Abstract
Background
Pendrin is a transport protein exchanging chloride for other anions, such as iodide in the thyroid gland or bicarbonate in the inner ear. Mutations in the SLC26A4 gene encoding for pendrin are responsible for both syndromic (Pendred syndrome) and non-syndromic (non-syndromic enlarged vestibular aqueduct, EVA) hearing loss. Besides clinical and radiological assessments, molecular and functional studies are essential for the correct diagnosis of Pendred syndrome and non-syndromic EVA. While a broad spectrum of mutations found in the Caucasian population has been functionally characterized, little is known about mutations specifically occurring in the populations of the Middle East. Here we show the characterization of the ion transport activity of three pendrin mutations previously found in deaf patients with EVA in the Israeli Jewish and Palestinian Arab populations, i.e. V239D, G334V X335 and I487Y FSX39.
Methods
Wild type and mutated pendrin allelic variants were functionally characterized in a heterologous over-expression system. The Cl−/I− and Cl−/OH− exchange activities were assessed by fluorometric methods suitable for measuring iodide fluxes and the intracellular pH.
Results
Both the Cl−/I− and the Cl−/OH− exchange activities of pendrin V239D, G334V X335 and I487Y FSX39 were significantly reduced with respect to the wild type, with V239D displaying a residual iodide transport.
Conclusion
Functional assays confirmed the diagnosis of non-syndromic EVA due to SLC26A4 mutations performed by radiological and molecular tests in deaf patients belonging to the Israeli Jewish and Palestinian Arab populations. The new finding that the V239D mutation displays residual function suggests that the symptoms caused by this mutation could be ameliorated by a pendrin ‘activator’, if available.
Key Words: Pendrin, Inherited hearing loss, Functional test, Ion transport, Pendred syndrome, EVA, SLC26A4
Introduction
Pendrin (PDS, SLC26A4) is an electroneutral anion exchanger [1, 2, 3, 4] mainly expressed in the thyroid gland, inner ear, kidney, and airways. In the thyroid gland pendrin is expressed on the apical membrane of thyrocytes [5] and acts as a chloride/iodide (Cl−/I−) exchanger allowing the transport of iodide into the follicular lumen [6]. In the inner ear, pendrin is expressed (i) in the epithelial cells of the endolymphatic sac and duct [7], (ii) on the apical membrane of transitional cells in the saccule, utricle and ampulla, and (iii) in different cochlear cell types [8, 9]. In the inner ear, pendrin exchanges Cl−/HCO3− and is responsible for bicarbonate secretion into the endolymph [10, 11], and thereby controls pH. Endolymph pH is crucial for the fluid [12] and ion [10, 13] homeostasis and hence, overall hearing function. In the kidney, pendrin is expressed on the apical membrane of β and non-α, non-β intercalated cells of the distal tracts of the nephron [14, 15] where once again it plays a role in pH homeostasis and blood pressure regulation [16, 17] through the secretion of bicarbonate and reabsorbtion of chloride [18]. In the airways, pendrin has been associated with mucus production [19] and control of airway surface liquid thickness [20], with a possible involvement in the pathogenesis of asthma and Chronic Obstructive Pulmonary Disease (COPD).
Mutations in the gene SLC26A4 encoding for pendrin are responsible for both syndromic and non-syndromic hearing loss. Syndromic hearing loss linked to mutations of the pendrin gene is called Pendred syndrome (OMIM#274600) [21]; this is an autosomal recessive disease characterized by the association of sensorineural hearing loss and a partial iodide organification defect, disclosed by a positive perchlorate discharge test [22], and associated with goiter in approximately 80% of patients of which a minority develops hypothyroidism [23]. Deafness is due to malformations of the inner ear, ranging from an enlarged vestibular aqueduct to the Mondini cochlea, which lacks the apical turn [24]. Monoallelic [25, 26, 27] or biallelic [26] mutations of the SLC26A4 gene could also lead to non-syndromic enlarged vestibular aqueduct (EVA) without thyroid gland involvement.
Functional tests of mutated pendrin allelic variants found in patients with Pendred syndrome or non-syndromic E VA revealed that the pathology is linked to a reduction or a loss of function in the ion transport activity of pendrin [28]. Subcellular localization studies revealed that the mutated proteins are often retained in subcellular compartments and are unable to reach the plasma membrane [29]. These mutations most likely cause protein misfolding, followed by impaired trafficking and subsequent degradation. Of the mutations that do reach the membrane, however, their transport function is impaired [27, 28, 30]. These latter mutations are of particular interest since they likely affect the ion binding and/or regulatory domains important for transporter function. Besides clinical and radiological assessments, the functional evaluation of pendrin mutations is essential for correct diagnosis, i.e. of Pendred syndrome and non-syndromic EVA [24]. Indeed, the clinical condition of pseudo-Pendred syndromes [31, 32, 33] and the high incidence in some populations for functional pendrin polymorphisms [34] could lead to an incorrect assignment of the pathological conditions in cases where proper functional characterizations are not possible. Whereas a broad spectrum of mutations found in the Caucasian populations have been functionally characterized [35], little is known about mutations occurring in the Caucasian populations of the Middle East. Here we characterized the ion transport activity of three pendrin mutations previously found in hearing impaired patients in the Israeli Jewish and Palestinian Arab populations, i.e. V239D, G334V X335 [36] and I487Y FSX39 [37]. The mutation V239D was found in one family of a cohort of 156 deaf probands belonging to the Palestinian population and their families. All four affected individuals were homozygous for V239D. The mutation G334V X335 was similarly found in one family in the above mentioned cohort of deaf Palestinian probands. These mutations were not detected in a control population of 100 Palestinian adults with normal hearing. The mutation I487Y FSX39 was found in one deaf individual of a cohort of 203 Jewish deaf probands. This mutation was not detected in a control population of 310 Jewish individuals with normal hearing.
