Abstract
The maintenance of cell volume homeostasis is critical for preventing pathological cell swelling that may lead to severe cellular dysfunction or cell death. Our earlier studies have shown that volume-regulated anion channels that play a major role in the regulation of cell volume are facilitated by a decrease in cellular cholesterol suggesting that cholesterol depletion should also facilitate regulatory volume decrease (RVD), the ability of cells to recover from hypotonic swelling. In this study, we test this hypothesis using a novel methodology developed to measure changes in cell volume using a microfluidics chamber. Our data show that cholesterol depletion of Chinese Hamster Ovary (CHO) significantly facilitates the recovery process, as is apparent from a faster onset of the RVD (162±10 s. vs. 114±5 s. in control and cholesterol depleted cells respectively) and a higher degree of volume recovery after 10 min of the hypotonic challenge (41%±6% vs. 65%±6% in control and cholesterol depleted cells respectively). In contrast, enriching cells with cholesterol had no effect on the RVD process. We also show here that similarly to our previous observations in endothelial cells, cholesterol depletion significantly increases the stiffness of CHO cells suggesting that facilitation of RVD may be associated with cell stiffening. Furthermore, we also show that increasing cell stiffness by stabilizing F-actin with jasplakinolide also facilitates RVD development. We propose that cell stiffening enhances cell mechano-sensitivity, which in turn facilitates the RVD process.
Key Words: Cell volume regulation, Regulatory volume decrease, Cholesterol, Cell stiffness, Microfluidics chamber for cell volume measurement
Introduction
The maintenance of cell volume homeostasis is critical for preventing pathological cell swelling that may lead to severe cellular dysfunction or cell death. In mammalian tissues, the osmotic environment is normally maintained within a narrow range but several pathological conditions, such as hypoxia, ischemia, hyponatremia and diabetic acidosis may create significant osmotic stresses [1, 2, 3]. Most cell types, therefore, developed mechanisms that prevent excessive swelling and maintain cell volume within a normal range. A recovery from cell swelling termed Regulatory Volume Decrease (RVD) is typically mediated by swelling-activated ion channels, that allow the osmolytes to flow out of the cell reducing the osmotic gradient across the cell membrane and allowing the cell volume to return to normal (reviewed by [4, 5, 6]). Our studies focus on understanding how cell volume regulation is affected by changes in the level of membrane cholesterol.
Cholesterol is one of the major lipid components of the plasma membrane in all mammalian cells and is essential for cell function and growth [7, 8]. It is also well known that changes in the levels of cellular cholesterol are associated with multiple diseases, most notably with the development of cardiovascular disease and diabetes. The basis for both cholesterol requirement and its cytotoxicity is its ability to alter the function of integral membrane proteins. Our earlier studies have shown that membrane cholesterol regulates the activity of volume-regulated anion channels (VRAC) [9, 10, 11], one of the main mechanisms of cell volume recovery after a hypotonic shock [4, 6]. Specifically, we have shown that cholesterol depletion significantly enhances the activity of VRAC in aortic endothelial cells whereas cholesterol enrichment has the opposite effect [9, 10]. Cholesterol depletion was also shown to facilitate VRAC in Ehrlich-Lettre ascites [12]. Furthermore, we have also shown that cholesterol depletion increases cellular stiffness and facilitates membrane-cytoskeleton adhesion in endothelial cells [13, 14] with a corresponding increase in VRAC activity [11]. These observations suggested that cholesterol depletion and an associated increase in cell stiffness should also facilitate the RVD process.
The main constraint in testing this prediction earlier, however, was a lack of available techniques to accurately measure real-time cell volume changes in substrate-attached cells. Indeed, until recently the two main experimental approaches to estimate dynamic changes in cell volume were based either on flow cytometry or fluorescent/confocal microscopy. The main constraint of the first approach, however, is that while it provides an excellent tool to measure cell volumes of cells in suspension, it cannot be used for substrate-attached cells without detaching them from the substrate, which clearly may have a major impact on the mechanisms of cell volume regulation. Optical methods to estimate cell volumes are based either on 3D reconstruction of confocal images, a method that is not very sensitive to small changes in cell height, or on changes of fluorescence intensity of fluorescent dyes as they get diluted during cell swelling, a method also of limited sensitivity. In this study, we use a novel methodology developed recently by Hua and colleagues to measure changes in cell volume of substrate-attached cells using a microfluidics chamber [15, 16, 17]. The main principle of this method is that when cells swell within a microchannel filled with an electrolyte solution, there is a matching reduction in the volume of electrolyte solution available for current flow which can be measured as a resistance increase under constant current conditions. The device has been characterized in previous studies demonstrating that it is sensitive to relatively small changes in cell volume in a precise and reproducible way [15, 16] and that the development of the RVD response can be suppressed by blocking mechanosensitive ion channels in several cell types [17]. In this study, we show that cholesterol depletion strongly facilitates the RVD process of Chinese Hamster Ovary (CHO) cells and that this effect is associated with an increase in cell stiffness. Furthermore, we also show that increasing cell stiffness by stabilizing F-actin also facilitates RVD. These studies support the hypothesis that cell stiffness plays an important role in cell volume regulation.
