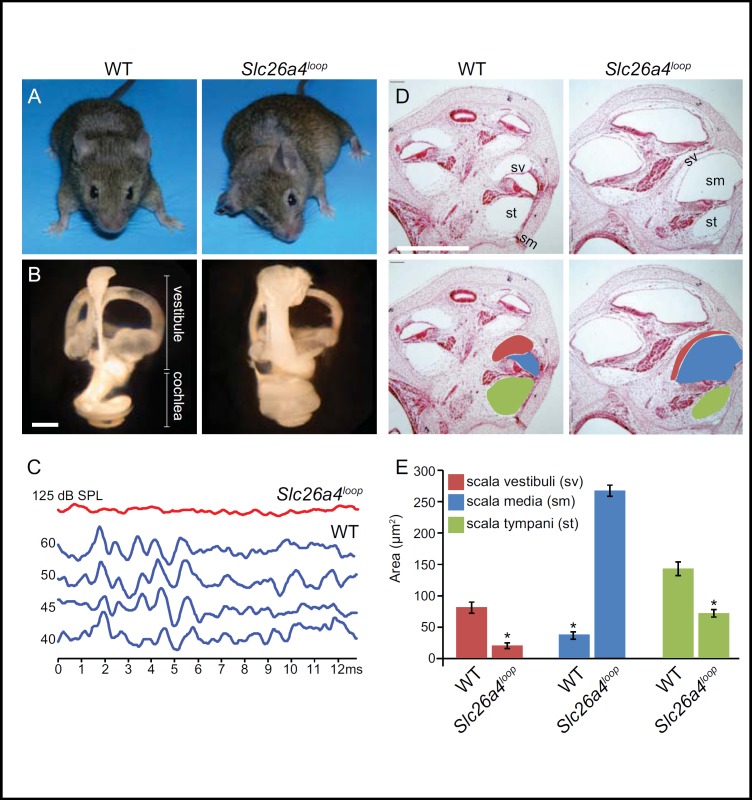
Fig. 2.
Auditory phenotypic characterization of Slc26a4loop is common to all known Slc26a4 mouse models. A: A representative image of Slc26a4loop mice shows the typical unsteady gait of the mutant mice that was determined by a panel of vestibular behavioral tests. B: Paint-filled inner ears of Slc26a4loop P0 mice show a bulged fluid filled compartment, with a prominent volume increase in the cochlea and the vestibular system. C: Auditory brainstem response (ABR) test on 8-week old mice reveals that Slc26a4loop mutants are profoundly deaf at three frequencies that were tested, 8Khz, 16Khz and 32Khz. An ABR recording for 8Khz is shown (red). D: Cross sections through the cochlea of Slc26a4loop mice reveals hydrops of the endolymphatic spaces of the scala media (sm), whereas the perilymphatic spaces of the scala vestibuli (sv) and scala tympani (st) are smaller. E: Histogram graphic representation shows the quantified area of the different cochlear compartments as compared between normal and mutant mice. A prominent enlargement of the endolymph field scala media is apparent in Slc26a4loop mice, at the expense of the smaller scala vestibuli and scala tympani. Scale bars equal 500 μm in panel B and D. Adapted from [42] with permission.
In the inner ear, pendrin is localized to the membrane of different cell types that face the endolymph, both in the cochlear and vestibular apparatus. Electrophysiological studies have shown that lack of pendrin leads to acidification of the endolymph, suggesting that pendrin mediates bicarbonate (HCO3–) secretion in the inner ear that buffers the accumulation of protons (H+) [11]. A subsequent increase of endolymphatic Ca2+ ion concentration in pendrin null mice is attributed to the lower pH level that inhibits the acid-sensitive TRPV5 and TRPV6 calcium channels [50]. Pendrin null mice also failed to develop endocochlear potential and hearing due to the loss of Kcnj10 protein expression after the age of P10 [35]. In the cochlea, Kcnj10, encoding a K+ channel, is normally expressed in intermediate cells of the stria vascularis and is sufficient for generating an endocochlear potential. An observed increased level of oxidative stress in pendrin null mice impairs normal function of the stria vascularis with subsequent loss of Kcnj10 protein expression and lack of endocochlear potential [51]. Interestingly, KCNJ10, together with mutations of SLC26A4, leads to digenic hearing loss with enlarged vestibular aqueduct [33]. Understanding that the pendrin mouse model fails to develop hearing due to the loss of the endocochlear potential defines window of opportunities for therapeutic approaches prior to the loss of KCNJ10 protein expression.