Materials and Methods
Cloning procedures and plasmid constructs
Standard procedures were used for DNA preparation, cloning, purification, and sequencing. The pTARGET (Promega Corporation) vector, containing the open reading frame (ORF) of full length human pendrin cloned from normal thyroid tissue, was originally provided by Prof. P. Beck-Peccoz, University of Milan (Italy). The pendrin mutants were made using the QuikChange® Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's protocol, using the following primers: for pendrin V239D: forward: 5’ CAC AGC TAA AGA TTG ACC TCA ATG TTT CAA CC 3’ and reverse: 5’ GGT TGA AAC ATT GAG GTC AAT CTT TAG CTG TG 3’, for pendrin G334V X335: forward: 5’ CCA TCC CAA GGG TGT GAT TGC CTC CTG AAC TTC CAC C 3'and reverse: 5’ GGT GGA AGT TCA GGA GGC AAT CAC ACC CTT GGG ATG G 3’, for pendrin I487Y FSX39: forward: 5’ CTG GGT GTT TAC GTG TTA TAG TGT CCA TCA TTC 3’ and reverse: 5’ GAA TGA TGG ACA CTA TAA CAC GTA AAC ACC CAG 3’.
The plasmid encoding for the enhanced yellow fluorescent protein H148Q, I152L (EYFP) was obtained by site-directed mutagenesis of the pEYFPN1 vector (Clontech) with the following primers: forward: 5’ GGA GTA CAA CTA CAA CAG CCA GAA CGT CTA TTT GAT GGC CGA CAA GCA GAA G 3’ and reverse: 5’ CTT CTG CTT GTC GGC CAT CAA ATA GAC GTT CTG GCT GTT GTA GTT GTA CTC C 3’. EYFP H148Q, I152L is an EYFP mutant with substantially improved sensitivity for iodide [38]. All plasmid inserts were sequenced prior to use in experiments (Microsynth AG, Switzerland).
Cell culture and transient transfection
HEK293 Phoenix cells [39] were cultured in Minimum Essential Eagle Medium (MEM, Sigma, Austria) supplemented with 10% fetal bovine serum (FBS, Cambrex Bio Science), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 1 mM pyruvic acid (sodium salt). The cells were maintained at 37°C, 5% CO2, 95% air and 100% humidity. Subcultures were routinely established every second to third day by seeding the cells into 100 mm diameter Petri dishes following trypsin/EDTA treatment. For functional tests, HEK293 Phoenix cells were seeded into poly-L-lysine-coated 96-multiwells, grown overnight and transfected with a total amount of 0.2 µg/well of plasmid DNA by the calcium phosphate co-precipitation method. The amount of plasmid DNA encoding for pendrin WT, V239D, G334V X335 and I487Y FSX39 was carefully quantified and kept rigorously constant throughout all the experiments. The transfection efficiency (determined as EYFP fluorescence intensity in the extracellular “high chloride solution”, described below) of cells transfected with pendrin V239D, G334V X335 and I487Y FSX39 was not reduced compared to WT (data not shown). Functional tests were performed 52 hours post-transfection.
Iodide transport
For measuring iodide transport, cells were co-transfected with 0.1 µg of pEYFPN1 plasmid and 0.1 µg of pTARGET plasmid bearing the cDNA of wild-type or mutated pendrin. The functional test was performed as already described, with minor modifications [4, 34, 35, 40, 41]. Shortly, cells were washed from the culture medium and bathed in 70 µl of “high chloride” solution (in mM: KCl 2, NaCl 135, CaCl2 1, MgCl2 1, D-glucose 10, HEPES 20, 308 mOsm with mannitol, pH 7.4). After measuring the fluorescent intensity (1 measurement/sec for 3 sec), 140 µl of”high iodide“ solution (in mM: KCl 2, NaI 135, CaCl2 1, MgCl2 1, D-glucose 10, HEPES 20, 308 mOsm with mannitol, pH 7.4) were injected in each well. Then, the fluorescence intensity was measured for 16 sec. Fluorescence measurements were done with the VICTORTM X3 Multilabel Plate Reader (Perkin Elmer) equipped with a liquid dispenser and the following filters: excitation: F485 (excitation center wavelength (CWL): 485 nm, bandwidth: 14 nm), emission: F535 (emission CWL: 535 nm, bandwidth: 25 nm). Experiments were performed at room temperature. From all the fluorescence measurements we subtracted the background fluorescence measured in cells transfected with the pTARGET vector only. Data were expressed as fluorescence variations in % (ΔF%). The percentage of fluorescence variation was always calculated between the beginning (average of 3 readings, 1 reading/sec) and the end (average of 3 readings, 1 reading/sec) of an experimental period of 19 sec.
