Abstract
Calcium sensing receptor (CaSR) mutations implicated in familial hypocalciuric hypercalcemia, pancreatitis and idiopathic epilepsy syndrome map to an extended arginine-rich region in the proximal carboxyl terminus. Arginine-rich motifs mediate endoplasmic reticulum retention and/or retrieval of multisubunit proteins so we asked whether these mutations, R886P, R896H or R898Q, altered CaSR targeting to the plasma membrane. Targeting was enhanced by all three mutations, and Ca2+-stimulated ERK1/2 phosphorylation was increased for R896H and R898Q. To define the role of the extended arginine-rich region in CaSR trafficking, we independently determined the contributions of R890/R891 and/or R896/K897/R898 motifs by mutation to alanine. Disruption of the motif(s) significantly increased surface expression and function relative to wt CaSR. The arginine-rich region is flanked by phosphorylation sites at S892 (protein kinase C) and S899 (protein kinase A). The phosphorylation state of S899 regulated recognition of the arginine-rich region; S899D showed increased surface localization. CaSR assembles in the endoplasmic reticulum as a covalent disulfide-linked dimer and we determined whether retention requires the presence of arginine-rich regions in both subunits. A single arginine-rich region within the dimer was sufficient to confer intracellular retention comparable to wt CaSR. We have identified an extended arginine-rich region in the proximal carboxyl terminus of CaSR (residues R890 - R898) which fosters intracellular retention of CaSR and is regulated by phosphorylation. Mutation(s) identified in chronic pancreatitis and idiopathic epilepsy syndrome therefore increase plasma membrane targeting of CaSR, likely contributing to the altered Ca2+ signaling characteristic of these diseases.
Key Words: Calcium sensing receptor, Pancreatitis, Carboxyl terminus, Arginine-rich motif, Membrane protein trafficking, 14-3-3 proteins
Introduction
The calcium sensing receptor (CaSR) is critical to systemic calcium homeostasis through its actions on the parathyroid, intestine, kidneys and bone [1]. Mutations in human CaSR cause calcium handling diseases which alter the Ca2+ setpoint for parathyroid hormone secretion. Loss-of-function mutations, characterized by a decrease in the apparent affinity of CaSR for extracellular calcium, are the most common, and result in benign familial hypocalciuric hypercalcemia (FHH; OMIM 14598) or in homozygous individuals, a more severe syndrome termed neonatal severe primary hyperparathyroidism (NSHPT; OMIM 239200). Less common are activating mutations of CaSR, which cause autosomal dominant hypoparathyroidism (ADH; OMIM 601298) or Bartter's syndrome type V (OMIM 601199.0035). CaSR is expressed in both the endocrine and exocrine pancreas [2], and mutations in CaSR [reviewed in 3, 4] as well as the common polymorphism R990G [5] have been linked to pancreatitis in the absence [5] or presence of mutations in other genes predisposing to premature trypsin activation, e.g. SPINK1(N34S) [3, 4, 6]. A link between CaSR mutations and idiopathic epilepsy syndrome was established by multi-generational linkage analysis [7]. The involvement of CaSR mutations in predisposition to chronic pancreatitis and epilepsy were observed in patients without the characteristic derangements in serum calcium or parathyroid hormone [6, 7] that are the signature of systemic Ca2+ handling diseases, suggesting tissue-specific effects of these mutations.
CaSR is a member of Family C/3 of the G proteincoupled receptor (GPCR) superfamily, which includes metabotropic glutamate, γ-aminoisobutyric acid, taste and pheromone receptors. The human CaSR carboxyl terminus is long (215 residues, from residue K863-S1078), and has no homologies with the carboxyl termini of other Family C/3 members. Disease causing mutations in the CaSR carboxyl terminus are relatively rare [8]. Point mutations linked to chronic pancreatitis as well as idiopathic epilepsy syndrome, FHH/NSHPT or ADH encompass an RXR motif, i.e., R896H [6] and R898Q [7], which is part of an extended arginine-rich region in the proximal carboxyl terminus of human CaSR, 886RATLRRSNVSRKR898. Mutations at R886 (R886P [9] and R886W [10]) cause FHH/NSHPT. A large inframe deletion of the CaSR carboxyl terminus (from S895 to V1075), which eliminates the RXR motif, causes ADH [11, 12]. We reasoned that since truncations of the CaSR carboxyl terminus have little effect on CaSR signaling to phospholipase Cβ [eg. 13] but increase the amount of CaSR at the plasma membrane [13, 14], mis-sense mutations most likely alter protein interactions required for regulated trafficking of CaSR through the secretory pathway and/or targeting of CaSR to the plasma membrane. In this report, we characterize the importance of the proximal arginine-rich region of the CaSR carboxyl terminus for regulated release of CaSR from the endoplasmic reticulum and targeting to the plasma membrane. We find that an extended arginine-rich region which includes both RR and RXR motifs contributes to retention of CaSR in the endoplasmic reticulum. A flanking protein kinase A phosphorylation site at S899 contributes to signaling-regulated recognition of the motif, since the phosphomutant S899D leads to increased plasma membrane targeting. All arginine and/or phosphorylation site mutants are functional, i.e., mediate Ca2+-stimulated ERK1/2 phosphorylation, leading to the conclusion that the arginine-rich region regulates CaSR signaling by controlling abundance at the plasma membrane. Mutation-mediated increases in plasma membrane CaSR in the concurrent presence of other genetic or environmental risk factors [3, 4], including moderate to heavy alcohol consumption [4, 5], may increase the risk for development of chronic pancreatitis. Likewise, increased plasma membrane abundance and/or differential targeting of CaSR in neurons may predispose to idiopathic epilepsy.
Materials and Methods
Generation of CaSR Mutants
Constructs were generated in the background of human CaSR in pEGFP-N1 with an amino terminal FLAG epitope [15] using Pfu ultra polymerase (Stratagene). Truncations in CaSR were generated by inserting a stop codon by PCR mutagenesis. Phosphorylation mutants S892A, S892D, S899A, S899D, S892A/S899A, and S892D/899D and point mutations R890A/R891A, R886P, R896H, and R898Q were generated in full length CaSR by primer-based mutagenesis. Similar approaches were used to generate the R890A/R891A mutant in CaSRΔ898. CaSR(3A) (CaSR(R896A/K897A/R898A)), and CaSR(5A) (CaSR(R890A/R891A/R896A/K897A/R898A)) were generated in the full length CaSR and the CaSRΔ898 truncation using seventy-five base pair complementary oligonucleotides with the appropriate mutations, an XmaI restriction site at the 5’ end and a BamHI restriction site at the 3’ end. Oligonucleotides were annealed 2 minutes at 94° and cooled to room temperature. Full length CaSR and duplexes were digested with XmaI and BamHI (Promega) for 3 hours at 37oC, run on 1% agarose gels and purified with the Qiagen QiaEXII kit. Digested and purified CaSR was then dephosphorylated with shrimp alkaline phosphatase (Promega M820A) according to the manufacturer's protocol, and ligated with T4 DNA Ligase (Promega M1801). The entire coding region was sequenced for all constructs (Genewiz).