Unique vestibular dysfunction in the Slc26a4loop mouse model
The vestibular system is comprised of five sensory organs (Fig. 3). Three cristae connected to semicircular canals are sensitive for angular movement and saccule and utricle are sensitive for linear acceleration and gravity [52]. The integrated input of these sensory patches is essential for three dimensional acquisition and gravity perception. Similar to the cochlea, the sensory cells of the vestibular system bathe in the (K+) rich endolymph and are elicited in response to a mechanical stimulation. However, unlike the cochlea, the vestibular sensory epithelia have a different morphology and depend on a unique inertial mass that is required for its proper stimulation. The utricle and saccule are two small sacs that contain an oval-shaped thick epithelium, known as maculae, that contains the sensory hair cells. The sensory epithelia of the utricle and saccule are anatomically positioned 90° to each other [53], correlating with their functional significance in sensing linear and vertical acceleration, respectively. The apical surface of the sensory epithelium is associated with an extracellular gelatinous matrix on which large number of calcium carbonate (CaCO3) crystals, also known as otoconia, are positioned. Otoconia are small highly dense calcitic minerals that associate exclusively with the saccule and utricle. Thousands of otoconia, partially embedded in a gelatinous matrix, are supported on the sensory epithelium and serve as an inertial mass that is critical for mechanical stimulation [54, 55]. Movement of the otoconial layer through action of gravitational acceleration forces activate the underlying mechanosensory hair cells to generate action potentials that are transmitted to the brain.
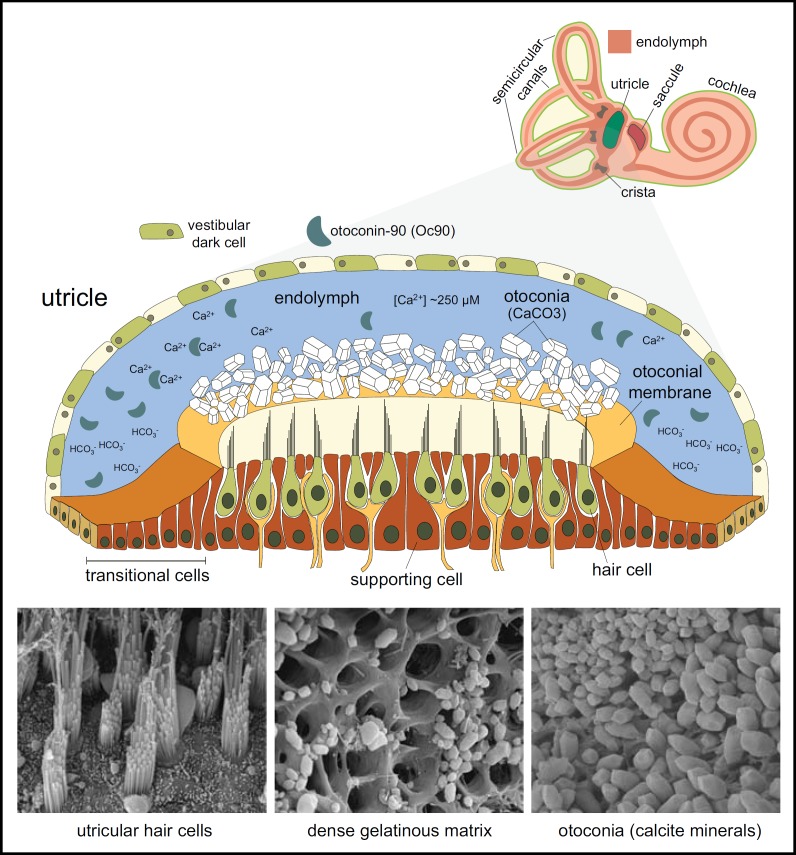
Fig. 3.