Intracellular pH measurements
For intracellular pH measurements, the fluorescent indicator 2’,7’-bis-(2- carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM, Invitrogen Molecular Probes) was used. HEK293 Phoenix cells were transiently transfected with 0.2 µg of pTARGET plasmid bearing the cDNA of wild-type or mutated pendrin and loaded (30 min, 37°C) with BCECF-AM 1 µM. Then, cells were washed twice and bathed for 30 min at room temperature in 70 µl of bicarbonate-free, “low chloride” solution (in mM: KCl 2, NaGluconate 135, CaCl2 1, MgCl2 1, D-glucose 10, HEPES 20, 308 mOsm with mannitol, pH 7.4). After measuring the fluorescent intensity (3 measurements every 5.6 sec), 140 µl of bicarbonate-free ”high chloride“ solution (in mM: KCl 2, NaCl 135, CaCl2 1, MgCl2 1, D-glucose 10, HEPES 20, 308 mOsm with mannitol, pH 7.4) were injected in each well. Then, the fluorescence intensity was measured again 16 times (one measurement every 5.6 sec). Fluorescence measurements were done with the VICTORTM X3 Multilabel Plate Reader (Perkin Elmer) equipped with a liquid dispenser and the following filters: excitation: P450 (excitation CWL: 450 nm, bandwidth: 8 nm), and P490 (excitation CWL: 492 nm, bandwidth: 8 nm), emission: F535 (emission CWL: 535 nm, bandwidth: 25 nm). Experiments were performed at room temperature. All the measurements were subtracted from that detected in cells transfected with the pTARGET vector and not loaded with BCECF-AM. Intracellular pH was calculated following the standard ratiometric method (http://probes.invitrogen.com/media/pis/mp01150.pdf). Shortly, titration of the intracellular pH was performed in nigericin (10 µM) treated HEK293 Phoenix cells in the presence of 150 mM potassium (in mM: KCl 150, MgCl2.6H2O 1, MES, HEPES or TRISMA base 20), at pHs of 5.0, 6.0, 6.5, 7.0, 7.5, 8.0 and 9.0. The resulting calibration curve fit with a sigmoidal dose-response equation:
Results were expressed as fluorescence ratio (λ490/λ450) variations (ΔRatio%), or the fluorescence ratios were interpolated on the calibration curve to determine the intracellular pH.
Salts, chemicals and drugs
All salts and chemicals used were of “pro analysis” grade.
Statistical Analysis
All data are expressed as arithmetic means ± S.E.M. For statistical analysis, GraphPad Prism software (version 4.00 for Windows, GraphPad Software, San Diego, California, USA) was used. Significant differences between means were tested by one way Analysis of Variance (ANOVA) with Bonferroni's post-test or paired Student's t-test, where appropriate. Statistically significant differences were assumed at p<0.05 (* p<0.05; ** p<0.01; *** p<0.001); (n) corresponds to the number of independent measurements.