Transfection and Immunoprecipitation
HEK293 cells (ATCC) were cultured in MEM supplemented with 10% fetal bovine serum and penicillin/ streptomycin in 5% CO2 and used within 25 passages. Cells were transfected with 2 or 3 µg total DNA in 35 mm dishes using NovaFector (Venn Nova) or FugeneHD (Roche) according to manufacturers’ protocol and cultured for 2-3 days. Cells were lysed in 5 mM EDTA, 0.5% Triton X-100, 10 mM iodoacetamide, and protease inhibitors (Roche Cømplete tablets) in PBS. For immunoprecipitation of CaSR, equal amounts of protein were precipitated overnight with M2 anti-FLAG antibody (Sigma) plus protein-G-agarose (Invitrogen). 14-3-3 immunoprecipitations were performed with pan-14-3-3 antibody (Santa Cruz SC-629) plus protein A-agarose (Invitrogen). Samples were eluted in SDS loading buffer ± 100 mM dithiothreitol, incubated at room temperature for 30 min, and run on 7.5% SDS polyacrylamide gels (Criterion, BioRad) and transferred to nitrocellulose for detection.
Western Blotting
Standard protocols were used. Primary antibodies include: rabbit polyclonal anti-LRG epitope for CaSR (custom-generated by Genemed Synthesis, Inc.) or mouse monoclonal anti-ADD epitope for CaSR (Abcam), phospho-p44/42 MAP Kinase (Thr202/Tyr204) antibody and p44/42 MAP Kinase antibody (Cell Signaling). ECL anti-Rabbit IgG, horseradish peroxidase linked whole antibody from donkey (GE Healthcare) or ECL anti-Mouse IgG, horseradish peroxidase linked whole antibody from sheep (GE Healthcare) was used as secondary antibody. SuperSignal West Pico Chemiluminescence Substrate (Pierce) was used to visualize proteins to film followed by scanning to computer and analysis with AlphaEaseFC V. 4.0.0 (Alpha Innotech) or FUJIFilm Luminescent Image Analyzer, LAS-4000mini and analysis software.
Enzyme-linked immunoabsorbance assays (ELISA) HEK293 cells were transfected with 2 or 3 µg total DNA in 6 well plates. Twenty-four or forty-eight hours after transfection, cells were split into 96 well poly-L-lysine coated plates and incubated overnight. A single well of transfected cells was split into 16 wells of a 96 well plate. Cells were fixed with either MeOH (total CaSR) or 4% paraformaldehyde (plasma membrane CaSR) for 15 minutes on ice. All subsequent steps were at room temperature. Cells were washed with TBS-T and blocked for 1 hour in 1% milk/TBS-T, followed by 1 hr incubation with monoclonal anti-FLAG-M2 peroxidase (HRP) antibody (Sigma A8592) according to manufacturer's protocol. Samples were developed with 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate solution (Sigma T8665) for 30 minutes. The reaction was stopped with 1M sulfuric acid, and absorbances were read at 450 nm. Eight replicates fixed in either paraformaldehyde or MeOH were averaged and background was subtracted (untransfected HEK293 cells fixed with paraformaldehyde or MeOH). Data were normalized to plasma membrane or total abundance of FLAG-CaSR as indicated for specific experiments.
MAPK phosphorylation assays
HEK293 cells transfected with 1 µg total DNA in 12 well plates were cultured for 48 hrs, then starved overnight in 0.5 mM Ca2+ with 0.2% BSA in DMEM. Cells were stimulated with either 0.5 mM or 5 mM Ca2+ for 10 minutes at 37oC and immediately harvested on ice into lysis buffer containing 25 mM HEPES, pH 7.5, 5 mM MgCl2, 5 mM EDTA, 1% Triton X-100, protease inhibitor (Roche Cømplete tablets), and protein phosphatase inhibitor solutions 1 and 2 (Sigma).
Equal amounts of supernatant protein were run on 4-15% gradient SDS polyacrylamide gels (Criterion, BioRad), and transferred to nitrocellulose for detection by Western blotting.
Data analysis
Data derived from western blot films were scanned to computer and analyzed with AlphaEaseFC V. 4.0.0 (Alpha Innotech) or FUJI Multigauge V3.0 software. Arbitrary units of immunoreactivity were normalized to wt CaSR from the same blot; normalized data from 3-6 experiments were averaged and plotted ± S.E.M. Significance was determined with the Student's t-test with significance defined as p < 0.05. For ELISA assays, 8 replicate wells were averaged and the intra-experiment standard deviation was less than 5%. Each experiment was corrected for background obtained in untransfected HEK293 cells, and normalized to results obtained for wt CaSR in the same experiment. Overall data from 3-5 independent experiments were averaged, and plotted ± S.E.M. (significance at p < 0.05).
Results
Patient mutations in an arginine-rich region alter plasma membrane abundance of CaSR
To characterize the effects of mutations identified in patients with FHH (R886P [9]), chronic pancreatitis (R896H [6]) or idiopathic epilepsy (R898Q [7]) that cluster in an arginine-rich region of the CaSR carboxyl terminus, we generated each in the background of amino terminal FLAG-tagged human CaSR, and compared protein abundance, function and surface localization relative to wt CaSR. Fig. 1A illustrates a western blot comparing wt CaSR and mutants 72 hrs after transient transfection in HEK293 cells. CaSR is a highly disulfide-linked protein and despite treatment with iodoacetamide during cell lysis and treatment of eluted samples with 0.1 M DTT, some dimer/oligomer is observed on the blots and therefore both regions of the blot are illustrated. The net abundance of all mutants was greater than wt CaSR, and closer examination of the monomeric region of the blot suggests that R896H and R898Q also showed increased abundance of the maturely glycosylated form of the receptor. The endoplasmic reticulum-localized form of CaSR (≈140 kDa) matures in the Golgi to ≈160 kDa, and the abundance of this form is considered synonymous with plasma membrane CaSR [16]. Commensurate with increased abundance of the maturely glycosylated form, both R896H and R898Q mediated extracellular Ca2+-stimulated ERK1/2 phosphorylation to levels equal to or greater than wt CaSR (Fig. 1B), arguing that these mutations induce a gain-of-function phenotype. CaSR mutant R886P showed lower levels of Ca2+-stimulated ERK1/2 phosphorylation relative to wt CaSR (Fig. 1B), despite higher expression (Fig. 1A), consistent with the diagnosis of FHH [9]. Mutation R898Q has been implicated in idiopathic epilepsy in individuals not displaying overt symptoms of FHH [7]. To explicitly define the effects of these mutations on plasma membrane abundance of CaSR, we used ELISA assays. HEK293 cells transiently expressing CaSR or mutants for 72 hrs were fixed with either 4% paraformaldehyde or ice cold methanol and probed with anti-FLAG antibody to define plasma membrane and total expression, respectively. Fig. 1C illustrates the results. Data for plasma membrane (black bars) and total (white bars) expression of each mutant was normalized to that of wt CaSR and plotted as % of wt abundance. If the mutations have an effect on net abundance but no differential effect on plasma membrane targeting, plasma membrane and total abundance should vary in parallel. If, however, the mutation alters the relative abundance of CaSR at the plasma membrane, there should be a statistically significant difference between surface and total abundance. As illustrated in Fig. 1C, all three mutations selectively increase plasma membrane relative to total abundance (*p<0.05), arguing that the arginine-rich region represents a functional retention motif in the context of the full length CaSR carboxyl terminus.
