Abstract
Lysis and extraction of cells are essential sample processing steps for investigations pertaining to metabolism of xenobiotics in cell culture studies. Of particular importance to these procedures are maintaining high lysis efficiency and analyte integrity as they influence the qualitative and quantitative distribution of drug and toxicant metabolites in the intra- and extracellular milieus. In this study we have compared the efficiency of different procedures viz. homogenization, sonication, bead beating, and molecular grinding resin treatment for disruption of HT-29 colon cells exposed to benzo(a)pyrene (BaP), a polycyclic aromatic hydrocarbon (PAH) compound and a suspected colon carcinogen. Also, we have evaluated the efficiency of various procedures for extracting BaP parent compound/metabolites from colon cells and culture media prior to High Performance Liquid Chromatography (HPLC) analyses. The extraction procedures include solid phase extraction, solid-supported liquid- liquid extraction, liquid-liquid extraction, and homogeneous liquid- liquid extraction. Our findings showed that bead-beating in combination with detergent treatment of cell pellet coupled with liquid-liquid extraction yielded greater concentrations of BaP metabolites compared to the other methods employed. Our method optimization strategy revealed that disruption of HT-29 colon cells by a combination of mechanical and chemical lysis followed by liquid-liquid extraction is efficient and robust enough for analyzing BaP metabolites from cell culture studies.
Key Words: Cell lysis, Liquid-liquid extraction, Sonication, Benzo(a)pyrene, HT-29 cells, Colon
Introduction
Benzo(a)pyrene (BaP), a member of the polycyclic aromatic hydrocarbon (PAH) family of compounds is an ubiquitous environmental contaminant to which people are exposed through several routes that include air, water, food, skin contact and occupational settings. Food ingestion is the major route of exposure for a great majority of the population exposed to BaP [1, 2]. Benzo(a)pyrene contamination of food arises from a number of sources, including environment, and food processing techniques. Global dietary intake of PAHs range from 0.02 to 3.6 μg/person/day. Foods that contributed to high PAH intake are red meat, dairy products, fats, oils, cereals and vegetables [1]. Epidemiological [3, 4] and laboratory [5] studies have reported a strong association between dietary exposure to BaP and colorectal cancer [3, 4].
Benzo(a)pyrene, subsequent to biotransformation is broken down to a variety of metabolites, some of which are reactive. These metabolites are bound to cellular macromolecules such as proteins, lipids and nucleic acids. While the metabolites released into the culture medium are amenable for liquid-liquid extraction using organic solvents, the metabolites that are present in the intracellular environment require techniques that must disrupt the cell for extraction of these compounds for further processing by High Performance Liquid Chromatography (HPLC) to determine their identity and quantification.
The various methods routinely employed for cell disruption includes homogenization, sonication, bead milling etc. Regardless of the technique used, the ability to extract either parent compound or metabolites when the cells are exposed to low concentrations of BaP present a challenge. This issue becomes more important when data derived from in vitro studies could be used for risk assessment purposes [6]. A variety of in vitro tools such as subcellular organelles, cell lines, and tissue slices are being used to study the biochemical or molecular mechanisms that underlie gastrointestinal carcinogenesis. The HT-29 cells, originally isolated from colon adenocarcinoma of a Caucasian woman have been used to study cell toxicity [7], chemical-induced differentiation [8], and cellular metabolism [9].
The purpose of this study is three fold; first, it is aimed at determining whether cultured HT-29 colon cells exposed to BaP could metabolically process this toxicant, and release B(a)P metabolites into the culture medium that could be quantified. Secondly, it assesses the efficacy of various cell lysis techniques prior to extracting and quantifying the BaP metabolites in intra- and extra cellular milieus. Finally, our approach also evaluates the efficiency of various extraction methods on the yield of BaP parent compound and metabolites from HT-29 colon cells and culture medium.
Materials and Methods
Preparation of lab ware
To prevent contamination, the following steps were taken. All glassware were acid (15% HNO3)-washed, followed by a detergent (Contrad 70, Fisher Scientific, GA) wash and subsequently rinsed with distilled water. The glassware was dried overnight in an oven at 100°C. All lab ware used for cell culture purposes were presterilized.
Cell culture
For the analytical method evaluation conditions, HT-29 cells were purchased from American Type Culture Collection (ATCC; catalog no. HTB-38; Manassas, Virginia). The cells were cultured in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) supplemented with 10% FBS (Invitrogen) and antibiotics (Penicillin/Streptomycin, Invitrogen). Initially cells were revived by the addition of 1ml of dimethyl sulfoxide (DMSO) stock (HT-29 cell frozen stocks) to 50 ml of media. Every 2 −3 days the media was changed until the cells achieved 80% conflueny. After cells were observed to be healthy, stocks were made using 90% FBS and 10% DMSO. Experiments using BaP were conducted as detailed below.