Materials and Methods
Cells and solutions
Chinese hamster ovary (CHO) K1 cell line was maintained at 37°C in a humidified 5% CO2 atmosphere in HAM's F-12 media (Gibco Invitrogen) supplemented with heat-inactivated 10% fetal bovine serum (Gemini BioProducts, Woodland, CA). Cells were fed or split every 2-3 days. Hypotonic solutions were prepared by diluting no serum F12 medium by 5%, 10% or 30% with distilled water. Isotonic solutions were prepared by supplementing the appropriate hypotonic solutions with sucrose to generate a solution of 300 mOsm while maintaining similar ionic strengths in hypotonic and isotonic solutions. Osmolarities of all the solutions were measured routinely by a VaPro Vapor Pressure Osmometer 5520 WESCOR device. To modulate cellular cholesterol, cells were incubated with 5 mM of MβCD or MβCD saturated with cholesterol for 1 hour. MβCD and cholesterol were purchased from Sigma Chemical (St. Louis, MO).
Volume measurements and analysis
Cells were seeded on 22×22 mm 1.5 thickness glass coverslips coated with Poly-L-lysine and grown to 70-90% confluency. Then, a coverslip with attached cells was placed and locked on CVC7000 stage to form a ceiling of a sealed microfluidic channel of 15 µm height. After mounting on the stage, cells were first equilibrated with isotonic solution for 5-10 minutes to achieve a stable baseline of the channel resistance and then the solution was switched to hypotonic for 10 min and then returned to isotonic. The principles and the technical properties of the CVC device are described in detail by [15]. CVC7000 is controlled by Cell Volume Analyzer 3.0.0.135/NanoVol 3.0.194 software packages, running under Ubuntu Linux 8.04 (Canonical Ltd, Germany/England). All the experiments were carried out at room temperature. Changes in cell volume were quantified as following: Cell swelling was quantified as ΔRsw= MaxRsw − MinRI-H where ΔRsw is the change of resistance during the swelling phase, calculated as the difference between maximum value during swelling (MaxRsw), and minimum value during the Isotonic to Hypotonic solutions’ switch (MinRI-H); Volume recovery was calculated as ΔRrc= MaxRsw − MinRrc where ΔRrc is change of resistance during the recovery phase, calculated as a difference between maximum value during swelling (MaxRsw), and minimum value during the recovery phase (MinRrc); The recovery to swelling ratio was calculated as: Recovery Ratio = ΔRrc/ΔRsw. All measurements are in kOhms. Recovery value is calculated for the 10 minutes hypotonic challenge.
Microaspiration
Microaspiration of CHO was performed as described in our earlier study [11, 13]. Briefly, cells were detached from the substrate by washing them with 80 µM EDTA and exposed to 5mM MβCD solution in serum-free media or to serum-free media only for 1 hour, then the membranes were visualized with a 50 µM fluorescent membrane dye carbocyanide DiIC18 (DI, Molecular probes) and then aspirated using micropipettes with 3-5 µm outer diameter pulled from borosilicate glass capillaries (SG10 glass; Richland Glass, Richland, NJ). Negative pressure was applied to a pipette by a pneumatic transducer tester (BioTek Instruments, Winooski, VT, USA). The experiments were carried out at room temperature. F-actin was visualized with Rhodamine Phalloidin.
Results
Quantitative assessment of RVD using microfluidic chamber
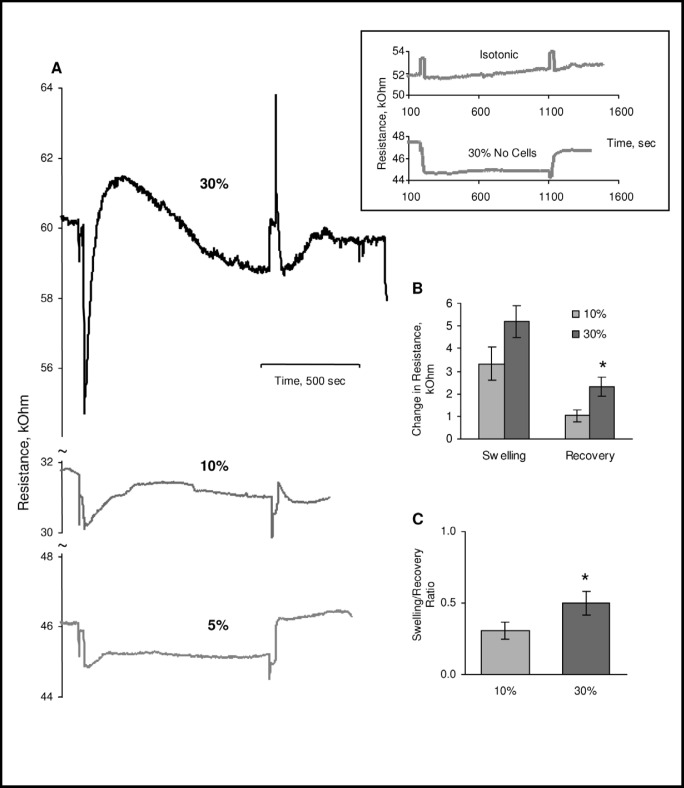
First, we tested the sensitivity of CHO cells to increased hypotonic challenge. Cells were challenged with osmotic gradients of 5%, 10% or 30% by decreasing the osmolarity of the extracellular medium while maintaining the ionic strength of the extracellular solutions. To achieve this goal, hypotonic solutions were prepared by diluting serum-free media (300 mOsm) by 5, 10 or 30% to make 285 mOsm, 270 mOsm and 210 mOsm solutions respectively and then each of these solutions was adjusted back to normal osmolarity (300 mOsm) by adding sucrose to create “ionic-strength matched” isotonic solutions for each of the hypotonic media. The rationale of this approach was to challenge the cells osmotically without dramatic changes of the ionic strength of the extracellular solution excluding the possibility that changes in ionic strength by itself might alter cell biomechanics and volume regulation. Thus, cells were first equilibrated with a “matched” isotonic solution and then challenged with a hypotonic solution of the same ionic strength.