Fig. 1.
Arginine-rich region mutations identified in patients enhance surface expression and function of CaSR. A. wt CaSR or R886P, R896H or R898Q mutants were immunoprecipitated from HEK293 cells with anti-FLAG antibody; blot was probed with anti-CaSR LRG antibody. Monomer (m) and dimer (d) regions of the western blot are indicated. B. ERK phosphorylation supported by arginine-rich region mutants. HEK293 cells expressing wt CaSR, R886P, R896H or R898Q mutants were stimulated with 5 mM extracellular Ca2+ for 10 min and lysates were analyzed for ERK1/2 phosphorylation as described in Methods. C. ELISA assay of arginine-rich region mutations was performed as described in Methods. Plasma membrane (black bars) and total (white bars) protein abundance were normalized to wt CaSR under the same fixation conditions (wt CaSR = 100%, dashed line). Results are the average ± S.E.M. of 3-4 independent experiments. Significant differences between surface and total protein expression for a given receptor mutant are indicated (* p<0.05).
Truncations confirm the importance of an arginine-rich region in CaSR carboxyl terminus
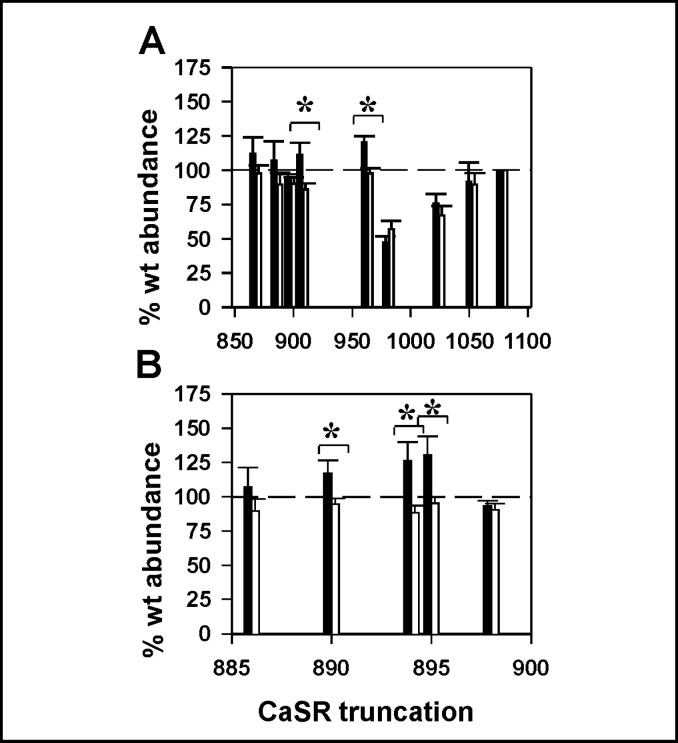
To confirm that the differential effects on plasma membrane targeting conferred by mutations within the arginine-rich region uniquely contributed to CaSR trafficking, we generated a series of truncation mutants in the background of wt N-terminal FLAG-tagged CaSR, including Δ868, Δ886, Δ898, Δ908, Δ963, Δ981, Δ1024, and Δ1052 (full length CaSR is 1078 amino acids). We used ELISA assays with anti-FLAG antibody to characterize both total and surface receptor abundance, and plotted data as described for Fig. 1C, i.e., normalized as % of wt abundance under each fixation condition. Fig. 2A illustrates that most truncations were expressed at levels equivalent to wt CaSR (dashed line), and exhibited plasma membrane abundance (black bars) proportional to total abundance (white bars). Only truncations Δ963 and Δ908 exhibited increased surface expression relative to total expression, indicating a differential effect on trafficking and/or plasma membrane stability upon elimination of distal residues. The Δ963 truncation is at the distal end of a low complexity disordered region at the proximal end of the filamin A binding site (minimal site 962-981 [17]). The Δ908 truncation, distal to the arginine-rich region in the proximal carboxyl terminus, is the only other hot spot resulting in differential plasma membrane abundance. To further refine this locus of enhanced plasma membrane abudance, we generated additional truncations between Δ886 and Δ908 (Fig. 2B). The plasma membrane relative to total abundance is increased for truncations Δ894 and Δ895 (which remove an RKR motif), and Δ890 (which disrupts an RR motif). Truncation at Δ898, which preserves the RKR motif and generates a truncated CaSR containing the entire argininerich region, does not alter plasma membrane relative to total abundance. Likewise truncation at Δ886, which removes the entire arginine-rich region, shows no differential plasma membrane abundance. These results suggest that the arginine-rich region containing both RR and RKR motifs may contribute to intracellular retention of CaSR and that the larger truncation at Δ908 may contain additional regulatory elements.
Fig. 2.
Plasma membrane relative to total receptor abundance of carboxyl terminal truncations of CaSR. A. The human CaSR carboxyl terminus extends from residue K863 to S1078. Truncations were generated over the range from CaSRΔ868 through CaSRΔ1052. ELISA assays were performed on replicate samples fixed in either 4% paraformaldehyde (plasma membrane, black bars) or methanol (total abundance, white bars) as described in Methods. Results were normalized to the abundance of wt CaSR under the same fixation conditions (wt CaSR = 100%, indicated with dashed line). B. Truncations between CaSRΔ886 and CaSRΔ908 were analyzed to resolve importance of arginine-rich region residues. For both A and B, significant differences between plasma membrane and total abundance for a given truncation are indicated (* p<0.05), and results are average ± S.E.M. of 5-8 independent experiments.