Benzo(a)pyrene treatment
The test chemical BaP (CAS No. 50-32-8; 98% pure; purity confirmed by gas chromatography–mass spectrometry as per the manufacturer) purchased from the Sigma Chemical Co. (St. Louis, MO) was used for preparation of stock solution. Working standard solutions were prepared by appropriate dilution of stock standard solution. The stock and working standard solutions were stored under refrigeration in either amber colored bottles or clear bottles wrapped with aluminum foil to prevent photodegradation of BaP. Because BaP and its metabolites are suspected carcinogens, they were handled in accordance with NIH guidelines for preventing exposure of lab personnel and equipment to this chemical [10].
Cells were seeded onto a 6 well plate or a T75 flask at a density of 20,000 cells/well or 1 × 106 cells respectively. The cells were then synchronized overnight by serum starvation (DMEM/F12 media, 1% FBS) method. HT-29 cells were treated with 10 & 25μM concentrations of BaP in DMSO (0.01%). Duration of HT-29 cell exposure to BaP lasted for 4 days.
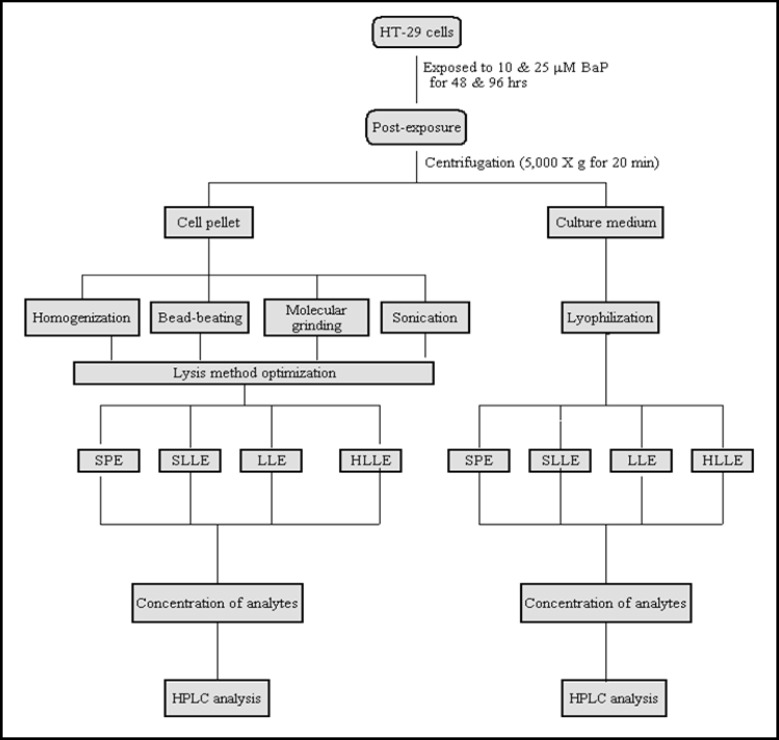
The culture conditions were kept at the same level (number of passages, fresh culture medium, actively growing cells, set duration of exposure, predetermined BaP exposure concentration, and same experimenter) to maintain consistency and avoid culture condition-imposed variables on data generation. The experimental procedures adopted are schematically shown in Fig. 1.
Fig. 1.
Schematic representation of the procedure for extraction of BaP metabolites from colon cells and culture medium. The samples generated from different extraction protocols were analyzed by HPLC. The acronyms used are: SPE: solid-phase extraction; LLE: liquid-liquid extraction; SLLE: solid-supported liquid-liquid extraction; HLLE: homogeneous liquid-liquid extraction; HPLC: high performance liquid chromatography.
Cell lysis
The following methods were used for cell lysis:
Homogenization
The cell pellet was reconstituted in 2 ml of ice-cold sucrose TKM buffer (sucrose 0.25 M, Tris 80 mM, KCl 25 mM, MgCl2 5 mM, pH 7.4) and kept on ice for 5 minutes. A volume of 20 μl of 0.5% SDS was added to the cells, swirled for 2 minutes and broken with a Dounce homogenizer (2 ml capacity; VWR International, Radnor, PA).
Sonication
Cells were subjected to sonication using a VirSonic 50 Ultrasonic Cell Disruptor (SP Scientific Co., Gardiner, NY) operated at 3 cycles of 20s on, 10s off, using a total volume of 1 ml of cell pellet in lysis buffer (G-Biosciences, MO). Care was taken to keep the cells on ice during sonication. The disrupted cell suspensions were stored at 4°C until extraction.