Figure 1A shows the typical recordings of the RVD process in cells challenged with 5, 10 or 30% osmotic gradients. In all experiments, the recording starts at isosmotic conditions and then switched to hypotonic which is accompanied with a drop of the resistance that reflects the mobility of the ions, which increases when sucrose is removed from the solution. An increase in ion mobility corresponds to higher conductivity/lower resistance of the solution, as demonstrated by a trace recorded in the absence of cells (inset, lower trace). As expected, no changes in resistance are observed when cells are maintained under the isoosmotic conditions for the duration of the experiment (inset, upper trace). After the initial drop we see gradual increase in resistance that reflects cell swelling and decrease in the conductivity of the chamber and then gradual recovery that reflects the RVD process. When the solutions are switched back to isotonic conditions, typically we see an initial decrease in resistance relative to the baseline level which most likely reflects cell shrinkage due to the loss of osmolytes during cell swelling and which gradually recovers toward the baseline. As expected, challenging the cells with increased osmotic gradient results is stronger and faster swelling, as is apparent from larger increases in the measured resistance. Interestingly, the recovery is enhanced when cells are challenged with stronger osmotic gradient: while after 10% osmotic challenge cells recover by 31%±5%, after 30% challenge recovery increases to 42%±5% suggesting that the magnitude of the RVD is dependent on the magnitude of the hypotonic challenge (1C).
Fig. 1.
Sensitivity of CHO cells to osmotic challenge. A: Typical recordings of cell swelling and recovery in CHO cells challenged with 5%, 10% or 30% osmotic gradients. The inset shows a trace recorded when cells were maintained under isosmotic conditions for the duration of the experiment and a trace showing changes in the resistance during isotonic/hypotonic solution exchange in the absence of cells. The initial drop in the resistance corresponds to the change of the solutions from isotonic to hypotonic, the subsequent increase in resistance reflects cell swelling and a gradual decrease of resistance, volume recovery. In all the experiments, we used the same perfusion protocol (in case of cells maintained under isotonic conditions, solutions were switched without altering the osmolarity of the solutions and resistance peaks indicate the switching of the solutions). Please note that there is significant variability in the amplitude of the individual traces and representative traces may not match the average amplitude. B: Average degree of swelling and recovery in cells challenged with 10 and 30% osmotic gradients. The degree of swelling is estimated as the height of the resistance peak and the degree of recovery is estimated by the decrease in resistance at the end time-point of hypotonic exposure. C: Average Recovery Ratios defined as the ratios between swelling and recovery for the two osmotic gradients. Data presented as means±SEM (n=5-11).