Disruption of the arginine-rich region enhances CaSR plasma membrane abundance
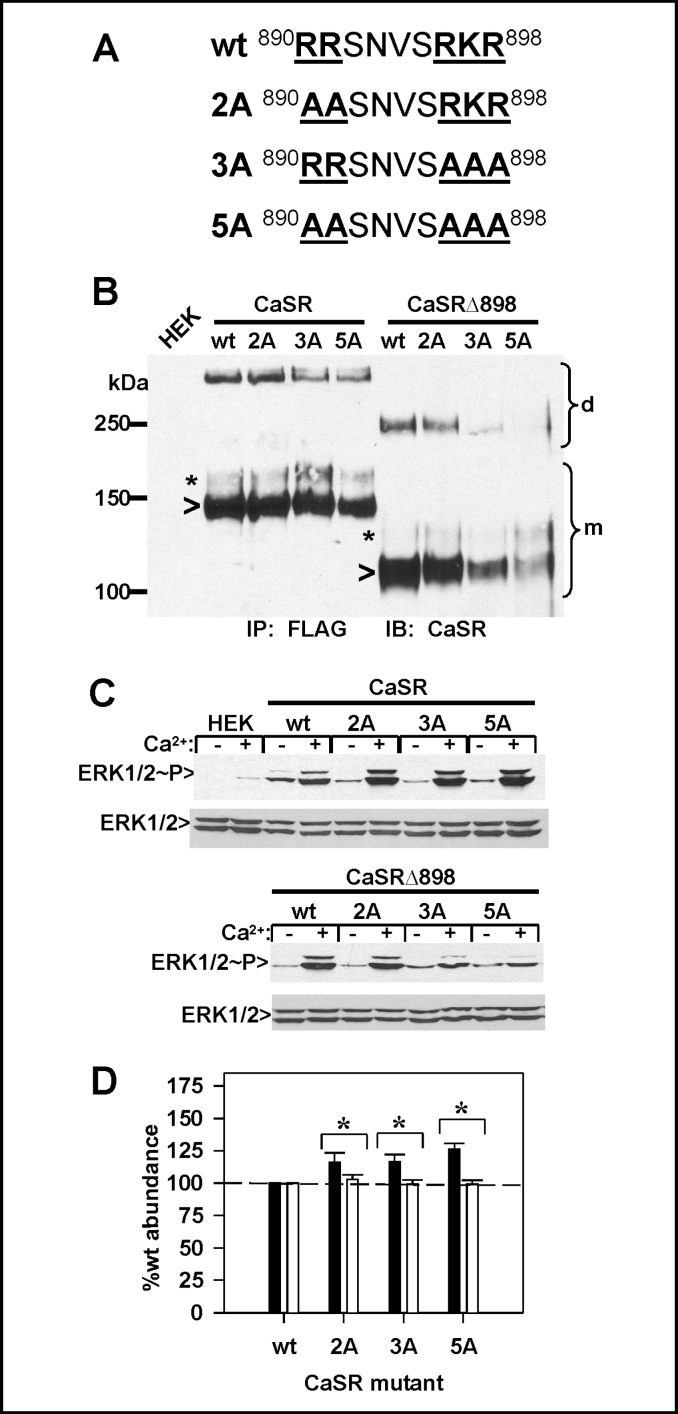
Truncations of the CaSR carboxyl terminus suggest that the proximal arginine-rich region contributes to regulation of plasma membrane CaSR abundance. Because truncations may remove distal modulatory elements which may control the function of the arginine rich region, we generated arginine/lysine to alanine mutations in the region from R890 through R898, including R890A/R891A (CaSR(2A)), R896A/K897A/R898A (CaSR(3A)) and R890A/R891A/R896A/K897A/R898A (CaSR(5A)). The locations of the mutations in the extended arginine-rich region are indicated in Fig. 3A. A similar series of mutants were generated in the background of the CaSRΔ898 truncation. We first examined the expression of the mutants by immunoprecipitation and western blotting, Fig. 3B. Truncation of CaSR at residue 898 reduces the masses of both immature and maturely glycosylated forms by ≈20 kDa. As illustrated on the blot, full length CaSR bearing the 2A, 3A or 5A mutations is expressed at levels similar to wt CaSR, while successive mutation of the arginine-rich motif in the CaSRΔ898 background causes a significant reduction in total expression. Because the arginine-rich region in the proximal carboxyl terminus is close to sequences critical to CaSR function [13, 18, 19], we determined whether the motif mutants were able to activate ERK1/2 phosphorylation in response to 5 mM extracellular Ca2+. Fig. 3C illustrates that in the full length CaSR background, the 2A, 3A and 5A mutants are more active that wt CaSR in mediating ERK1/2 phosphorylation, consistent with the increased amount of mature receptor observed on the western blot (Fig. 3B). In the CaSRΔ898 background, however, the 2A mutant had activity comparable to wt CaSRΔ898, while the 3A and 5A mutants had significantly reduced activities (Fig. 3C), likely due to reduced expression levels (Fig. 3B).
Fig. 3.
Disruptions of arginine-rich region motifs alter CaSR plasma membrane abundance. A. Arginine to alanine mutations were generated in the arginine-rich region (R890A/R891A = 2A; R896A/K897A/R898A = 3A; and R890A/R891A/R896A/ K897A/R898A = 5A) in either full length CaSR or the CaSRΔ898 truncation as described in Methods. B. HEK293 cells transfected with the FLAG-tagged constructs of arginine-rich region mutants were immunoprecipitated with anti-FLAG antibody, and blots were probed with anti-CaSR LRG antibody as described in Methods. Monomer (m) and dimer (d) regions of the blot are indicated. C. HEK293 cells were transfected with full length CaSR, CaSRΔ898 or arginine mutants and stimulated with 5 mM extracellular Ca2+ for 10 min as described in Methods. Lysates were run on 4-15 % gradient gels, blotted to nitrocellulose, probed for phosphorylated ERK1/2, stripped, and reprobed for total ERK. D. Arginine mutants (2A, 3A, 5A) of full length CaSR were expressed in HEK293 cells and assayed by ELISA at 3 days after transfection. All data were normalized to the abundance of wt CaSR under comparable fixation conditions, as described in Fig. 1 and Methods. Black bars, plasma membrane abundance; white bars, total abundance; dashed line indicates wt CaSR (= 100%). Results are average ± S.E.M. Significant differences between plasma membrane or total abundance for a given receptor mutant are indicated (* p<0.05).
Both the immunoprecipitation results of Fig. 3B and the ERK1/2 phosphorylation assay of Fig. 3C suggest that CaSR mutants 2A, 3A and 5A may have increased plasma membrane abundance. We explored this possibility directly using ELISA assays of transiently transfected HEK293 cells. Fig. 3D illustrates the results, analyzed as described for Fig. 1C, i.e., both plasma membrane (black bars) and total (open bars) abundance of CaSR and mutants were normalized to respective wt CaSR values (dotted lines). All motif mutants in the full length CaSR background, 2A, 3A and 5A, were increased at the plasma membrane relative to wt CaSR, thus the individual motifs within the arginine-rich region between R890 and R898 contribute to the intracellular retention of CaSR. Comparable studies were not done with the CaSRΔ898 mutants, since western blots (Fig. 3B) indicate drastically reduced abundance.