Results
The iodide transport of pendrin mutants V239D, G334V X335 and I487Y FSX39 is significantly impaired compared to wild type
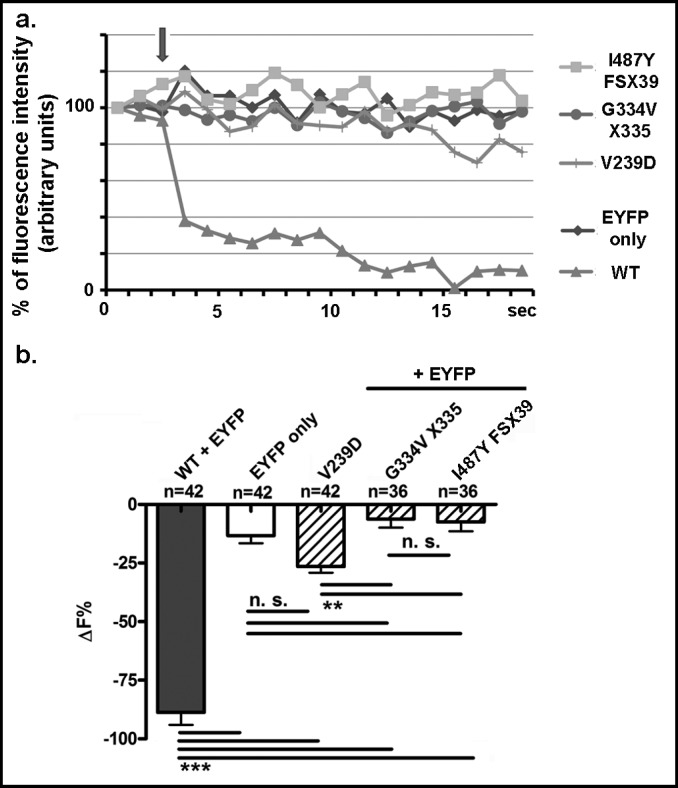
In order to test the halide transport activity of wildtype (WT) pendrin and its mutants (Fig. 1), we measured the EYFP fluorescence in cells over-expressing WT pendrin or its mutants before and after addition of iodide to the extracellular solution. Since pendrin acts as an iodide transporter [42, 43], the presence of extracellular iodide should induce an iodide flux into the cytoplasm. Iodide is a much better quencher of EYFP fluorescence than chloride, therefore, an increase of intracellular iodide should lead to a decrease of EYFP fluorescence [38]. Indeed, as shown in Fig. 2a and b, addition of iodide to the extracellular solution (indicated by an arrow in Fig. 2a) leads to a marked decrease (88 ± 5%, n = 42) of EYFP fluorescence in cells expressing WT pendrin. In cells overexpressing only EYFP, the addition of iodide to the extracellular solution leads, as expected, to a significantly smaller decrease (13 ± 3%, n = 42) of the relative fluorescence. This small amount of quenching is probably due to endogenous, scarcely represented ion channels or transporters able to sustain a small iodide influx. These results confirm that pendrin is an iodide transporter [30, 42, 43] most likely acting as a Cl−/I− anion exchanger in our system [4].
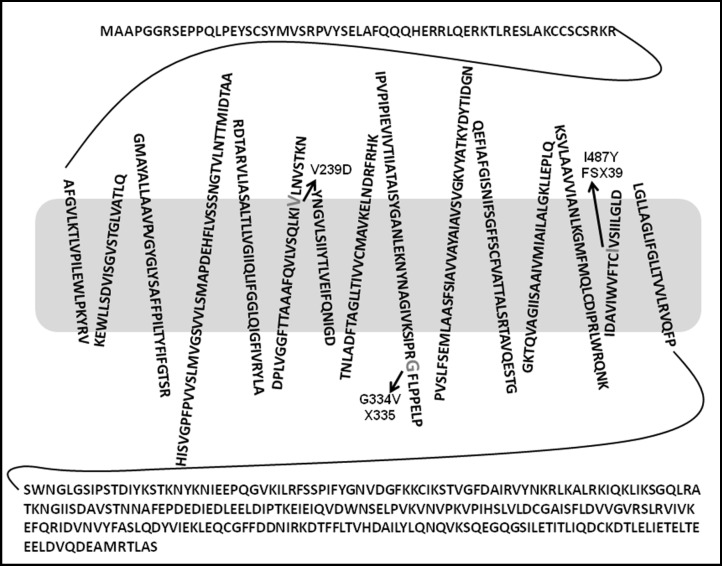
Fig. 1.
Putative topology of human pendrin and location of the V239D, G334V X335 and I487Y FSX39 mutations. The 15-transmembrane model, as proposed in [35] shows the position of the mutations V239D, G334V X335 and I487Y FSX39; the amino acids involved in substitutions are indicated in enlarged gray letters.
Fig. 2.
Iodide transport of WT pendrin and the mutants V239D, G334V X335 and I487Y FSX39. a, Representative original recording of the intracellular fluorescence intensity measured in cells transfected with WT or mutated pendrin and EYFP or EYFP alone as a control (EYFP only). The arrow indicates the addition of iodide to the extracellular solution. b, Percentage of fluorescence decrease (ΔF%) determined over an experimental period of 19 sec. n indicates the number of independent samples collected over 5 experimental days. ***: p<0.001, **: p<0.01, n. s.: not statistically significant, one way ANOVA with Bonferroni's post-test.
As in cells overexpressing only EYFP, the addition of iodide to the extracellular solution lead only to a small decrease in the intracellular fluorescence of cells over-expressing the mutated pendrin isoforms (for V239D: 26 ± 3%, n = 42; for G334V X335: 6 ± 4%, n = 36; for I487Y FSX39: 7 ± 4%, n = 36). Only V239D displayed transport activity that was significantly higher compared to the G334V X335 and the I487Y FSX39 mutants (Fig. 2b) but heavily impaired with respect to WT pendrin.