Cholesterol depletion facilitates RVD: association between RVD and cell stiffness
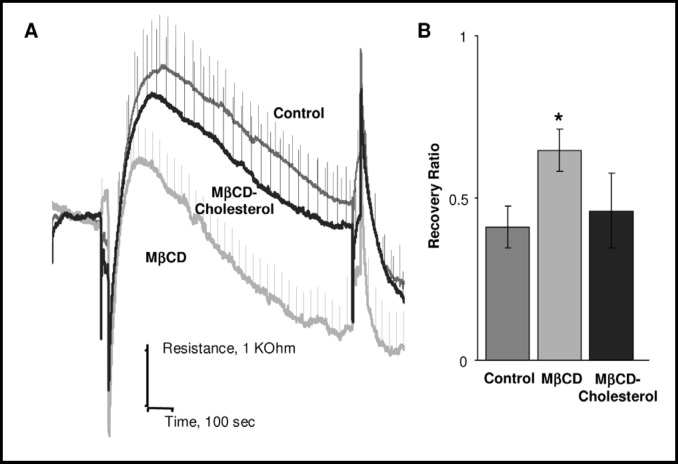
As described in our earlier studies, cholesterol levels in CHO cells were modulated by exposing the cells to 5 mM methyl-β-cyclodextrin (MβCD) or to MβCD saturated with cholesterol for 1 hour, resulting in ~50% decrease or increase in the level of membrane cholesterol, respectively [9, 18]. Figure 2 shows the impact of cholesterol on the time-courses of cell swelling and recovery in cells challenged with 30% osmotic gradient. Cells that were cholesterol depleted swell less (lower resistance peaks) and recover sooner (the peak of cell volume occurs at 162 s ±10 s in control cells at 114 s ±5 s in cholesterol-depleted cells, p<0.05) with a clearly higher rate of recovery (2A). As a result, recovery ratio increases from 41%±5% in control cells to 65%±6% in cholesterol depleted cells (p<0.05) (2B). A similar but less pronounced trend was observed when cells were challenged with 10% osmotic gradient: the recovery ratio increased from 31%±5% to 48%±6%. In contrast, cholesterol enrichment had no effect on the kinetics of cell swelling nor on the extent of RVD.
Fig. 2.
Facilitation of cell volume-recovery by cholesterol depletion. A: Average time courses of cell swelling and recovery in response to 30% osmotic gradient in control cells, cells depleted of cholesterol and cells enriched with cholesterol. B: Average recovery ratios for the same populations of cells. Both in the time-course and for the bar graph, the data are presented as means±SEM (n=6-10).
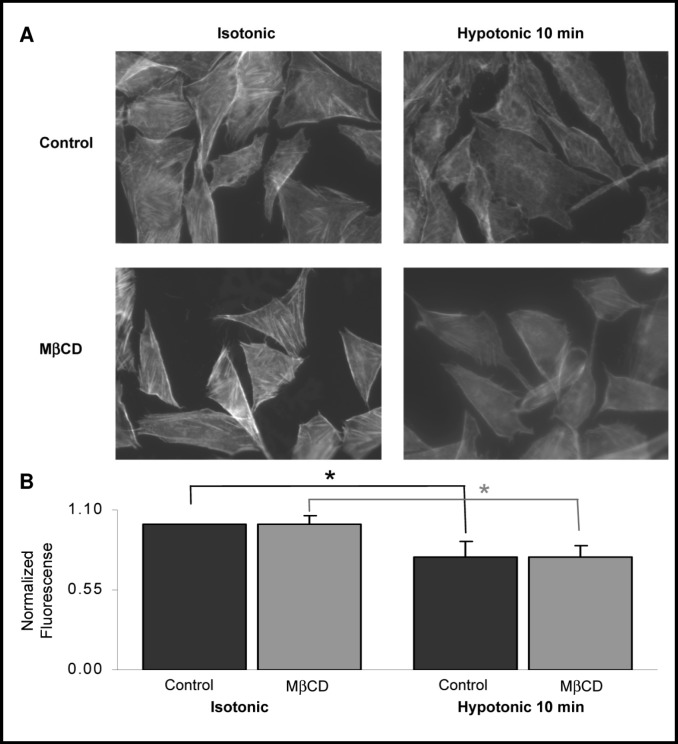
Next, we tested whether cholesterol depletion affects the structure and the abundance of F-actin in CHO cells. This question arose from previous observations in our lab and in labs of other investigators that cell swelling is accompanied with a decrease in cellular F-actin content and that disruption of F-actin facilitates VRAC activity [3, 19]. These observations led to a hypothesis that disruption of F-actin may play the role of a triggering mechanism to initiate cell volume recovery. In this study we show that while hypotonic challenge indeed results in a decrease in F-actin specific fluorescence, cholesterol depletion had no effect on this response (Fig. 3).
Fig. 3.
Cholesterol depletion has no effect on F-actin. A: Typical images of F-actin specific fluorescence visualized with rhodamine-phalloidin in control and cholesterol-depleted cells under isotonic and hypotonic conditions. B: Quantification of F-actin specific fluorescence. Data presented as means±SEM (n= 5 independent experiments with 20-30 cells measured in each experiment).