A single arginine-rich region per CaSR dimer is sufficient for intracellular retention
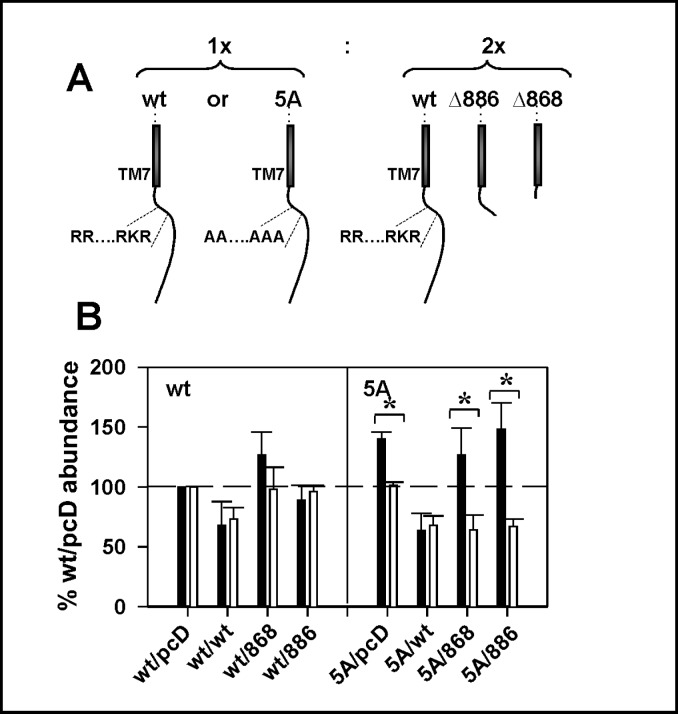
While the combined results of Fig. 1 through 3 argue that elimination or mutation of part or all of the argininerich region increases plasma membrane abundance of the resulting mutants, the effects are modest, i.e., at most ≈25-50% increase in plasma membrane CaSR. Since CaSR is a disulfide-linked dimer, these results are consistent with the notion that the arginine-rich region may represent a “quality control” signal which results in retention of only incorrectly folded or dimerized receptors in which the motif(s) are exposed. We tested this hypothesis using ELISA assays to quantify plasma membrane and total abundance of FLAG-CaSR or FLAG-CaSR(5A) when they were expressed as heterodimers with CaSR truncations which eliminate the arginine-rich region. To drive heterodimerization, FLAG-CaSR or FLAG-CaSR(5A) were cotransfected at a 1:2 ratio with the test cDNAs (CaSR, CaSRΔ868 or CaSRΔ886-EGFP), as illustrated in Fig. 4A. The assay quantified only FLAG-CaSR or FLAG-CaSR(5A) plasma membrane or total abundance, since cotransfected cDNAs did not contain the FLAG epitope. Regardless of cotransfection partner, FLAG-CaSR showed no significant increase in plasma membrane relative to total abundance (Fig. 4B, left), suggesting that a single exposed arginine-rich region was sufficient for normal trafficking of CaSR. FLAG-CaSR(5A) plasma membrane abundance was increased relative to total abundance, (labeled 5A/pcDNA, Fig. 4B, right), as was seen in Figure 3D. Interestingly, co-expression with wt CaSR was able to attenuate plasma membrane relative to total abundance, again suggesting that a single arginine-rich region on one of the monomers in the dimer is sufficient to confer the normal trafficking phenotype. Heterodimers with no arginine-rich regions, obtained upon coexpression of FLAG-CaSR(5A) with either CaSRΔ868 or CaSRΔ886-EGFP, have significantly enhanced plasma membrane relative to total abundance (Fig. 4B, right). These results provide unequivocal support for a model in which a single exposed arginine-rich region within the context of the dimer is sufficient for retention/ retrieval of receptors.
Fig. 4.
A single arginine-rich domain is sufficient for retention equivalent to wt CaSR. A. wt CaSR or CaSR(5A) were coexpressed at a 1:2 cDNA ratio with pcDNA3.1, wt CaSR, CaSRΔ868 or CaSRΔ886-EGFP to facilitate heterodimerization. B. ELISA assays were performed on day 3 after transfection as described in A, and plasma membrane (black bars) and total (white bars) protein abundance were expressed relative to wt CaSR under the same fixation conditions (=100%). Left portion of graph indicates wt CaSR and right portion indicates CaSR(5A) mutant experiments. Results are average ± S.E.M. for 3-5 experiments. Significant differences between plasma membrane and total protein abundance for a given receptor mutant are indicated (* p<0.05).
Regulation of arginine-rich region-mediated retention by phosphorylation
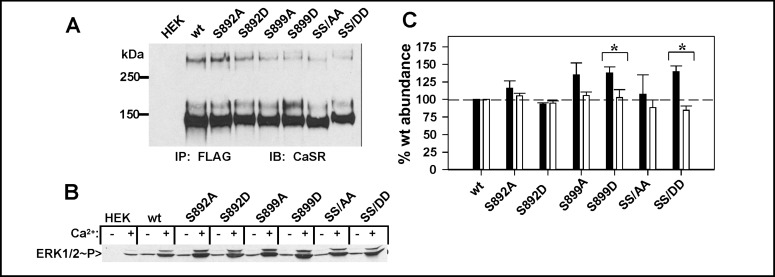
The arginine-rich region is flanked by two phosphorylation sites, S892 and S899. S892 is a canonical PKC phosphorylation site, conserved in mammals but not in fish, while S899 is a PKA site conserved throughout evolution. To determine whether phosphorylation at either site regulates recognition or utilization of the argininerich region, we mutated both residues individually or in combination to alanine to eliminate phosphorylation or aspartic acid to mimic phosphorylation. First, we examined expression of the mutants by immunoprecipitation, illustrated in Fig. 5A. All mutants expressed at levels comparable to wt CaSR, although the S899D mutant had a stronger band at ≈160 kDa, consistent with enhanced maturation and surface localization. Because phosphorylation at a nearby protein kinase C-specific site, T888, influences CaSR activation and signaling [18, 20], we determined whether the S892 or S899 mutants affected CaSR-mediated activation of ERK1/2 phosphorylation. As illustrated in Fig. 5B, all mutants were capable of mediating ERK1/2 phosphorylation in response to 5 mM extracellular Ca2+ to levels equal to or greater than wt CaSR. We therefore used ELISA assays with anti-FLAG antibody to examine the plasma membrane versus total abundance of each mutant relative to wt CaSR, Fig. 5C. Significant effects on plasma membrane abundance relative to totals were observed for the S899D mutant (p<0.05) but not for the S892A or S892D mutants. The effects of S899D were recapitulated in the double phosphomimic mutant, CaSR(SS/DD), while CaSR(SS/ AA) had no effect. These results suggest that phosphorylation at S899 increases CaSR at the plasma membrane and must therefore prevent recognition of the arginine-rich motif(s). Either disruption of the extended arginine-rich region by mutation(s) to alanine (Fig. 3) or the S899D mutation in the presence of an intact argininerich region (Fig. 5) leads to increased plasma membrane abundance of CaSR, confirming that the arginine-rich region represents a functionally important, phosphorylation-regulated retention motif.
Fig. 5.
Phosphorylation sites flanking the arginine-rich region regulate recognition. A. Serine to alanine or serine to aspartic acid mutants at residues S892 or S899 or both (SS/AA or SS/DD) were transfected in HEK293 cells and immunoprecipitated after 3 days using anti-FLAG antibody. Western blots were probed with anti-CaSR LRG antibody. B. ERK phosphorylation supported by wt CaSR or phosphorylation site mutants. HEK293 cells expressing wt CaSR or S892 or S899 mutants were stimulated with 5 mM extracellular Ca2+ for 10 min and lysates were analyzed for ERK1/2 phosphorylation as described in Methods. C. ELISA assay performed as described in Methods and Figure 1 after 3 days of transfection for phosphorylation site mutants described in A. Plasma membrane (black bars) and total (white bars) protein abundance relative to wt CaSR under the same fixation conditions (wt CaSR = 100%, dashed line) are plotted. Results are average ± S.E.M. of 5-7 independent experiments. Significant differences between surface and total protein expression for a given receptor mutant are indicated (* p<0.05).