Bead-beating
Cell pellet stored in 2 ml of sucrose-TKM buffer and 5% SDS was placed in a polypropylene tube. Spherical, lead-free, acid washed and double distilled water-rinsed soda lime glass beads (0.1 mm diameter) were added to the pellet. The pellet was lysed with a Disruptor Genie (Scientific Industries, New York, USA) at 4°C. Lysates were clarified by centrifugation at 5,000X g for 20 minutes. The particles (beads) settled to the bottom were discarded and the supernatant fraction was removed using a Pasteur pipette and stored at −4°C until extraction.
Molecular grinding resin
Molecular grinding resin (G-Biosciences, Maryland Heights, MO) was added to cell pellet. The resin consists of high tensile micro particles, which effectively disrupt nuclei and other cellular organelles. The pellet was placed in a 1.5 ml microcentrifuge tube and disrupted using a matching pestle affixed to a hand-held homogenizer. After disruption, the contents were subjected to centrifugation. The supernatant fraction was carefully removed and stored at −4°C until extraction.
Lyophilization of culture medium
After separation of cells, the culture medium was lyophilized for 18 h in a VirTis 12EL Freezemobile (SP Scientific Co., Gardiner, New York) and stored at −70°C until used. The lyophilized culture medium was reconstituted in 5 ml of sucrose TKM buffer and subjected to the extraction protocols detailed in Fig. 1.
Liquid-liquid extraction (LLE)
The lysates of HT-29 colon cells obtained from the bead-beating method were transferred to a 10 ml Corning screw cap tube. A volume of 1 ml HPLC grade water was added, followed by 3 ml of methanol and 1.5 ml of chloroform. The tubes were stirred using a vortexer for 60 s and then centrifuged at 5,000 X g for 20 minutes. After centrifugation, the organic and aqueous layers were separated. The organic layer was carefully retrieved into another tube. The tube with the aqueous layer was subjected to a second extraction with water, methanol and chloroform as mentioned above. The organic layer was retrieved again. Organic phases from both extractions were combined and dried under a steady stream of nitrogen N2. The residue was reconstituted in 300 μl of methanol, passed through Acrodisc filters (0.45 μm; 25 mm diameter; Gelman Sciences, Ann Arbor, MI) to remove particulates. A volume of 30 μl was injected onto HPLC.
The lyophilized culture medium was reconstituted in 1 ml of sucrose-TKM buffer, subjected to LLE as mentioned above. After extraction, the samples were prepared for HPLC analysis.
Homogeneous liquid-liquid extraction (HLLE)
An aliquot of 4 mL supernatant solution of sample was placed in a 10 mL screw cap tube with 0.5 mL hexane as extraction solvent and stirred for 1 minute. Then 2 mL of HPLC grade water was added to it. The extraction solvent was separated from the sample via formation of a distinct water-immiscible phase at the top of the vial. The extraction solvent was drawn out by a 1000 μl Hamilton syringe, transferred to a conical tube and evaporated to near dryness under a steady stream of N2. The residue was reconstituted in 300 μl methanol, filtered to remove particulates and 30 μl of it was injected on to a HPLC column.
Solid phase extraction (SPE)
The SPE cartridges (capacity 3.0 ml; particle size 55μm; Waters Corporation, Milford, MA) were affixed to the Supelco VisiprepTM vacuum manifold (Sigma) were used for solid phase extraction. The cartridges were conditioned according to the manufacturer instructions. Next, the cartridges were equilibrated with 3ml of water at a flow rate of 1ml/min. The column was allowed to air dry and analytes were eluted with 3 ml of dichloromethane at a flow rate of 1ml/min. The eluate was collected in glass tubes wrapped with aluminum foil to prevent photodegradation of BaP and/or metabolites. The eluates collected were individually dried under a gentle stream of N2 at room temperature. The residue was reconstituted in 300μl of methanol, filtered to remove particulates before injecting onto a HPLC column.
Solid supported liquid-liquid extraction (SLLE)
Cleanert SLE Plus cartridges containing specially treated diatomite material were purchased from Bonna-Agela Technologies Inc. (Wilmington, DE). They were fixed to the Supelco VisiprepTM vacuum manifold. Samples of lysed cell pellet or reconstituted lyophilized culture media were loaded onto the column by gentle vacuum. After 3 minutes, methanolchloroform was applied onto the column and the components were let to elute under gentle vacuum. The eluates were collected into glass tubes wrapped with aluminum foil and were individually dried under a gentle stream of N2 at room temperature. The residue was reconstituted with 500μl of methanol, filtered to remove particulates before injecting onto a HPLC column.