The sensory maculae of the vestibular system. Out of the five sensory organs of the vestibular system, the utricle and saccule share similar anatomical and morphological structures. Top: A cross section through the utricular sac illustrates the thick sensory epithelium creating the floor of the maculae. The hair cells (green) surrounded by supporting cells (brown) are connected into nerve fibers (yellow) in their basilar pole. The apical surface of the hair cells is composed of hair bundles of actin rich stereocilia (black) that protrude into dense gelatinous matrix. A large number of calcite crystals, known as otoconia (white), are positioned on top of the epithelium. The inertial mass of these mineralized crystals triggers the hair cells by transferring mechanical energy in response to linear movement of the body. The sac is surrounded by epithelial cells and contains endolymphatic fluids. Bottom: SEM showing the hair cells, dense gelatinous matrix and otoconia. Adapted from [42] with permission.
In addition to the auditory deficit, all Slc26a4 mouse models show prominent vestibular dysfunction with variable manifestations [40, 42, 43]. Among the various vestibular deficits, a prominent abnormal gait, circling behavior and abnormal reaching response clearly distinguishes the mutant mice from their littermate controls. A closer look at the utricle and saccule of these mouse models reveals an abnormal giant otoconia associated with the sensory epithelium [40]. The formation of the giant stones breaks the delicate balance between otoconia and the underlying hair cells. While in normal conditions the otoconia sustain an equally distributed mass to elicit the hair cells, in the presence of Slc26a4 mutations a sporadic distribution of the giant minerals leads to a differential mass over the sensory cells. Whereas some hair cells are overloaded with the weight of the giant stones, others lack any viable load of otoconia minerals. As a result, both cell populations are neutralized from being active in vestibular sensation and leads to severely disturb vestibular perception. Interestingly, out of the three Slc26a4 mouse models, the Slc26a4loop mice shows a unique composition of the pathological mineralized bodies [42]. A gradual change in composition of the Slc26a4loop giant otoconia leads to the formation of calcium oxalate minerals, determinately different from the calcium carbonate constituent of the wild-type otoconia (Fig. 4). The changes in chemical structure of the minerals are suggested to be attributed to an ongoing progressive acidification of the surrounding fluids as a result of impaired pendrin function. A lower pH leads to dissolution of the native calcium carbonate mineral while it is in favor of calcium oxalate mineral aggregations that are more stable in acidic environment. Prominently the formation of calcium oxalate stones are most known in the kidney under pathological conditions [56]. A recent study shows that Slc26a4 ablation leads to calcium wasting in urine due to down regulation of calcium absorbing protein in the kidney [57]. This evidence supports the leading framework that extracellular pH affects homeostasis in other organs via pH sensitive calcium channels [50]. The nucleation of oxalate crystals in Slc26a4loop inner ears is a unique example that links a genetic mutation to this aberrant pathophysiology [42]. Interestingly, in addition to the aberrant composition of Slc26a4loop giant minerals this strain distinguishes from other Slc26a4 mouse models in the morphology of the vestibular hair cells. At early progressive ages both Slc26a4−/− and Slc26a4tm1Dontuh/ tm1Dontuh mice show massive degeneration of utricular and saccular sensory cells [40, 43], whereas in Slc26a4loop mouse the vestibular hair cells are intact at the same time point [42]. Although the molecular mechanism for this variation is yet to be determined, it highlights the importance of having several mouse models to illuminate the function of pendrin through different genetic mutations.