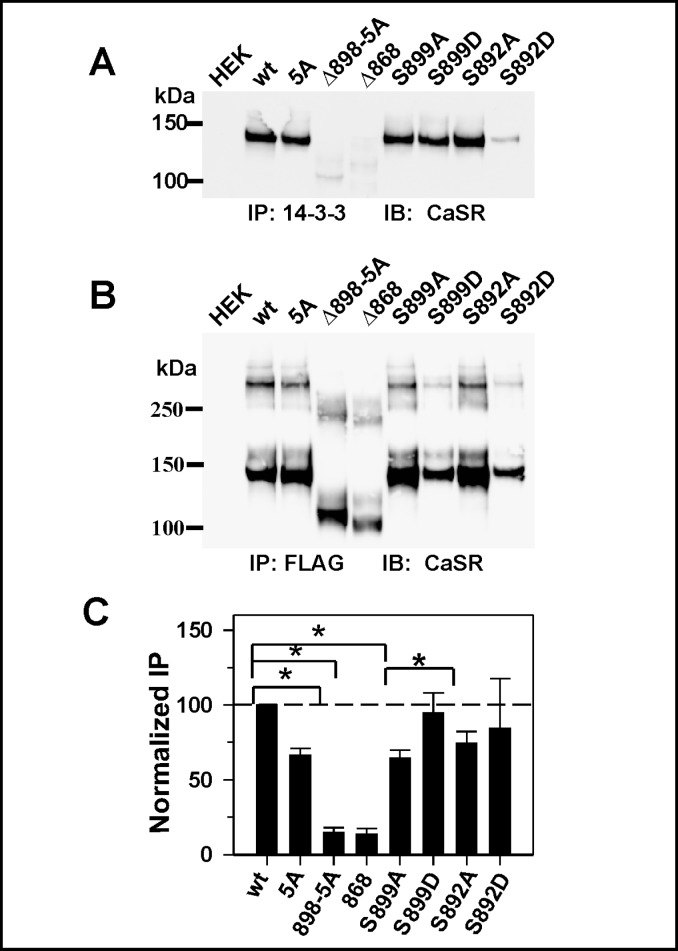
14-3-3 proteins interact with CaSR via the arginine-rich region
14-3-3 proteins have been implicated in the phosphorylation-regulated release of RXR motif-containing membrane proteins from the ER [e.g., 21, 22]. Here we determined whether CaSR interacts with 14-3-3 proteins through the arginine-rich region, and whether the interaction is modulated by phosphorylation at flanking sites. Fig. 6A illustrates the results of immunoprecipitation of endogenous 14-3-3 proteins from HEK293 cells using a pan-14-3-3 antibody, and probing the blot with anti-CaSR antibody. We compared wt CaSR, CaSR(5A), the CaSRΔ898(5A) and CaSRΔ868 truncations. Of note is that CaSR coimmunoprecipitating with anti-14-3-3 antibody is the immature form, ≈140 kDa (Fig. 6A), which suggests CaSR and 14-3-3 proteins interact at the ER and do not remain in a stable complex throughout the trafficking to the plasma membrane. On first inspection, elimination of the arginine-rich region does not appear to significantly decrease the interaction (Fig. 6A). However, the expression levels of the various mutants varied significantly, and we therefore performed a parallel immunoprecipitation of the same samples with anti-FLAG antibody to assess total CaSR or mutant expression (Fig. 6B). To quantify coprecipitation, we calculated the ratio of CaSR immunoreactivity precipitated by anti-14-3-3 antibody to CaSR immunoreactivity precipitated by anti-FLAG antibody, and normalized that to the ratio obtained for wt CaSR. As illustrated in Fig. 6C, the arginine-rich region mutant (5A) exhibited significantly lower coimmunoprecipitation with 14-3-3 proteins than wt CaSR (≈45%, *p<0.05). The CaSR carboxyl terminus distal to R898 may contain additional binding site(s) for 14-3-3 proteins, since the truncation mutant CaSRΔ898(5A) shows binding of 14-3-3 equivalent to background, as does CaSRΔ868. The current results suggest that the argininerich region represents a bona fidé site mediating 14-3-3 interactions. Since phosphorylation modulates recognition of the arginine-rich region (Fig. 5), we determined whether mutations at S892 or S899 affected CaSR interaction(s) with 14-3-3 proteins. We compared the normalized immunoprecipitation of S892A/D and S899A/ D (Fig. 6C). The phosphorylation mutants showed greater variability in coimmunoprecipitation (large standard deviations), but there was significantly more coimmunoprecipitation of CaSR with 14-3-3 proteins for the S899D mutant than the S899A mutant, while there were no significant differences between the S892A and S892D mutants. The combined results of Fig. 5 and 6 suggest that the protein kinase A consensus phosphorylation site S899 is involved in regulation of the recognition of the arginine-rich region.
Fig. 6.
The immature form of CaSR coimmunoprecipitates with 14-3-3 proteins. HEK293 cells transiently expressing wt CaSR, CaSR(5A), or truncations CaSRΔ898(5A) or CaSRΔ868, or the phosphorylation site mutants, S892A/D and S899A/D were subjected to immunoprecipitation with anti-14-3-3 antibody (A) or anti-FLAG antibody (B) as described in methods. Blots from both immunoprecipitations were probed with anti-CaSR LRG antibody or anti-CaSR ADD antibody. C. CaSR immunoreactivity from anti-14-3-3 blot was normalized to that of the same sample from the anti-FLAG blot and plotted as % of wt CaSR. Results are average ± S.E.M. of 3-5 independent transfections.
Discussion
In this report we have identified a physiologically important arginine-rich region, containing both RR and RXR motifs, in the CaSR proximal carboxyl terminus, which contributes to regulation of plasma membrane targeting. Mutations in the motif increase plasma membrane relative to total receptor abundance, suggesting that the arginine-rich region mediates retention in intracellular compartment(s), most likely the ER. Both motifs have been shown to mediate retention and/or retrieval of membrane proteins to the ER, particularly for multisubunit receptors or channels where they can act as sensors of multimeric assembly [23]. GPCRs use such retention/retrieval motifs as either dimer or folding sensors, allowing release from the ER of only properly folded and dimerized receptors [reviewed in 24, 25]. For wild type receptors therefore, the motifs do not act in an all-or-none fashion, but rather result in retention of only misfolded or improperly assembled receptors [25]. A prime example of this type of quality control is the vasopressin V2 receptor, which is targeted to the plasma membrane despite the presence of an RXR motif in the third intracellular loop, suggesting that the motif is only exposed in receptor mutants [26]. GABAB1 receptors, on the other hand, are retained in the ER through an RXR motif in the carboxyl terminus [27]. Shielding of the motif upon heterodimerization with GABAB2 receptors makes the motif on GABAB1 receptors unavailable to the retention/ retrieval machinery, releasing only heterodimers to the plasma membrane [27]. The arginine-rich region of wt CaSR is a modulatory site. Disruption of the argininerich region results in ≈40-50% increase in plasma membrane-localized CaSR. The presence of a single motif within the context of the CaSR dimer is sufficient to invoke intracellular retention equivalent to wt CaSR. These results are consistent with the possibility that the carboxyl termini of the monomers in the dimer are independently shielded by interaction with the core transmembrane domain in properly folded receptors and/or by specific interacting proteins. Alternatively, higher order interactions among CaSR dimers or phosphorylation-regulated protein interactions may shield the arginine-rich region from the retention machinery. Additional approaches will be required to resolve this issue.