HPLC analysis
The BaP parent compound and metabolites from the cells and culture medium were resolved by a HPLC Model 1200 (Agilent Technologies, Wilmington, DE) equipped with a fluorescence detector. The chromatograph was operated through a ChemStation (Agilent Technologies) for instrument control, data acquisition and analyses. Fifty μl of samples were injected onto a C18 reverse phase column (ODS Pinnacle II, PAH column 4 μm, 250 × 2.1 mm; Restek Corporation, Bellefonte, PA). The column (temperature 33°C) was eluted for 45 minutes at a flow-rate of 1 ml/min with a ternary gradient of water: methanol: ethanol (40: 40: 20%) for 20 minutes, followed by the same gradient at a ratio of 30: 46: 24 for 10 minutes, 100% methanol for 10 min and returning to the initial gradient of 40:40:20 for 5 min. The excitation and emission wavelengths for the detector were 244 and 410 nm respectively. Benzo(a)pyrene metabolite standards were purchased from the National Cancer Institute Chemical Carcinogen Repository (Midwest Research Institute, Kansas City, MO). As BaP and its metabolite standards are potential carcinogens/mutagens, they were handled in accordance with NIH guidelines [10]. Identification of the metabolites was accomplished by comparison of retention times and peak areas of the samples with that of standards.
Recovery experiments for lysis and extraction methods
To the cells, appropriate dilutions of 3H BaP (76 Ci/mmol; GE Healthcare, Piscataway, NJ) was added and incubated for 6 hrs. The radioactivity in the cells and culture medium after incubation were counted. The cells were lysed by the techniques mentioned above. Aliquots of samples prior to- and post lysis were taken for liquid scintillation counting (Beckman Coulter, CA) to compute lysis recovery. Similarly, before extraction, the culture medium was spiked with 3H [BaP]. After extraction, the recovery values were calculated.
Statistics
Every analysis for method optimization was carried out at least three times. A total of 8 samples per method were used. Mean, standard deviation, and standard errors were determined. If relative standard deviation was unusually high, additional measurements were carried out and data outliers were identified by the Grubbs outlier test [11]. The statistical significance of the results (across different treatments) was determined through analyses of variance. The criterion for statistical significance was p < 0.05 in all cases. Error bars are given as ±1 standard error of the mean (SEM).
Results & Discussion
Though sample processing and quantification of toxicants and their metabolites from cell culture systems is not a daunting task as those of whole animal tissues that generate potential interferents, it still is an arduous task owing to the low concentrations of these compounds present in cells or culture media. Therefore, sample processing is a critical step in bioanalysis, which must ensure not only the recovery but also the integrity of sample analytes. Since several methods are used for cell lyses and extraction from normal and human cancerous cell cultures [12], it is necessary to compare/evaluate the extraction efficiency of these methods, and apply the most appropriate method for the quantification of BaP metabolites in colon cell cultures.
As a first step towards cell lysis optimization step, experiments were conducted to select the most appropriate technique (Fig. 1). The cell pellets were subjected individually to homogenization, sonication, bead-beating and molecular resin grinding procedures. The bead-beating of the resuspended pellet coupled with detergent treatment yielded a greater recovery of BaP compared to homogenization, sonication and molecular resin grinding methods (Table 1). The differences in BaP metabolite concentrations were statistically significant (p < 0.005; Figs. 2-5).
Table 1.
Recovery of spiked 3H [BaP] from cell lysis methods. Appropriate dilutions of 3H [BaP] was added to the cultures and incubated for 6 h. After incubation, cells were lysed as mentioned in “Materials and Methods” section. Radioactivity in samples before and after cell lysis were measured using a liquid scintillation counter.
| Method | Recovery (%) |
|---|---|
| Homogenization | 85 |
| Sonication | 83 |
| Bead-beating | 94 |
| Molecular grinding | 87 |
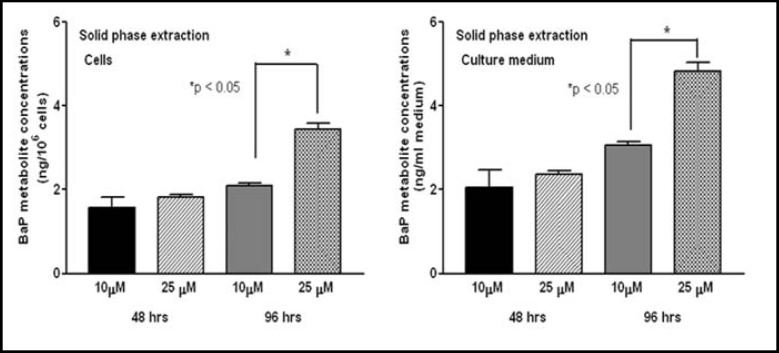
Fig. 2.
Effect of liquid-liquid extraction (LLE) on BaP metabolite concentrations in culture medium and lysates of HT-29 colon cells (106/ml) incubated for 48 & 96 hrs with 10 & 25 μM of benzo(a)pyrene. Post-incubation, cells were lysed by bead-beating treatment; lysates were extracted by LLE and analyzed by HPLC.