The Cl−/OH− exchange activity of pendrin mutants V239D, G334V X335 and I487Y FSX39 is significantly impaired compared to WT
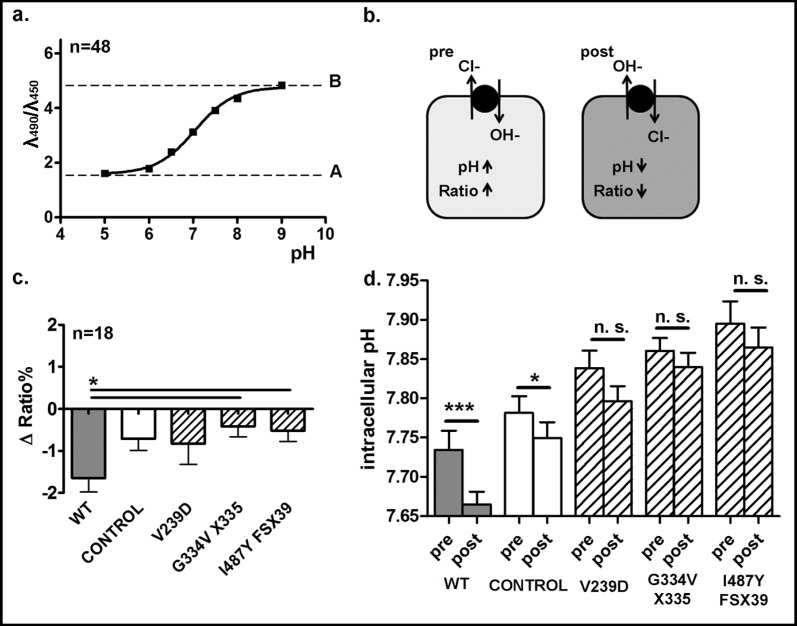
In order to test the Cl−/OH− exchange activity of WT pendrin and its mutants, the BCECF-AM fluorescence was measured in chloride-depleted control and pendrin-transfected cells before and after addition of chloride to the extracellular solution. A titration curve of the intracellular pH determined in non-transfected HEK293 Phoenix cells loaded with BCECF-AM was produced (Fig. 3a). In our conditions, the calculated pKa for BCECF-AM was 7.04. The treatment to induce the chloride depletion (30 min at room temperature in bicarbonate-free, “low chloride” solution, see Materials and Methods) should induce a chloride efflux following the concentration gradient in exchange for OH− (Fig. 3b, “pre”), most likely via endogenous exchangers. This would induce a substantial basification of the intracellular pH, as observed in Fig. 3d, “pre”. As pendrin acts as an Cl−/ OH−, Cl−/HCO3− exchanger [1] the presence of extracellular chloride should induce a chloride flux into the cytoplasm in exchange for OH−, leading, as a consequence, to a decrease of the intracellular pH (Fig. 3b, “post”). A decrease in the BCECF-AM fluorescence ratio would be indicative of such a pH change. Indeed, as shown in Fig. 3c, addition of chloride to the extracellular solution led to a significant fluorescence ratio decrease, corresponding to an intracellular acidification (Fig. 3d) in cells transfected with WT pendrin. In contrast, in cells over-expressing the pendrin mutants, the addition of chloride to the extracellular solution led only to a small, non-significant decrease in intracellular pH. These results demonstrate that, similarly to the Cl−/I− exchange activity, the Cl−/OH− exchange activity of the pendrin mutants (V239D, G334V X335 and I487Y FSX39) is also deeply impaired. The small acidification observed in control cells is most likely due to endogenous Cl−/OH− exchangers.
Fig. 3.
Cl−/OH− exchange activity of WT pendrin and the mutants V239D, G334V X335 and I487Y FSX39. a, Titration curve of the intracellular pH determined in non-transfected HEK293 Phoenix cells loaded with BCECF-AM 1 µM maintained in solutions containing 150 mM K+ and 10 µM nigericin at the pH of 5.0, 6.0, 6.5, 7.0, 7.5, 8.0 and 9.0. n indicates the number of independent samples, collected over 4 experimental days. b, Cells transfected with WT or mutated pendrin or with the pTARGET empty vector as a control were loaded with BCECF-AM 1 µM and bathed for 30 min in a bicarbonate-free, low chloride solution (pre) and then exposed to a high chloride solution (post). Fluorescence emissions at 535 nm following excitation at 450 and 490 nm were measured over time, subtracted for their respective backgrounds and expressed as ratios. c, Percentage of fluorescence decrease (Δ Ratio%) determined over the experimental period (107 sec). n indicates the number of independent samples, collected over three experimental days. *: p<0.05, one way ANOVA with Bonferroni's post-test. d, The ratios values were interpolated on the calibration curve shown in a, to determine the intracellular pH before and after exposure of cells to chloride. ***: p<0.001, *: p<0.05, n. s.: not statistically significant, paired Students t-test. The number of experiment is the same as in c.