The arginine-rich domain of CaSR is flanked by PKC (S892) and PKA (S899) phosphorylation sites, suggesting that recognition of the arginine-rich domain may be regulated by cellular signaling. Complex regulation by flanking PKA/PKC sites has been demonstrated for NMDA receptors in neurons [28], requiring both signaling pathways to be activated for efficient trafficking to the plasma membrane. Here we demonstrate that the S899 consensus site for PKA-mediated phosphorylation is critical for increasing CaSR abundance at the plasma membrane. PKA inhibition has only minor effects on CaSR-mediated signaling at the plasma membrane [29], suggesting that the primary role for PKA-mediated phosphorylation may be in CaSR trafficking rather than function. Plasma membrane-localized CaSR couples to both Gαq- and/or Gαi-mediated pathways, depending upon cell type [30]. Global increases in cellular cAMP may stimulate trafficking of nascent CaSR through the secretory pathway, to ultimately increase plasma membrane CaSR and moderate cAMP levels. Alternatively, a number of adenylyl cyclases are sensitive to Ca2+ (AC1, AC8 and perhaps AC3 are stimulated by Ca2+/calmodulin; AC5 and AC6 are inhibited by Ca2+) [31]. CaSR trafficking to the plasma membrane may be modulated by the balance of stimulatory and inhibitory inputs to adenylyl cyclase, perhaps coupled to subcellular targeting of PKA to the ER by A-kinase anchoring proteins [32, 33]. Finally, CaSR has recently been shown to couple to Gαs in malignant breast epithelial cells [34] and normal pituitary cells [35], suggesting that CaSR activation may regulate its own release from the ER. The signaling pathways and subcellular localization of protein complexes which regulate PKA-mediated trafficking of CaSR remain to be determined but may represent a novel locus for intersection of Ca2+ and cAMP signaling pathways.
Phosphorylation-dependent binding of dimeric 14-3-3 proteins has been shown to mediate release from the ER of membrane proteins containing arginine-rich motifs [21]. The general model posits that COPI subunits recognize exposed arginine-rich motifs and retrieve proteins to the ER. Phosphorylation at flanking sites facilitates binding of dimeric 14-3-3 proteins to the arginine-rich motifs and, by competition, results in release of membrane proteins to the Golgi and subsequently the plasma membrane [21, 22]. Here we demonstrate the importance of the arginine-rich motif in the CaSR carboxyl terminus for interaction with 14-3-3 proteins, since anti-14-3-3 antibody specifically precipitates the immature form of CaSR. Disruption of the motif by mutation or truncation reduces or eliminates 14-3-3 coprecipitation and enhances plasma membrane abundance of CaSR. Several aspects of 14-3-3 interactions with CaSR are therefore contrary to the developing model for 14-3-3-mediated forward trafficking of membrane proteins, i.e., interaction with 14-3-3 proteins causes retention rather than release of CaSR and further, phosphorylation at a flanking site, S899, does not alter 14-3-3 interactions but does foster forward movement of CaSR to the plasma membrane. Further studies are required to determine whether CaSR interacts directly with 14-3-3 proteins or is targeted by a bridging protein interaction to a larger complex containing 14-3-3 proteins. The physiological role of such a complex in the ER is also an open question. Nevertheless, the current data highlight the importance of the arginine-rich region in formation of a complex which controls release of CaSR from the ER, and which fosters interaction with 14-3-3 proteins.
There is ample evidence for intracellular retention of endogenously expressed CaSR in many cell types, including pancreatic and renal epithelial cells, keratinocytes and neurons [36, 37, 38, 39, 40, 41]. Exit from the ER may therefore be a rate limiting step in CaSR transit to the plasma membrane in vivo as well as in heterologous cells. Many loss-of-function mutations in CaSR prevent trafficking of the receptor to the plasma membrane [42] thereby causing a reduction in CaSR signaling capacity. Gain-of-function mutations are less common, but may produce their phenotype as a result of an intrinsic increase in agonist affinity and/or signaling efficacy or an increase in the number of receptors at the plasma membrane [43, 44]. Here we demonstrate the latter. Both the R896H and R898Q mutations were identified in patients with unconventional diagnoses with respect to CaSR mutations, i.e. chronic pancreatitis [6] or idiopathic epilepsy [7], respectively. Our results suggest that the mutations disrupt the distal RKR motif of the arginine-rich region, and increase plasma membrane targeting of the mutated receptors, consistent with a gain-of-function phenotype. This conclusion is corroborated by the larger deletion mutation, from S895 to V1075, which was identified in patients with ADH [11, 12]. These results support the physiological relevance of the RKR motif, within the context of the larger arginine-rich region, in the modulation of plasma membrane CaSR expression. The remaining challenge is to characterize the cellular signaling events which regulate CaSR targeting to the plasma membrane.
Acknowledgements
We thank Drs. Alice Cavanaugh and Michael Grant for helpful comments. Supported by the Geisinger Clinic, Weis Center for Research Summer Internship Program (O.M.), and NIH GM077563 and funds from the Geisinger Clinic (G.E.B.).