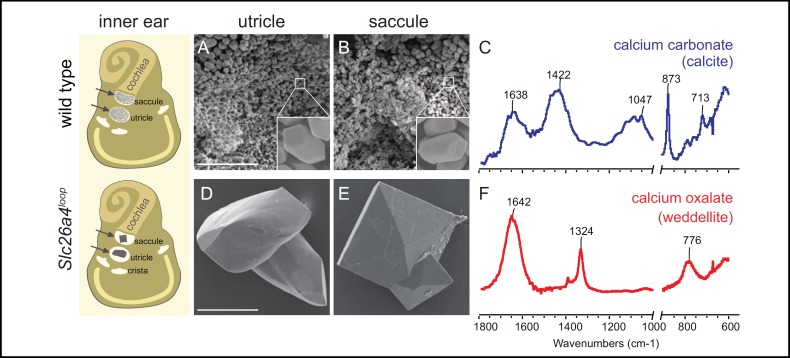
Fig. 4.
Calcium oxalate minerals in the Slc26a4loop vestibular system is a unique phenotype among Slc26a4 mouse models. Left panel: Schematic illustration of the inner ear summarizing the morphological differences of the inner ear stones at the age of 10 months old. The two sensory maculae of the vestibular system, utricle and saccule are indicated (arrows). A, B: SEM images of the utricle and saccule of a wild-type mouse shows the small (~7 μm) otoconia with typical morphology (inset in A and B). C: The spectrum of wild-type otoconia, showing the typical calcite vibrations at 713, 875 and 1422 cm-1. D, E: Minerals extracted from the utricle and saccule of Slc26a4loop mutant mice. The utricle minerals are larger relative to wild-type otoconia. In the saccule, a new type of mineral can be found with crystal morphology typical of Ca-oxalate dihydrate (weddelite) crystals. F: Spectrum of the mineral extracted from the saccule in Slc26a4loop mutant mice shows vibrations at 1324 and 776 cm-1 that are typical of weddelite crystals. Scale bars equal 50 μm in panel A applies to B, 100 μm in panel D applies to E. Adapted from [42] with permission.
Summary
The field of genetics and its associated area of genomics have evolved tremendously over the past years. Since the discovery of SLC26A4 mutations and association with disease, there has been remarkable progress in understanding pendrin function due to technological genomic advances. The wide expression of pendrin in a variety of systems initiated studies that illuminated the role of pendrin in different clinical conditions including deafness [11], asthma and chronic obstructive pulmonary disease (COPD) [58], kidney and chloride reabsorption [59] and iodide homeostasis of the thyroid [10]. However, despite progress, there are aspects of the role of pendrin that still remain to be explored. The absence of precise genotype-phenotype correlations, as well as modifiers of disease, of the known pendrin mutations highlight intriguing questions. The overview provided in this summary lays the groundwork for these questions and points to a promising future in the pendrin field.
Acknowledgements
Research in the Avraham laboratory is funded by the Israel Science Foundation Grant 1486/07, the National Institutes of Health (NIDCD) R01DC011835, and the Israel Ministry of Health.
Abbreviations
- PS
(Pendred syndrome)
- EVA
(enlarged vestibular aqueduct)
- NSHL
(non-syndromic hearing loss)
- ER
(endoplasmic reticulum)
- EN
(N-ethyl-N-nitrosourea)
- ABR
(auditory brainstem response)
References
- 1.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 2.Reardon W, Coffey R, Chowdhury T, Grossman A, Jan H, Britton K, Kendall-Taylor P, Trembath R. Prevalence, age of onset, and natural history of thyroid disease in Pendred syndrome. J Med Genet. 1999;36:595–598. [PMC free article] [PubMed] [Google Scholar]
- 3.Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3-secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett LA, Morsli H, Wu DK, Green ED. Expression pattern of the mouse ortholog of the Pendred's syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci USA. 1999;96:9727–9732. doi: 10.1073/pnas.96.17.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 7.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl-/OH-/HCO3-exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 9.Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED, Izuhara K. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008;180:6262–6269. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- 10.Bizhanova A, Kopp P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150:1084–1090. doi: 10.1210/en.2008-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pera A, Dossena S, Rodighiero S, Gandia M, Botta G, Meyer G, Moreno F, Nofziger C, Hernandez-Chico C, Paulmichl M. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci USA. 2008;105:18608–18613. doi: 10.1073/pnas.0805831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dossena S, Rodighiero S, Vezzoli V, Nofziger C, Salvioni E, Boccazzi M, Grabmayer E, Botta G, Meyer G, Fugazzola L, Beck-Peccoz P, Paulmichl M. Functional characterization of wildtype and mutated pendrin (SLC26A4), the anion transporter involved in Pendred syndrome. J Mol Endocrinol. 2009;43:93–103. doi: 10.1677/JME-08-0175. [DOI] [PubMed] [Google Scholar]
- 14.Brownstein ZN, Dror AA, Gilony D, Migirov L, Hirschberg K, Avraham KB. A novel SLC26A4 (PDS) deafness mutation retained in the endoplasmic reticulum. Arch Otolaryngol Head Neck Surg. 2008;134:403–407. doi: 10.1001/archotol.134.4.403. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Griffith AJ. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert S, Blons H, Jonard L, Feldmann D, Chauvin P, Loundon N, Sergent-Allaoui A, Houang M, Joannard A, Schmerber S, Delobel B, Leman J, Journel H, Catros H, Dollfus H, Eliot MM, David A, Calais C, Drouin-Garraud V, Obstoy MF, Tran Ba Huy P, Lacombe D, Duriez F, Francannet C, Bitoun P, Petit C, Garabedian EN, Couderc R, Marlin S, Denoyelle F. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet. 2006;14:773–779. doi: 10.1038/sj.ejhg.5201611. [DOI] [PubMed] [Google Scholar]
- 17.Griffith AJ, Wangemann P: Hearing loss associated with enlargement of the vestibular aqueduct: Mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 2011; in press. [DOI] [PMC free article] [PubMed]
- 18.Merchant SN, Nakajima HH, Halpin C, Nadol JB, Jr., Lee DJ, Innis WP, Curtin H, Rosowski JJ. Clinical investigation and mechanism of air-bone gaps in large vestibular aqueduct syndrome. Ann Otol Rhinol Laryngol. 2007;116:532–541. doi: 10.1177/000348940711600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell C, Cucci RA, Prasad S, Green GE, Edeal JB, Galer CE, Karniski LP, Sheffield VC, Smith RJ. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat. 2001;17:403–411. doi: 10.1002/humu.1116. [DOI] [PubMed] [Google Scholar]
- 20.Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet. 1999;104:188–192. doi: 10.1007/s004390050933. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto K, Suzuki H, Harada D, Namba A, Abe S, Usami S. Distribution and frequencies of PDS (SLC26A4) mutations in Pendred syndrome and nonsyndromic hearing loss associated with enlarged vestibular aqueduct: a unique spectrum of mutations in Japanese. Eur J Hum Genet. 2003;11:916–922. doi: 10.1038/sj.ejhg.5201073. [DOI] [PubMed] [Google Scholar]
- 22.Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, Nance WE, Yang Y, Zalewski CK, Brewer CC, Butman JA, Griffith AJ. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42:159–165. doi: 10.1136/jmg.2004.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azaiez H, Yang T, Prasad S, Sorensen JL, Nishimura CJ, Kimberling WJ, Smith RJ. Genotype-phenotype correlations for SLC26A4-related deafness. Hum Genet. 2007;122:451–457. doi: 10.1007/s00439-007-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Oshima A, Tsukamoto K, Abe S, Kumakawa K, Nagai K, Satoh H, Kanda Y, Iwasaki S, Usami S. Clinical characteristics and genotype-phenotype correlation of hearing loss patients with SLC26A4 mutations. Acta Otolaryngol. 2007;127:1292–1297. doi: 10.1080/00016480701258739. [DOI] [PubMed] [Google Scholar]