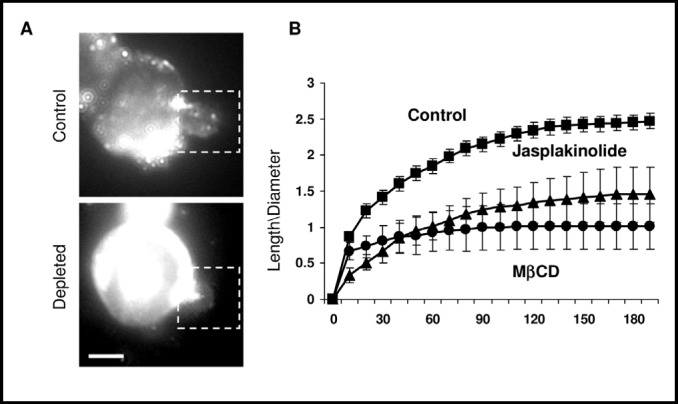
We suggested previously, however, that cholesterol depletion may facilitate cellular responses to mechanical stimuli by an increase in cell stiffness [11, 20] and our earlier studies have shown that cholesterol depletion increases the stiffness of endothelial cells [13]. To test the hypothesis that the cholesterol depletion-induced increase in RVD may be associated with an increase in cell stiffness, we estimated cell stiffness of CHO cells using microaspiration. The general principle of this technique is that the more stiff the cells, the more difficult it is to deform them with negative pressure that is applied through a micropipette pressed into the cell surface and typically maintained for 3 min. Images are acquired before the application of the pressure and then every 30 sec. The length of membrane protusion (L) is measured at each time point and normalized for the diameter of the pipette (D) to account for the variability between individual pipettes, as described earlier [13]. Figure 4 shows the representative images and the average time-courses of progressive membrane deformation induced by negative pressure of 15 mmHg in control and cholesterol-depleted cells. Here we show that, similarly to the effect we observed earlier in endothelial cells, cholesterol depletion significantly increased the stiffness of CHO cells; there was less membrane deformation in cholesterol-depleted cells. Furthermore, we also tested whether an increase in cell stiffness may also be induced independently of cholesterol depletion by exposing the cells to Jasplakinolide (JASP), an agent that stabilizes F-actin. Cells were exposed to JASP for 2 hours and the uptake was verified by staining F-actin with rhodamine-phalloidin: since JASP competes with phalloidin for binding to F-actin, its uptake and binding decreases rhodamine-phalloidin specific fluorescence (not shown). Our observations show that exposure to JASP also results in significant increase in cell stiffness, comparable to the effect of cholesterol depletion.
Fig. 4.
Cholesterol depletion results in cell stiffening. A: Representative images of a control and a cholesterol depleted cells as the membrane is being pulled into the pipette using 15mm Hg of negative force. The membrane is visualized with a membrane fluorescent dye DiI18. The bar is 10 µm. B: The average time courses of aspirated lengths of control, cholesterol depleted cells and cells exposed to Jasplakinolide. Time measurements are in seconds (x-axis) and stiffness, (y-axis) is a measurement of length pulled into the pipette divided by the diameter of the pipette. Data presented as means±SEM (n=6-9 cells in 3 independent experiments).
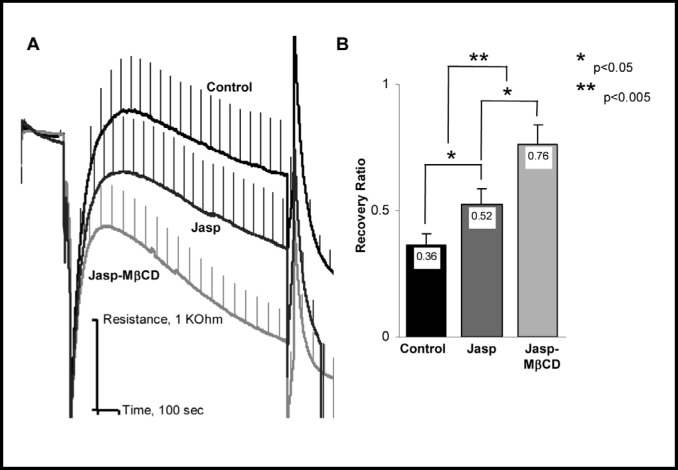
Stabilization of F-actin also facilitates RVD
It is well known that cell stiffness is primarily dependent on the submembrane cytoskeleton and that disruption of F-actin results in a significant decrease in cell stiffness (e.g. [14, 21, 22, 23, 24]). In this study, we tested whether stabilization of F-actin with Jasplakinolide (JASP) has an effect on the RVD development. Our observations show that exposing the cells to JASP can also facilitate RVD even though the effect was less pronounced that that of cholesterol depletion (Fig. 5). Interestingly, pre-exposure to JASP did not abrogate the effect of cholesterol depletion: cells that were both pre-exposed to JASP and then cholesterol depleted generated significantly stronger and faster RVD response than cells exposed either to JASP alone or to MβCD alone. We propose, therefore, that both stabilization of F-actin and cholesterol depletion facilitate RVD by increasing cell stiffness.
Fig. 5.
Stabilization of F-actin facilitates cell volume recovery. A: Average time courses of cell swelling and recovery in response to 30% osmotic gradient in control cells, cells treated with Jasplakinolide or first cholesterol depleted and then treated with Jasplakinolide. B: Average recovery ratios for the same populations of cells. Data presented as means±SEM (n=20-30).
Discussion
While numerous studies have explored the mechanisms of cell volume regulation and the impact of different pathophysiological conditions [3, 6], surprisingly little is known about the role of membrane cholesterol in RVD. This study shows that cholesterol depletion significantly facilitates RVD in CHO cells and that this effect is correlated with an increase in cell stiffness.