Fig. 5.
Effect of solid supported liquidliquid extraction (SLLE) on BaP metabolite concentrations in culture medium and lysates of HT-29 colon cells (106/ml) incubated for 48 & 96 hrs with 10 & 25 μM of benzo(a)pyrene. Post-incubation, cells were lysed by bead-beating treatment; lysates were extracted by SLLE and analyzed by HPLC.
Though homogenization is one of the most common cell disruption methods [13], the recovery of BaP or its metabolite concentrations in homogenized samples were found to be low in our study. Sonication was advocated as the preferred cell disruption method for increased product recovery [14]. While the recovery of BaP was high using this method, the metabolite concentrations registered were lower than homogenization. It has been reported that ultra-sonic waves created by the probe in a liquid medium generate tiny gas bubbles that implode (cavitation process) [15]. The temperature and pressure produced as a result of cavitation may have contributed to lysis and disrupted the integrity of metabolites [15]. Both bead-beating and molecular grinding resin methods yielded appreciable recoveries of BaP than homogenization and sonication. The BaP metabolite concentrations were greater than homogenization and sonication. Even though both molecular grinding resin and bead-beating employ micro-particles for cell disruption, bead beating was found to be more effective than molecular grinding resin.
After optimizing the cell lysis method, we next sought to optimize the extraction technique. As part of this strategy we employed different extraction methods to determine the yield of BaP in both cells and culture medium. Liquid-liquid extraction gave the highest recovery of BaP compared to SPE, SLLE, and HLLE. In the order of percent recovery, LLE > HLLE > SPE > SLLE (Table 2). This trend is reflected in BaP parent compound and organic metabolite concentrations as well.
Table 2.
Recovery of spiked 3H [BaP] from cell culture extraction methods. Appropriate dilutions of 3H [BaP] was added to the cultures and incubated for 6 h. After incubation, cells were lysed; cells and media were extracted as mentioned in “Materials and Methods” section. Radioactivity in samples before and after extraction were measured using a liquid scintillation counter.
| Method | Recovery (%) |
|---|---|
| Solid phase liquid extraction | 88 |
| Supported liquid extraction | 83 |
| Liquid- liquid extraction | 95 |
| Homogeneous liquid- liquid extraction | 90 |
Both cells and culture media yielded greater concentrations of BaP metabolites when the samples were subjected to LLE (Fig. 2), rather than HLLE (Fig. 3), SPE (Fig. 4) and SLLE (Fig. 5).
Fig. 3.
Effect of homogeneous liquidliquid extraction (HLLE) on BaP metabolite concentrations in culture medium and lysates of HT-29 colon cells (106/ml) incubated for 48 & 96 hrs with 10 & 25 μM of benzo(a)pyrene. Postincubation, lysed by bead-beating treatment; lysates were extracted by HLLE and analyzed by HPLC.
Fig. 4.
Effect of solid phase extraction (SPE) on BaP metabolite concentrations in culture medium and lysates of HT-29 colon cells (106/ml) incubated for 48 & 96 hrs with 10 & 25 μM of benzo(a)pyrene. Post-incubation, cells were lysed by bead-beating treatment; lysates were extracted by SPE and analyzed by HPLC.
Findings of our studies reinforced the notion that liquid-liquid extraction is an effective sample preparation method. This method is based on the principle of differential distribution of analyte between the aqueous medium and an immiscible organic solvent. This process includes thorough and vigorous mixing of both phases and subsequent separation of phases facilitated by mechanical shaking and centrifugation, respectively [16]. Even though several solvent mixtures are used for liquid-liquid extraction purposes, chloroform-methanol mixture (Folch agent) used in our study may have exhaustively extracted BaP and its metabolites. Methanol has been known to denature cell membrane proteins and thereby facilitate the extraction of lipids from inside the cells. As a result, the lipid-bound BaP and/or metabolites were partitioned into chloroform [17]. In addition to lipids, chloroformmethanol can successfully retrieve PAHs from other binding sites such as proteins [18]. It needs to be mentioned that chloroform-methanol mixture has also been found to be effective in extracting BaP and fluoranthene (another PAH compound) from colon and liver tissues of mice and rats respectively in our laboratory [19, 20, 21].
Recently, homogenous liquid-liquid extraction (HLLE) has been touted as an efficient technique for the extraction and determination of PAHs. This method has been employed for detection of PAHs in waste water [22] and vegetables [23]. Unlike LLE, during extraction (phase separation), no interface exists between the water consolute and the extracting solvents. As a result, the surface area of the interface is large enough to hold analytes of interest. The recovery of BaP compound from this method was somewhat lower than LLE.