- 25.Napiontek U, Borck G, Muller-Forell W, Pfarr N, Bohnert A, Keilmann A, Pohlenz J. Intrafamilial variability of the deafness and goiter phenotype in Pendred syndrome caused by a T416P mutation in the SLC26A4 gene. J Clin Endocrinol Metab. 2004;89:5347–5351. doi: 10.1210/jc.2004-1013. [DOI] [PubMed] [Google Scholar]
- 26.Masmoudi S, Charfedine I, Hmani M, Grati M, Ghorbel AM, Elgaied-Boulila A, Drira M, Hardelin JP, Ayadi H. Pendred syndrome: phenotypic variability in two families carrying the same PDS missense mutation. Am J Med Genet. 2000;90:38–44. [PubMed] [Google Scholar]
- 27.Sugiura M, Sato E, Nakashima T, Sugiura J, Furuhashi A, Yoshino T, Nakayama A, Mori N, Murakami H, Naganawa S. Long-term follow-up in patients with Pendred syndrome: vestibular, auditory and other phenotypes. Eur Arch Otorhinolaryngol. 2005;262:737–743. doi: 10.1007/s00405-004-0884-z. [DOI] [PubMed] [Google Scholar]
- 28.Wu CC, Yeh TH, Chen PJ, Hsu CJ. Prevalent SLC26A4 mutations in patients with enlarged vestibular aqueduct and/or Mondini dysplasia: a unique spectrum of mutations in Taiwan, including a frequent founder mutation. Laryngoscope. 2005;115:1060–1064. doi: 10.1097/01.MLG.0000163339.61909.D0. [DOI] [PubMed] [Google Scholar]
- 29.Fugazzola L, Cirello V, Dossena S, Rodighiero S, Muzza M, Castorina P, Lalatta F, Ambrosetti U, Beck-Peccoz P, Botta G, Paulmichl M. High phenotypic intrafamilial variability in patients with Pendred syndrome and a novel duplication in the SLC26A4 gene: clinical characterization and functional studies of the mutated SLC26A4 protein. Eur J Endocrinol. 2007;157:331–338. doi: 10.1530/EJE-07-0263. [DOI] [PubMed] [Google Scholar]
- 30.Rotman-Pikielny P, Hirschberg K, Maruvada P, Suzuki K, Royaux IE, Green ED, Kohn LD, Lippincott-Schwartz J, Yen PM. Retention of pendrin in the endoplasmic reticulum is a major mechanism for Pendred syndrome. Hum Mol Genet. 2002;11:2625–2633. doi: 10.1093/hmg/11.21.2625. [DOI] [PubMed] [Google Scholar]
- 31.Walsh T, Abu Rayan A, Abu Sa'ed J, Shahin H, Shepshelovich J, Lee MK, Hirschberg K, Tekin M, Salhab W, Avraham KB, King MC, Kanaan M. Genomic analysis of a heterogeneous Mendelian phenotype: multiple novel alleles for inherited hearing loss in the Palestinian population. Hum Genomics. 2006;2:203–211. doi: 10.1186/1479-7364-2-4-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87:1778–1784. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Gurrola JG, 2nd, Wu H, Chiu SM, Wangemann P, Snyder PM, Smith RJ. Mutations of KCNJ10 together with mutations of SLC26A4 cause digenic nonsyndromic hearing loss associated with enlarged vestibular aqueduct syndrome. Am J Hum Genet. 2009;84:651–657. doi: 10.1016/j.ajhg.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T, Vidarsson H, Rodrigo-Blomqvist S, Rosengren SS, Enerback S, Smith RJ. Transcriptional control of SLC26A4 is involved in Pendred syndrome and nonsyndromic enlargement of vestibular aqueduct (DFNB4) Am J Hum Genet. 2007;80:1055–1063. doi: 10.1086/518314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brownstein Z, Friedman LM, Shahin H, Oron-Karni V, Kol N, Abu Rayyan A, Parzefall T, Lev D, Shalev S, Frydman M, Davidov B, Shohat M, Rahile M, Lieberman S, Levy-Lahad E, Lee M, Shomron N, King M-C, Walsh T, Kanaan M, Avraham KB. Targeted genomic capture and massively parallel sequencing to identify genes for hereditary hearing loss in Middle Eastern families. Genome Biol. 2011;12:R89. doi: 10.1186/gb-2011-12-9-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dror AA, Avraham KB. Hearing impairment: a panoply of genes and functions. Neuron. 2010;68:293–308. doi: 10.1016/j.neuron.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Anagnostopoulos AV. A compendium of mouse knockouts with inner ear defects. Trends Genet. 2002;18:499. doi: 10.1016/s0168-9525(02)02753-1. [DOI] [PubMed] [Google Scholar]