Discussion
Pendred syndrome is described as an autosomal recessive disease characterized by the association of sensorineural hearing loss and a partial iodide organification defect [22]. Mutations in the SLC26A4 gene, which encodes the pendrin protein, are the most common cause of syndromic deafness [44] and, in addition, are present in up to 4% of patients with non-syndromic hearing loss [26]. Recessively inherited phenotypes are frequent in endogamous communities and are particularly suitable for identifying genes responsible for clinically important phenotypes. Therefore, we set out to functionally characterize three pendrin mutations, i.e. (i) V239D, (ii) G334V X335 and (iii) I487Y FSX39, which have been described in hearing impaired patients in the Middle East. The amino acid substitution V329D is due to the nucleotide change 716T>A, and is located within the 6th putative transmembrane segment of the pendrin transporter (Fig. 1). Subcellular localization studies showed that the mutated protein is mainly retained in the endoplasmic reticulum [36]. The second mutation, i.e. pendrin G334V X335, results from a splicing mutation (1001G>T) that results in a premature truncation of the protein (Fig. 1). These two mutations were identified in Palestinian Arab families where the homozygous subjects had prelingual, bilateral, severe-to-profound hearing loss with EVA but no clinical evidence of thyroid abnormalities (Table 1). The third mutation studied, i.e. pendrin I487Y FSX39, results from the insertion 1458_1459insT, leading to a prematurely truncated protein, with a scrambled sequence of 39 amino acids at the C terminus (Fig. 1). It seems that this mutation, as the V329D isoform, also localizes predominantly in the endoplasmic reticulum [37]. This mutation was identified in homozygosity in a deaf Israeli resident of Iranian ethnic origin with EVA and no overt signs of thyroid dysfunction (Table 1).
Table 1.
Pendrin mutations identified in the Palestinian Arab and Israeli Jewish populations.
| Nucleotide change | Amino acid change | Subcellular localization | Patient phenotype | Patient ethnicity | Reference |
|---|---|---|---|---|---|
| 716T>A | V239D | ER | EVA | Palestinian | [36] |
| 1001G>T | G334V X335 | - | EVA | Palestinian | [36] |
| 1458_1459insT | I487Y FSX39 | ER | NSHL with EVA | Iranian Jewish | [37] |
ER = endoplasmic reticulum, NSHL = non-syndromic hearing loss, E VA = enlarged vestibular aqueduct
Fluorometric assays suitable for measuring iodide fluxes and the intracellular pH showed that both the Cl−/ I− and the Cl−/OH− exchange activities of pendrin V239D, G334V X335 and I487Y FSX39 were dramatically reduced compared to the WT. The lack of transport activity shown by the two truncation-mutations (G334V X335 and I487Y FSX39) could be explained by the low amount of transporter that would be expressed at the cellular membrane, as was shown for the I487Y FSX39 mutation by Brownstein et al. [37]. For the V239D mutation, similarly to the I487Y FSX39, a severely hampered translocation of the protein into the cellular membrane was assumed [36], however, our experimental data show a residual transport activity of this isoform. A possible explanation for the residual Cl−/I− exchange could be the presence of a small amount of protein that does reach the plasma membrane. The severely hampered translocation of pendrin V239D into the cellular membrane is most likely caused by a “processing” defect, i.e. the misfolded mutated protein is retained in the intracellular compartment. In addition, the possibility that the impaired trafficking to the plasma membrane is due to an impaired interaction with yet unidentified chaperones or adaptor proteins is an intriguing and unexplored hypothesis and deserves further investigation.
Conclusion
Functional assays confirmed the diagnosis of non-syndromic EVA due to pendrin mutations performed by radiological and molecular tests in deaf patients belonging to the Israeli Jewish and Palestinian Arab populations.
The new finding that the V239D mutation displays residual function suggests that the symptoms caused by this mutation could be ameliorated by a pendrin ‘activator’, if available.
Acknowledgements
Charity Nofziger is supported by the Lise Meitner stipend of the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) (M11108-B11). K.B.A. and M.K. are supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (NIH) (R01DC011835). This work was further supported by the FWF and the FP-7 to M.P. (P18608; PIRSES-GA-2008-230661).