Next to LLE, SPE provided good recovery for BaP. In recent years, SPE has gained support as an alternative to conventional liquid-liquid extraction due to its reduced solvent use, extraction time, and low costs [24, 25]. In this extraction, BaP is trapped in the C-18 stationary phase because of their chemical nature (non polar) and a stronger affinity for this phase [26]. Using an appropriate solvent, the adsorbed BaP is washed off into an amber tube for further concentration.
We have tested another method, the solid-supported LLE (SLLE), also called as supported liquid extraction (SLE) to examine its efficiency in extracting BaP and/or its metabolites. In this method, the cartridges contain an inert medium such as a high-purity diatomaceous earth with a high surface area and high capacity for aqueous adsorption. When the sample is loaded onto the extraction cartridge, the aqueous phase is absorbed while the analytes are spread over the surface of the support in a very thin layer. When a suitable water immiscible organic solvent is applied, liquid-liquid extraction occurs. The high surface area at the interface between the organic and aqueous phases ensures an efficient extraction. This method has been used for routine extraction of PAHs from water samples and human body fluids [27]. However, its usefulness in extraction of PAHs from cell cultures has not been known.
Our studies have revealed that both LLE & HLLE were effective extraction methods compared to SPE & SLLE. While the principle of SPE is based on adsorption of analytes onto a chemically active packing material, LLE operates on the basis of partitioning equilibrium. Probably during extraction, the breakthrough volume of BaP may have been affected by the concentration of this toxicant in samples and unknown interfering molecules in the packing material.
We also measured the concentrations of different BaP metabolites in cells and culture media to determine whether extraction methods had a bearing on the distribution of BaP metabolite types in these two matrices. Both the cultured HT-29 cells and the culture media registered BaP metabolites. Cultured colon cells were reported to biotransform BaP to an array of metabolites that are egressed into the culture medium [28]. Irrespective of the method used in our study, the total concentration of BaP metabolites were more in culture medium compared to cells. The greater concentrations of BaP metabolites in the medium relative to cultured cells reflect an increased likelihood of metabolic activation of BaP and subsequent excretion into the medium.
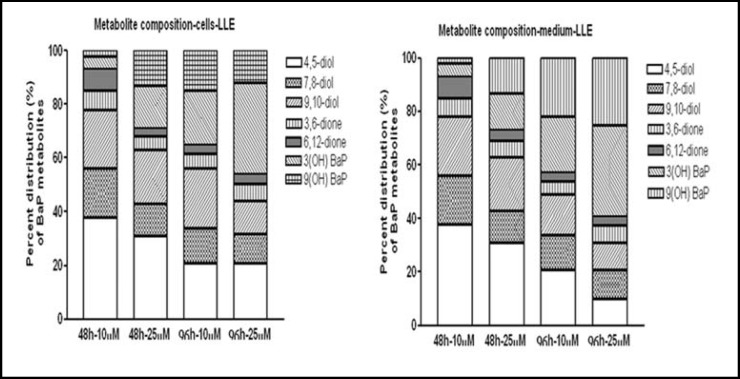
Regardless of the extraction methods employed, the following BaP metabolite types were common to all cells and culture media samples tested. The BaP metabolites were BaP 4,5-diol; BaP 7,8-diol; BaP 9,10-diol; BaP 3,6-dione; BaP 6,12-dione; 3(OH) BaP & 9(OH) BaP. As a representative example, the percent distribution of metabolite types in cells and culture media obtained by LLE is shown in Fig. 6. Neither BaP exposure concentration nor exposure time were found to influence the percent composition of BaP metabolite types in cells and culture media.
Fig. 6.
Distribution (percent of total metabolites) of individual BaP metabolite types in culture medium and lysates of HT-29 colon cells (106/ml) incubated for 48 & 96 hrs with 10 & 25 μM of benzo(a)pyrene. Post-incubation, cells were lysed by bead-beating treatment; lysates were extracted by LLE and analyzed by HPLC.
There were remarkable differences among the extraction techniques in the relative proportion of BaP metabolite types in cells and culture media. The presence of 7,8-diol and 3,6-; 6,12-quinone metabolites in culture medium suggests the egression of these reactive metabolites from the colon cells into the culture medium. However, compared to the concentration of quinone metabolites, the concentration of BaP 7, 8-diol metabolites were low in the medium. Since BaP diol epoxide (BPDE) is unstable in the aqueous medium [29], hydrolysis of this metabolite cannot be ruled out.