- 39.Acevedo-Arozena A, Wells S, Potter P, Kelly M, Cox RD, Brown SD. ENU mutagenesis, a way forward to understand gene function. Annu Rev Genomics Hum Genet. 2008;9:49–69. doi: 10.1146/annurev.genom.9.081307.164224. [DOI] [PubMed] [Google Scholar]
- 40.Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet. 2001;10:153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- 41.Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl-/HCO3- exchanger activity and impairs bicarbonate secretion in kidney collecting duct. American journal of physiology Cell physiology. 2010;299:C33–41. doi: 10.1152/ajpcell.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dror AA, Politi Y, Shahin H, Lenz DR, Dossena S, Nofziger C, Fuchs H, Hrabe de Angelis M, Paulmichl M, Weiner S, Avraham KB. Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J Biol Chem. 2010;285:21724–21735. doi: 10.1074/jbc.M110.120188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu YC, Wu CC, Shen WS, Yang TH, Yeh TH, Chen PJ, Yu IS, Lin SW, Wong JM, Chang Q, Lin X, Hsu CJ. Establishment of a knock-in mouse model with the SLC26A4 c.919-2A>G mutation and characterization of its pathology. PLoS One. 2011;6:e22150. doi: 10.1371/journal.pone.0022150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- 45.Corti A. Recherches sur l'organe de l'ouië des mammifères. Ztschr wissensch Zool. 1851;3:109–169. [Google Scholar]
- 46.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tiplink filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 47.Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 48.Wangemann P, Schacht J. Cochlear homeostasis. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea Handbook of Auditory Research. Vol. 8. New York: Springer; 1996. pp. 130–185. [Google Scholar]
- 49.Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–316. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol. 2007;292:F1314–1321. doi: 10.1152/ajprenal.00432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh R, Wangemann P. Free radical stress-mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2008;294:F139–148. doi: 10.1152/ajprenal.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- 53.Curthoys IS, Betts GA, Burgess AM, MacDougall HG, Cartwright AD, Halmagyi GM. The planes of the utricular and saccular maculae of the guinea pig. Ann N Y Acad Sci. 1999;871:27–34. doi: 10.1111/j.1749-6632.1999.tb09173.x. [DOI] [PubMed] [Google Scholar]
- 54.Thalmann R, Ignatova E, Kachar B, Ornitz DM, Thalmann I. Development and maintenance of otoconia: biochemical considerations. Ann N Y Acad Sci. 2001;942:162–178. doi: 10.1111/j.1749-6632.2001.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 55.Carlstrom D. A crystallographic study of vertebrate otoliths. Biol Bull. 1963;125:441–463. [Google Scholar]
- 56.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25:831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barone S, Amlal H, Xu J, Soleimani M: Deletion of the Cl-/HCO3- exchanger pendrin downregulates calcium-absorbing proteins in the kidney and causes calcium wasting. Nephrol Dial Transplant 2011; in press. [DOI] [PMC free article] [PubMed]
- 58.Nofziger C, Vezzoli V, Dossena S, Schonherr T, Studnicka J, Nofziger J, Vanoni S, Stephan S, Silva ME, Meyer G, Paulmichl M. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2011;90:399–405. doi: 10.1038/clpt.2011.128. [DOI] [PubMed] [Google Scholar]
- 59.Wagner CA. The emerging role of pendrin in renal chloride reabsorption. Am J Physiol Renal Physiol. 2007;292:F912–913. doi: 10.1152/ajprenal.00449.2006. [DOI] [PubMed] [Google Scholar]