Abbreviations
- EVA
(enlarged vestibular aqueduct)
- ORF
(open reading frame)
- EYFP
(enhanced yellow fluorescent protein)
- FBS
(fetal bovine serum)
- EDTA
(ethylenediaminetetraacetic acid)
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- BCECF-AM
(2’,7’-bis-(2- carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester)
- MES
(2-(N-morpho-lino)ethanesulfonic acid)
- TRISMA
base (2-amino-2-(hydroxymethyl)propane-1,3-diol)
References
- 1.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: An apical Cl-/OH-/HCO3-exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 2.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: Role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dossena S, Maccagni A, Vezzoli V, Bazzini C, Garavaglia ML, Meyer G, Furst J, Ritter M, Fugazzola L, Persani L, Zorowka P, Storelli C, Beck-Peccoz P, Botta G, Paulmichl M. The expression of wildtype pendrin (SLC26A4) in human embryonic kidney (HEK 293 Phoenix) cells leads to the activation of cationic currents. Eur J Endocrinol. 2005;153:693–699. doi: 10.1530/eje.1.02018. [DOI] [PubMed] [Google Scholar]
- 4.Dossena S, Rodighiero S, Vezzoli V, Bazzini C, Sironi C, Meyer G, Furst J, Ritter M, Garavaglia ML, Fugazzola L, Persani L, Zorowka P, Storelli C, Beck-Peccoz P, Botta G, Paulmichl M. Fast fluorometric method for measuring pendrin (SLC26A4) Cl-/I- transport activity. Cell Physiol Biochem. 2006;18:67–74. doi: 10.1159/000095164. [DOI] [PubMed] [Google Scholar]
- 5.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K. Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J Clin Endocrinol Metab. 2002;87:3356–3361. doi: 10.1210/jcem.87.7.8679. [DOI] [PubMed] [Google Scholar]
- 7.Dou H, Xu J, Wang Z, Smith AN, Soleimani M, Karet FE, Greinwald JH, Jr., Choo D. Co-expression of pendrin, vacuolar H+-ATPase alpha4-subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. J Histochem Cytochem. 2004;52:1377–1384. doi: 10.1177/002215540405201014. [DOI] [PubMed] [Google Scholar]
- 8.Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC, Green ED. Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in Pendred syndrome. J Assoc Res Otolaryngol. 2003;4:394–404. doi: 10.1007/s10162-002-3052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith AJ, Wangemann P: Hearing loss associated with enlargement of the vestibular aqueduct: Mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res 2011;in press. [DOI] [PMC free article] [PubMed]
- 10.Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol. 2007;292:F1314–F1321. doi: 10.1152/ajprenal.00432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HM, Wangemann P. Failure of fluid absorption in the endolymphatic sac initiates cochlear enlargement that leads to deafness in mice lacking pendrin expression. PLoS One. 2010;5:e14041. doi: 10.1371/journal.pone.0014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F229–F241. doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 16.Wall SM, Pech V. Pendrin and sodium channels: Relevance to hypertension. J Nephrol. 2010;23(Suppl 16):S118–S123. [PubMed] [Google Scholar]
- 17.Eladari D, Chambrey R, Frische S, Vallet M, Edwards A. Pendrin as a regulator of ECF and blood pressure. Curr Opin Nephrol Hypertens. 2009;18:356–362. doi: 10.1097/MNH.0b013e32832c91f4. [DOI] [PubMed] [Google Scholar]
- 18.Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl-/HCO3- exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol. 2010;299:C33–C41. doi: 10.1152/ajpcell.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED, Izuhara K. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008;180:6262–6269. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- 20.Nakagami Y, Favoreto S, Jr., Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC, Erle DJ. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181:2203–2210. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 22.Bizhanova A, Kopp P. Genetics and phenomics of Pendred syndrome. Mol Cell Endocrinol. 2010;322:83–90. doi: 10.1016/j.mce.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Reardon W, Coffey R, Chowdhury T, Grossman A, Jan H, Britton K, Kendall-Taylor P, Trembath R. Prevalence, age of onset, and natural history of thyroid disease in Pendred syndrome. J Med Genet. 1999;36:595–598. [PMC free article] [PubMed] [Google Scholar]
- 24.Fugazzola L, Mannavola D, Cerutti N, Maghnie M, Pagella F, Bianchi P, Weber G, Persani L, Beck-Peccoz P. Molecular analysis of the Pendred's syndrome gene and magnetic resonance imaging studies of the inner ear are essential for the diagnosis of true Pendred's syndrome. J Clin Endocrinol Metab. 2000;85:2469–2475. doi: 10.1210/jcem.85.7.6694. [DOI] [PubMed] [Google Scholar]
- 25.Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, Nance WE, Yang Y, Zalewski CK, Brewer CC, Butman JA, Griffith AJ. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): Evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42:159–165. doi: 10.1136/jmg.2004.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert S, Blons H, Jonard L, Feldmann D, Chauvin P, Loundon N, Sergent-Allaoui A, Houang M, Joannard A, Schmerber S, Delobel B, Leman J, Journel H, Catros H, Dollfus H, Eliot MM, David A, Calais C, Drouin-Garraud V, Obstoy MF, Tran Ba HP, Lacombe D, Duriez F, Francannet C, Bitoun P, Petit C, Garabedian EN, Couderc R, Marlin S, Denoyelle F. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in caucasian populations. Eur J Hum Genet. 2006;14:773–779. doi: 10.1038/sj.ejhg.5201611. [DOI] [PubMed] [Google Scholar]