References
- 1.Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. doi: 10.1007/978-1-4020-6191-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Bruce JI, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 3.Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2009;10:63–87. doi: 10.1146/annurev-genom-082908-150009. [DOI] [PubMed] [Google Scholar]
- 4.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–424. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 5.Muddana V, Lamb J, Greer JB, Elinoff B, Hawes RH, Cotton PB, Anderson MA, Slivka A, Whitcomb DC. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor Kazal 1type and alcohol. World J Gastroenterol. 2008;14:4486–4491. doi: 10.3748/wjg.14.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felderbauer P, Klein W, Bulut K, Ansorge N, Dekomien G, Werner I, Epplen JT, Schmitz F, Schmidt WE. Mutations in the calcium-sensing receptor: A new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006;41:343–348. doi: 10.1080/00365520510024214. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor A, Satishchandra P, Ratnapriya R, Reddy R, Kadandale J, Shankar SK, Anand A. An idiopathic epilepsy syndrome linked to 3q13.3-q21 and missense mutation in the extracellular calcium sensing receptor gene. Ann Neurol. 2008;64:158–167. doi: 10.1002/ana.21428. [DOI] [PubMed] [Google Scholar]
- 8.Pdasheva S, D'Souza-Li L, Canaff L, Cole DE, Hendy GN. CASRdb: calcium-sensing receptor locus-specific database for mutations causing familial (benign) hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2004;24:107–111. doi: 10.1002/humu.20067. [DOI] [PubMed] [Google Scholar]
- 9.Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ. Familial isolated hyperparathyroidism. Clinical and genetic characteristics of 36 kindreds. Medicine. 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Nissen PH, Christensen SE, Heickendorff L, Brixen K, Mosekilde L. Molecular genetic analysis of the calcium sensing receptor gene in patients clinically suspected to have familial hypocalciuric hypercalcemia: Phenotypic variation and mutation spectrum in a Danish population. J Clin Endocrinol Metab. 2007;92:4373–4379. doi: 10.1210/jc.2007-0322. [DOI] [PubMed] [Google Scholar]
- 11.Lienhardt A, Garabédian M, Bai M, Sinding C, Zhang Z, Lagarde J-P, Boulesteix J, Rigaud M, Brown EM, Kottler M-L. A large homozygous or heterozygous in-frame deletion within the calcium-sensing receptor's carboxylterminal cytoplasmic tail that causes autosomal dominant hypocalcemia. J Clin Endocrinol Metab. 2000;85:1695–1702. doi: 10.1210/jcem.85.4.6570. [DOI] [PubMed] [Google Scholar]
- 12.Lienhardt A, Bai M, Lagarde J-P, Rigaud M, Zhang Z, Jiang Y, Kottler M-L, Brown EM, Garabédian M. Activating mutations of the calcium-sensing receptor: Management of hypocalcemia. J Clin Endocrinol Metab. 2001;86:5313–5323. doi: 10.1210/jcem.86.11.8016. [DOI] [PubMed] [Google Scholar]
- 13.Gama L, Breitwieser GE. A carboxyl-terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem. 1998;273:29712–29718. doi: 10.1074/jbc.273.45.29712. [DOI] [PubMed] [Google Scholar]
- 14.Ray K, Fan G-F, Goldsmith PK, Spiegel AM. The carboxyl terminus of the human calcium sensing receptor. Requirements for cell-surface expression and signal transduction. J Biol Chem. 1997;272:31355–31361. doi: 10.1074/jbc.272.50.31355. [DOI] [PubMed] [Google Scholar]
- 15.Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 16.Ray K, Clapp P, Goldsmith PK, Spiegel AM. Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem. 1998;273:34558–34567. doi: 10.1074/jbc.273.51.34558. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Breitwieser GE. High affinity interaction with filamin A protects against calcium sensing receptor degradation. J Biol Chem. 2005;280:11140–11146. doi: 10.1074/jbc.M412242200. [DOI] [PubMed] [Google Scholar]
- 18.Chang W, Pratt S, Chen T-H, Bourguignon L, Shoback D. Amino acids in the cytoplasmic C terminus of the parathyroid Ca2+-sensing receptor mediate efficient cell-surface expression and phospholipase C activation. J Biol Chem. 2001;276:44129–44136. doi: 10.1074/jbc.M104834200. [DOI] [PubMed] [Google Scholar]
- 19.Miedlich S, Gama L, Breitwieser GE. Calcium sensing receptor activation by a calcimimetic suggests a link between cooperativity and intracellular calcium oscillations. J Biol Chem. 2002;277:49691–49699. doi: 10.1074/jbc.M205578200. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y-F, Zhang Z, Kifor O, Lane CR, Quinn SJ, Bai M. Protein kinase C (PKC) phosphorylation of the Ca2+-sensing receptor (CaR) modulates functional interaction of G proteins with the CaR cytoplasmic tail. J Biol Chem. 2002;277:50543–50549. doi: 10.1074/jbc.M205798200. [DOI] [PubMed] [Google Scholar]
- 21.O'Kelly I, Butler MH, Zilberberg N, Goldstein SAN. Forward transport: 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- 22.Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 23.Mrowiec T, Schwappach B. 14-3-3 proteins in membrane protein transport. Biol Chem. 2006;387:1227–1236. doi: 10.1515/BC.2006.152. [DOI] [PubMed] [Google Scholar]
- 24.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermosilla R, Ouestlati M, Donalies U, Schonenberg E, Krause E, Oksche A, Rosenthal W, Schulein R. Disease-causing V2 vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 27.Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prezeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation supresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacol. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 29.Bösel J, John M, Freichel M, Blind E. Signaling of the human calcium-sensing receptor expressed in HEK293-cells is modulated by protein kinases A and C. Exp Clin Endocrinol Diabetes. 2003;111:21–26. doi: 10.1055/s-2003-37496. [DOI] [PubMed] [Google Scholar]
- 30.Brennan SC, Conigrave AC. Regulation of cellular signal transduction pathways by the extracellular calcium-sensing receptor. Curr Pharm Biotechnol. 2009;10:270–281. doi: 10.2174/138920109787847484. [DOI] [PubMed] [Google Scholar]
- 31.Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 32.Huang LJ, Wang L, Ma Y, Durick D, Perkins G, Deerinck TJ, Ellisman MH, Taylor SS. NH2-terminal targeting motifs direct dual specificity A-Kinase-anchoring Protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J Cell Biol. 1999;145:951–959. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 34.Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamillapalli R, Wysolmerski J. The calcium-sensing receptor couples to Galpha(s) and regulates PTHrP and ACTH secretion in pituitary cells. J Endocrinol. 2010;204:287–297. doi: 10.1677/JOE-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chattopadhyay N, Légrádi G, Bai M, Kifor O, Ye C, Vassilev PM, Brown EM, Lechan RM. Calcium-sensing receptor in the rat hippocampus: a developmental study. Dev Brain Res. 1997;100:13–21. doi: 10.1016/s0165-3806(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 37.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/ polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 38.Bruce JIE, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 39.Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflugers Arch. 2000;441:379–387. doi: 10.1007/s004240000436. [DOI] [PubMed] [Google Scholar]
- 40.Tu C-L, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J Invest Dermatol. 2007;127:1074–1083. doi: 10.1038/sj.jid.5700633. [DOI] [PubMed] [Google Scholar]
- 41.Vizard TN, O'Keeffe GW, Gutierrez H, Kos CH, Riccardi D, Davies AM. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat Neurosci. 2008;11:285–291. doi: 10.1038/nn2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White E, McKenna J, Cavanaugh A, Breitwieser GE. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol. 2009;23:1115–1123. doi: 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 44.Vezzoli G, Terranegra A, Arcidiacono R, Biasion R, Coviello D, Syren ML, Paloschi V, Giannini S, Mignogna G, Rubinacci A, Ferraretto A, Cusi D, Gianchi G, Soldati L. R990G polymorphism of calcium-sensing receptor does produce a gain-of-function and predispose to primary hypercalciuria. Kid Int. 2007;71:1155–1162. doi: 10.1038/sj.ki.5002156. [DOI] [PubMed] [Google Scholar]