For all the extraction techniques used in this study, the presence of unmetabolized BaP in cells was more when exposed to 25 μM BaP, compared to 10 μM BaP at 48 h. However, at 96 hrs of exposure to 10 μM BaP, no parent compound could be detected either in cells or culture media, while at 25 μM BaP, only culture media registered BaP parent compound (Table 3). The presence of BaP parent compound in cells and culture medium at high BaP exposure concentration could be due to the fact that the capacity of cells to metabolize BaP may have reached a saturation point at 25 μM BaP exposure concentration. Our results are in agreement with that of other researchers [28, 30, 31] who also detected BaP parent compound in various human cell cultures exposed to BaP concentrations used in this study.
Table 3.
Concentrations (ng/106 cells or ng/ml medium) of BaP parent compound (unmetabolized) in cell cultures. Required concentrations of BaP was added to the cultures and incubated for specified time periods. After incubation, cells were lysed; cells and media were extracted as mentioned in “Materials and Methods” section. The sample extracts were concentrated, reconstituted in methanol, filtered to remove particulates and injected onto a reverse phase HPLC equipped with a fluorescence detector for quantification of BaP.
| Cells | Culture media | |||||||
|---|---|---|---|---|---|---|---|---|
| Method | 10μM | 25μM | 10μM | 25μM | ||||
| 48 hrs | 96hrs | 48 hrs | 96 hrs | 48 hrs | 96hrs | 48 hrs | 96hrs | |
| Solid phase liquid extraction | 0.20 | ND | 0.34 | ND | 0.30 | ND | 0.42 | 0.23 |
| Supported liquid extraction | 0.25 | ND | 0.40 | ND | 0.18 | ND | 0.40 | 0.21 |
| Liquid-liquid extraction | 0.40 | ND | 0.53 | ND | 0.12 | ND | 0.30 | 0.13 |
| Homogeneous liquid-liquid extraction | 0.40 | ND | 0.55 | ND | 0.15 | ND | 0.34 | 0.19 |
Given the different types of BaP metabolites detected in cell lysates and culture media, a brief account of their generation and relevance to carcinogenesis and toxicity is deemed appropriate. Initial oxidation of BaP is catalyzed by cytochrome P450 (CYP) family of enzymes (CYP1A1, CYP1A2, and CYP1B1), which yield arene oxides (9-OH-BaP, 7-OH-BaP, 6-OH-BaP, 3-OH-BaP, and 1-OH-BaP). These arene oxides either rearrange to phenols or undergo hydration catalyzed by epoxide hydrolase generating BaP-9,10-diol; BaP-7,8-diol; and BaP-4,5-diol [32, 33]. Some of the metabolites such as 7,8-dihydrodiol is activated into 7,8-dihydrodiol 9,10-epoxide, a highly electrophilic chemical species, which binds with cellular nucleophiles such as DNA to cause damage to the cells. Also, during BaP metabolism, some unstable metabolites such as 6-hydroxy-BaP are formed that undergo autooxidation to generate 1,6-, 3,6- and 6,12-quinone metabolites, which are stable [34]. The radical cataions formed as intermediates during BaP metabolism react with water to form quinones and phenols that are highly reactive and interact with DNA to initiate carcinogenesis [35]. The role of the above-mentioned metabolites in carcinogenesis is well known [34]. However, during the conversion of quinones to quinols, reactive oxygen species (ROS) are also produced [36]. Production of ROS leads to severe oxidative stress, eventually contributing to toxicity [37].
The aim of this study was to evaluate the proficiency of various cell lysis and extraction methods and optimize a method for quantifying BaP metabolites from colon cells. Hence we have only focused on providing quantitative and qualitative distribution for BaP organic metabolites only. Subsequent to method optimization, we intend to delineate BaP concentration-response relationship with regard to temporal variations in extracellular and intracellular BaP metabolite levels and types from both organic and aqueous fractions. These aspects together with the activity and expression profiles of BaP biotransformation enzymes will provide a comprehensive picture of BaP metabolism in HT-29 cells and will be the subject of future studies.
In conclusion, our studies revealed that bead-beating coupled liquid-liquid extraction are the ideal cell lysis and extraction methods respectively vis-á-vis other methods for studying BaP metabolism in colon cell cultures. Even though HT-29 cells were employed, the cell lysis and extraction techniques adopted in the present study could be deployed for other PAH-exposed colon tumor cells such as Caco-2, DLD-1, HCT 116, KM12SM, LOVO, LS174T, LT97, SW480, SW620, lung, breast, prostate, ovarian tumor cells, explants of tumors and non-tumor cells such as alveolar cells, aortic endothelial cells, hepatocytes etc.
Supplementary Material
Erratum
Acknowledgements
This publication was made possible by grants 1R01CA142845-01A1 from the National Cancer Institute (NCI), 5T32HL007735-12 from the National Heart, Lung and Blood Institute (NHLBI), 5R25GM059994-11 from the National Institute of General Medical Sciences (NIGMS), and 5 S11ES014156 02 from the National Institute of Environmental Health Sciences (NIEHS) all of which are components of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official to thank Mrs. Aramandla Radhika and Mr. Greg views of NIH. Our thanks are also due to the Southern Poffenberger, Diabetes, Endocrinology, & Metabolism Regional Education Board, Atlanta, Georgia for a Division of Vanderbilt University School of Medicine for Dissertation Award to Mr. Jeremy Myers. We would like assistance with lyophilization of culture media.