During the last decade, cholesterol emerged as a major regulator of a variety of ion channels [25, 26, 27]. The predominant effect of cholesterol is suppression of the channel function, an effect that has been described for multiple types of K+ channels [28, 29, 30, 31], voltage-gated Ca2+ and Na+ channels [32, 33, 34] and Cl− channels [9]. More specifically, in terms of the transport mechanisms that contribute to RVD, membrane cholesterol has a negative effect on VRAC, shown by an increase in amplitude and/or rate of swelling-induced current development in depleted cells [9, 10, 11, 12]. Likewise, swelling-induced efflux of anionic osmolytes in several cell types is enhanced by cholesterol depletion and suppressed by cholesterol enrichment [35, 36, 37, 38]. Cholesterol also inhibits the large-conductance Ca2+-sensitive K+ channel [31, 39] known to be osmotically sensitive in several cell types [3]. In contrast, however, Ca2+ entry channels belonging to the superfamily of Transient Receptor Potential Channels (TRP) that were also shown to be sensitive to cell swelling and contribute to the RVD process [3, 40] are suppressed rather than enhanced by cholesterol depletion [41, 42]. Thus, clearly, cholesterol has complex effects on different swelling-activated ion channels. Specific mechanisms, however, that underlie cholesterol sensitivity of ion channels are poorly understood. It is also possible that changes in membrane cholesterol may affect the hydraulic water permeability of the cell membrane but the impact of this effect on the relationship between swelling and recovery is not clear. This study shows that cholesterol depletion significantly facilitates RVD in CHO cells whereas cholesterol enrichment has no significant effect. These observations suggest that cholesterol sensitivity of VRAC alone cannot explain the impact of cholesterol on the RVD process.
An alternative possibility to consider is that depletion-induced facilitation of RVD may be associated with an increase in cell stiffness. The finding that cholesterol depletion resulted in an increase rather than decrease of cell stiffness was surprising [13], but accumulating evidence shows that this effect is consistent across an array of biomechanical measurements, including [13], stiffening of “deep” cytoskeleton, as estimated by particle tracking [43], an increase in membrane-cytoskeleton adhesion [44] and an increase in cell force generation [45]. All previous studies, however, were performed in vascular endothelial cells leading to a suggestion that cholesterol depletion-induced cell stiffening may be a peculiar feature of endothelial cells. Here we show that cholesterol depletion also increases the stiffness of CHO cells suggesting that it may be a general phenomenon extending to other cell types.
Furthermore, we have also shown earlier that an increase in cell stiffness in endothelial cells is associated with an increased sensitivity of endothelial cells to shear stress, as manifested by enhanced realignment of the cells in the direction of the flow [20]. We proposed, therefore, that an increase in stiffness may sensitize the cells to mechanical forces generated by shear stress. Here we show that there is a clear correlation between the effects of membrane cholesterol on cell stiffening and on the RVD. More specifically, we show that cholesterol depletion increases cell stiffness and facilitates the RVD response whereas cholesterol enrichment had no effect on either of these parameters. Furthermore, we also show that an increase in cell stiffness by an independent approach, stabilization of F-actin fibers, also facilitates RVD. Taken together these observations suggest that cell stiffness may play an important role in the regulation of RVD. It is not possible, however, to completely exclude the possibility that changes in stiffness and RVD are associative rather than causative and further studies elucidating molecular mechanisms underlying changes in cell stiffness are needed to address this question.
In terms of the mechanism, an increase in cell stiffness critically depends on the integrity of F-actin [13, 44]. We investigated further, therefore, the impact of cholesterol depletion and osmotic challenge on F-actin organization in CHO cells and, more importantly, the impact of F-actin stabilization on the RVD. Previous studies have shown that osmotic challenge typically disrupts the integrity of F-actin in different cell types [3, 19, 46]. A small but significant decrease in F-actin specific fluorescence in osmotically challenged CHO cells is fully consistent with these studies. It is also consistent with our previous studies [13, 20] that cholesterol depletion does not have an apparent effect on F-actin structure suggesting that an increase in cell stiffness should be attributed to the stabilization of F-actin rather than an increase in F-actin content. We therefore, tested how stabilization of F-actin affects the RVD process in CHO cells under both control and cholesterol depletion conditions. Previous studies addressing the role of F-actin in RVD have been controversial. In most cases, disrupting F-actin with cytochalasins or latrunculin was shown to inhibit RVD (reviewed by [3] but in some cases the opposite effect was observed [47]. In the latter study, it was also shown that stabilization of F-actin with Jasplakinolide suppresses RVD in MDCK cells. In this study, however, we clearly show that stabilization of F-actin in CHO cells results in significant upregulation of the RVD process supporting our hypothesis that an increase in cell stiffness facilitates RVD, which is consistent with a more common observation that RVD is inhibited by F-actin depolymerization. The most likely explanation for the variability in the effects of actin agents on the RVD is that the stress in the cytoskeleton is nonuniform in space and between different cell types. The role of local actin network as the primary load bearing element might vary between cell types and different experimental conditions. We propose that when actin constitutes the primary bearing element an increase in stiffness facilitates the responsiveness of the cells to mechanical stimuli.