- 27.Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, Kittles R, Eisenman D, Kim HJ, Niparko J, Thomsen J, Arnos KS, Nance WE, King KA, Zalewski CK, Brewer CC, Shawker T, Reynolds JC, Butman JA, Karniski LP, Alper SL, Griffith AJ. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: Genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat. 2009;30:599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: Implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87:1778–1784. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- 29.Rotman-Pikielny P, Hirschberg K, Maruvada P, Suzuki K, Royaux IE, Green ED, Kohn LD, Lippincott-Schwartz J, Yen PM. Retention of pendrin in the endoplasmic reticulum is a major mechanism for Pendred syndrome. Hum Mol Genet. 2002;11:2625–2633. doi: 10.1093/hmg/11.21.2625. [DOI] [PubMed] [Google Scholar]
- 30.Dossena S, Vezzoli V, Cerutti N, Bazzini C, Tosco M, Sironi C, Rodighiero S, Meyer G, Fascio U, Furst J, Ritter M, Fugazzola L, Persani L, Zorowka P, Storelli C, Beck-Peccoz P, Botta G, Paulmichl M. Functional characterization of wild-type and a mutated form of SLC26A4 identified in a patient with Pendred syndrome. Cell Physiol Biochem. 2006;17:245–256. doi: 10.1159/000094137. [DOI] [PubMed] [Google Scholar]
- 31.Fugazzola L, Cerutti N, Mannavola D, Crino A, Cassio A, Gasparoni P, Vannucchi G, Beck-Peccoz P. Differential diagnosis between Pendred and pseudo-Pendred syndromes: Clinical, radiologic, and molecular studies. Pediatr Res. 2002;51:479–484. doi: 10.1203/00006450-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Kara C, Kilic M, Ucakturk A, Aydin M. Congenital goitrous hypothyroidism, deafness and iodide organification defect in four siblings: Pendred or pseudo-Pendred syndrome? J Clin Res Pediatr Endocrinol. 2010;2:81–84. doi: 10.4274/jcrpe.v2i2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis N, Lunardi C, Shield JP. Sensori-neural deafness and hypothyroidism: Autoimmunity causing ‘pseudo-Pendred syndrome’. Horm Res. 2006;65:267–268. doi: 10.1159/000092513. [DOI] [PubMed] [Google Scholar]
- 34.Pera A, Dossena S, Rodighiero S, Gandia M, Botta G, Meyer G, Moreno F, Nofziger C, Hernandez-Chico C, Paulmichl M. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci USA. 2008;105:18608–18613. doi: 10.1073/pnas.0805831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dossena S, Rodighiero S, Vezzoli V, Nofziger C, Salvioni E, Boccazzi M, Grabmayer E, Botta G, Meyer G, Fugazzola L, Beck-Peccoz P, Paulmichl M. Functional characterization of wildtype and mutated pendrin (SLC26A4), the anion transporter involved in Pendred syndrome. J Mol Endocrinol. 2009;43:93–103. doi: 10.1677/JME-08-0175. [DOI] [PubMed] [Google Scholar]
- 36.Walsh T, Abu RA, Abu SeJ, Shahin H, Shepshelovich J, Lee MK, Hirschberg K, Tekin M, Salhab W, Avraham KB, King MC, Kanaan M. Genomic analysis of a heterogeneous mendelian phenotype: Multiple novel alleles for inherited hearing loss in the palestinian population. Hum Genomics. 2006;2:203–211. doi: 10.1186/1479-7364-2-4-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownstein ZN, Dror AA, Gilony D, Migirov L, Hirschberg K, Avraham KB. A novel SLC26A4 (PDS) deafness mutation retained in the endoplasmic reticulum. Arch Otolaryngol Head Neck Surg. 2008;134:403–407. doi: 10.1001/archotol.134.4.403. [DOI] [PubMed] [Google Scholar]
- 38.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 39.DiCiommo DP, Duckett A, Burcescu I, Bremner R, Gallie BL. Retinoblastoma protein purification and transduction of retina and retinoblastoma cells using improved alphavirus vectors. Invest Ophthalmol Vis Sci. 2004;45:3320–3329. doi: 10.1167/iovs.04-0140. [DOI] [PubMed] [Google Scholar]
- 40.Dror AA, Politi Y, Shahin H, Lenz DR, Dossena S, Nofziger C, Fuchs H, Hrabe de AM, Paulmichl M, Weiner S, Avraham KB. Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J Biol Chem. 2010;285:21724–21735. doi: 10.1074/jbc.M110.120188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fugazzola L, Cirello V, Dossena S, Rodighiero S, Muzza M, Castorina P, Lalatta F, Ambrosetti U, Beck-Peccoz P, Botta G, Paulmichl M. High phenotypic intrafamilial variability in patients with Pendred syndrome and a novel duplication in the SLC26A4 gene: Clinical characterization and functional studies of the mutated SLC26A4 protein. Eur J Endocrinol. 2007;157:331–338. doi: 10.1530/EJE-07-0263. [DOI] [PubMed] [Google Scholar]
- 42.Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- 43.Gillam MP, Sidhaye AR, Lee EJ, Rutishauser J, Stephan CW, Kopp P. Functional characterization of pendrin in a polarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem. 2004;279:13004–13010. doi: 10.1074/jbc.M313648200. [DOI] [PubMed] [Google Scholar]
- 44.Coyle B, Reardon W, Herbrick JA, Tsui LC, Gausden E, Lee J, Coffey R, Grueters A, Grossman A, Phelps PD, Luxon L, Kendall-Taylor P, Scherer SW, Trembath RC. Molecular analysis of the PDS gene in Pendred syndrome. Hum Mol Genet. 1998;7:1105–1112. doi: 10.1093/hmg/7.7.1105. [DOI] [PubMed] [Google Scholar]