Acknowledgements
We thank Reichert Techmologies for giving us the opportunity to perform these experiments on the Cell Volume Cytometer through Developmental Testing Agreement. This work was supported by National Institutes of Health grants HL073965 and HL083298 (to IL) and HL054887 (to FS).
References
- 1.Law RO. Regulation of mammalian brain cell volume. J Exper Zool. 1994;268:90–96. doi: 10.1002/jez.1402680204. [DOI] [PubMed] [Google Scholar]
- 2.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haeussinger D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of Cell Volume Regulation in Vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 4.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 5.Stutzin A, Hoffmann EK. Swelling-activated ion channels: functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol (Oxf) 2006;187:27–42. doi: 10.1111/j.1748-1716.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann EK, Pedersen SF. Cell volume homeostatic mechanisms: effectors and signalling pathways. Acta Physiol. 2011;202:465–485. doi: 10.1111/j.1748-1716.2010.02190.x. [DOI] [PubMed] [Google Scholar]
- 7.Yeagle PL. Modulation of membrane function by cholesterol. Biochimie. 1991;73:1303. doi: 10.1016/0300-9084(91)90093-g. [DOI] [PubMed] [Google Scholar]
- 8.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 9.Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current (VRAC) in bovine endothelial cells. J Gen Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanenko VG, Rothblat GH, Levitan I. Sensitivity of volume-regulated anion current to cholesterol structural analogues. J Gen Physiol. 2004;123:77–88. doi: 10.1085/jgp.200308882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byfield FJ, Hoffman BD, Romanenko VG, Fang Y, Crocker JC, Levitan I. Evidence for the role of cell stiffness in modulation of volume-regulated anion channels. Acta Physiol. 2006;187:285–294. doi: 10.1111/j.1748-1716.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 12.Kjaer-Klausen T, Hougaard C, Hoffmann EK, Pedersen SF. Cholesterol modulates the volume regulated anion current in Ehrlich Lettre Ascites cells via effects on Rho and F-actin. Am J Physiol Cell Physiol. 2006;291:C757–771. doi: 10.1152/ajpcell.00029.2006. [DOI] [PubMed] [Google Scholar]
- 13.Byfield F, Aranda-Aspinoza H, Romanenko VG, Rothblat GH, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J. 2004;87:3336–3343. doi: 10.1529/biophysj.104.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Northup N, Marga F, Byfield FJ, Levitan I, Forgacs G. Cellular cholesterol effects on membrane-cytoskeleton adhesion. J Cell Sci. 2007;120:2223–2231. doi: 10.1242/jcs.001370. [DOI] [PubMed] [Google Scholar]
- 15.Ateya DA, Sachs F, Gottlieb PA, Besch S, Hua SZ. Volume Cytometry: Microfluidic Sensor for High-Throughput Screening in Real Time. Analyt Chem. 2005;77:1290–1294. doi: 10.1021/ac048799a. [DOI] [PubMed] [Google Scholar]
- 16.Heo J, Meng F, Sachs F, Hua S. Dynamic Effects of Hg2+-induced Changes in Cell Volume. Cell Biochem Biophys. 2008;51:21. doi: 10.1007/s12013-008-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua SZ, Gottlieb PA, Heo J, Sachs F. A mechanosensitive ion channel regulating cell volume. Am J Physiol Cell Physiol. 2010;298:C1424–C1430. doi: 10.1152/ajpcell.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tikku S, Epshtein Y, Collins H, Travis AJ, Rothblat GH, Levitan I. Relationship between Kir2.1/Kir2.3 activity and their distribution between cholesterol-rich and cholesterol-poor membrane domains. Am J Physiol Cell Physiol. 2007;293:C440–450. doi: 10.1152/ajpcell.00492.2006. [DOI] [PubMed] [Google Scholar]
- 19.Levitan I, Almonte C, Mollard P, Garber SS. Modulation of a volume-regulated chloride current by F-Actin. J Membr Biol. 1995;147:283–294. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- 20.Kowalsky GB, Byfield FJ, Levitan I. oxLDL facilitates flow-induced realignment of aortic endothelial cells. Am J Physiol Cell Physiol. 2008;295:C332–340. doi: 10.1152/ajpcell.00335.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pourati J, Maniotis A, Spiegel D, Schaffer JL, Butler JP, Fredberg JJ, Ingber DE, Stamenovic D, Wang N. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am J Physiol Cell Physiol. 1998;274:C1283–1289. doi: 10.1152/ajpcell.1998.274.5.C1283. [DOI] [PubMed] [Google Scholar]
- 22.Rotsch C, Radmacher M. Drug-Induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Theret DP, Wheeler LT, Ohshima N, Nerem RM. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J Biomech Eng. 1990;112:263–268. doi: 10.1115/1.2891183. [DOI] [PubMed] [Google Scholar]
- 24.Byfield F, Aranda-Aspinoza H, Romanenko VG, Rothblat GH, Levitan I. Cholesterol depletion constraints mechanical deformation of aortic endothelial cells. In: Hamza MH, editor. IASTED International Conference on Biomechanics. Rhodos, Greece: ACTA Press; 2003. pp. 86–91. [Google Scholar]
- 25.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovas Res. 2006;69:798. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Levitan I. Cholesterol and Kir channels. IUBMB Life. 2009;61:781–790. doi: 10.1002/iub.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and Ion Channels. In: Harris JR, editor. Cholesterol Binding and Cholesterol Transport Proteins. Subcellular Biochemistry. Vol. 51. Springer Science; 2010. pp. 509–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abi-Char JL, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel JL, Nattel S, Hatem SN. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol. 2007;582:1205. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenhouse-Dantsker A, Leal-Pinto E, Logothetis DE, Levitan I. Comparative analysis of cholesterol sensitivity of Kir channels: Role of the CD loop. Channels. 2010;4:63–66. doi: 10.4161/chan.4.1.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukiya AN, Belani JD, Rychnovsky S, Dopico AM. Specificity of cholesterol and analogs to modulate BK channels points to direct sterol-channel protein interactions. J Gen Physiol. 2011;137:93–110. doi: 10.1085/jgp.201010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, Bruno MJ, Tape SE, Egebjerg J, Greathouse DV, Mattice GL, Koeppe RE, II, Andersen OS. Regulation of Sodium Channel Function by Bilayer Elasticity: The Importance of Hydrophobic Coupling. Effects of Micelle-forming Amphiphiles and Cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toselli M, Biella G, Taglietti V, Cazzaniga E, Parenti M. Caveolin-1 Expression and Membrane Cholesterol Content Modulate N-Type Calcium Channel Activity in NG108-15 Cells. Biophys J. 2005;89:2443–2457. doi: 10.1529/biophysj.105.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundbaek JA, Koeppe RE, Andersen OS. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc Nat Acad Sci USA. 2011;107:15427. doi: 10.1073/pnas.1007455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema TA, Fisher SK. Cholesterol regulates volume-sensitive osmolyte efflux from human SH-SY5Y neuroblastoma cells following receptor activation. J Pharmacol Exp Ther. 2008;324:648–657. doi: 10.1124/jpet.107.131110. [DOI] [PubMed] [Google Scholar]
- 36.Lambert IH. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem Res. 2004;29:27–63. doi: 10.1023/b:nere.0000010433.08577.96. [DOI] [PubMed] [Google Scholar]
- 37.Lim CH, Schoonderwoerd K, Kleijer WJ, de Jonge HR, Tilly BC. Regulation of the cell swelling-activated chloride conductance by cholesterol-rich membrane domains. Acta Physiol. 2006;187:295–303. doi: 10.1111/j.1748-1716.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- 38.Ortenblad N, Young JF, Oksbjerg N, Nielsen JH, Lambert IH. Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol. 2003;284:C1362–1373. doi: 10.1152/ajpcell.00287.2002. [DOI] [PubMed] [Google Scholar]
- 39.Bolotina V, Omelyanenko V, Heyes B, Ryan U, Bregestovski P. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 1989;415:262–268. doi: 10.1007/BF00370875. [DOI] [PubMed] [Google Scholar]
- 40.Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 42.Brownlow SL, Harper AGS, Harper MT, Sage SO. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium. 2004;35:107. doi: 10.1016/j.ceca.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Byfield FJ, Tikku S, Rothblat GH, Gooch KJ, Levitan I. OxLDL increases endothelial stiffness, force generation and network formation. J Lipid Res. 2006;47:715–723. doi: 10.1194/jlr.M500439-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Sun M, Northup N, Marga F, Huber T, Byfield FJ, Levitan I, Forgacs G. The effect of cellular cholesterol on membrane-cytoskeleton adhesion. J Cell Sci. 2007;120:2223–2231. doi: 10.1242/jcs.001370. [DOI] [PubMed] [Google Scholar]
- 45.Norman LL, Oetama RJ, Dembo M, Byfield F, Hammer DA, Levitan I, Aranda-Espinoza H. Modification of Cellular Cholesterol Content Affects Traction Force, Adhesion and Cell Spreading. Cell Mol Bioeng. 2010;3:151–162. doi: 10.1007/s12195-010-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen SF, Beisner KH, Hougaard C, Willumsen BM, Lambert IH, Hoffmann EK. Rho family GTP binding proteins are involved in the regulatory volume decrease process in NIH3T3 mouse fibroblasts. J Physiol. 2002;541:779–796. doi: 10.1113/jphysiol.2002.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerrigan MJ, Hall AC. Stimulation of regulatory volume decrease (RVD) by isolated bovine articular chondrocytes following F-actin disruption using latrunculin B. Biorheology. 2005;42:283–293. [PubMed] [Google Scholar]