References
- 1.Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider H, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh A, Archibong AE, Hood DB, Guo Z, Loganathan BG. Global Environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In: Loganathan BG, Sam PKS, editors. Global Contamination Trends of Persistent Organic Chemicals. Boca Raton, Florida: CRC Press; 2011. pp. 95–124. [Google Scholar]
- 3.Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. Dietary benzo(a)pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2030–2034. doi: 10.1158/1055-9965.EPI-04-0854. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R, Peters U, Cross AJ, Kulldorff M, Weissfield JL, Pinsky PF, Rothman N, Hayes RB. Meat, meat cooking methods and preservation and risk for colorectal adenoma. Cancer Res. 2005;65:8034–8041. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- 5.Harris DL, Washington MK, Hood DB, Roberts LJ, II, Ramesh A. Dietary fat-influenced development of colon neoplasia in ApcMin mouse exposed to benzo(a)pyrene. Toxicol Pathol. 2009;37:938–946. doi: 10.1177/0192623309351722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacGregor JT, Collins JM, Sugiyama Y, Tyson CA, Dean J, Smith L, Andersen M, Curren RD, Houston JB, Kadlubar FF, Kedderis GL, Krishnan K, Li AP, Parchment RE, Thummel K, Tomaszewski JE, Ulrich R, Vickers AEM, Wrighton SA. In vitro human tissue models in risk assessment: report of a concensus building workshop. Tox Sci. 2001;59:17–36. doi: 10.1093/toxsci/59.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Sambruy Y, Ferruzza S, Ranaldi G, DeAngelis I. Intestinal cell culture models. Applications in Toxicology and Pharmacology. Cell Biol Toxicol. 2001;17:301–317. doi: 10.1023/a:1012533316609. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell DM, Ball JM. Characterization of a spontaneously polarizing HT-29 cell line, HT-29/c/f8. Invitro Cell Dev Biol-Animal. 2004;40:297–302. doi: 10.1290/04100061.1. [DOI] [PubMed] [Google Scholar]
- 9.Ebert MN, Klinder A, Peters WHM, Schaferhenrich A, Sendt W, Scheele J, Pool-Zobel BL. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–1644. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- 10.NIH: NIH guidelines for the laboratory use of chemical carcinogens . NIH Publication No. 81-2385. Washington, DC: Government Printing Office; 1981. [Google Scholar]
- 11.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 12.Van Hee P, Middelberg AP, Van Der Lans RG, Van Der Wielen LA. Relation between cell disruption conditions, cell debris particle size, and inclusion body release. Biotechnol Bioeng. 2004;88:100–110. doi: 10.1002/bit.20343. [DOI] [PubMed] [Google Scholar]
- 13.Balasundaram B, Harrison S, Bracewell DG. Advances in product release strategies and impact on bioprocess design. Trends Biotechnol. 2009;27:477–485. doi: 10.1016/j.tibtech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Wenger MD, DePhillips P, Bracewell DG. A microscale yeast cell disruption technique for integrated process development strategies. Biotechnol Prog. 2008;24:606–614. doi: 10.1021/bp070359s. [DOI] [PubMed] [Google Scholar]
- 15.Capelo JL, Galesio MM, Felisberto GM, Vaz C, Pessoa JC. Micro-focused ultrasonic solid-liquid extraction (muFUSLE) combined with HPLC and fluorescence detection for PAHs determination in sediments: optimization and linking with the analytical minimalism concept. Talanta. 2005;66:1272–1280. doi: 10.1016/j.talanta.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Mitra S, Brukh R. Sample preparation: an analytical perspective. In: Mitra S, editor. Sample Preparation Techniques in Analytical Chemistry. Hoboken: John Wiley & Sons, Inc.; 2003. pp. 1–36. [Google Scholar]
- 17.Simko P. Polycyclic aromatic hydrocarbons in smoked meats. In: Toldrá F, editor. Safety of meat and processed meat-food microbiology and food safety. Springer Science & Business Media LLC; 2009. pp. 343–364. [Google Scholar]
- 18.Kwack SJ, Lee BM. Correlation between DNA or protein adducts and benzo(a)pyrene diol epoxide I-triglyceride adduct detected in vitro and in vivo. Carcinogenesis. 1999;21:629–632. doi: 10.1093/carcin/21.4.629. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(a)pyrene in F344 rats following oral administration. Exp Toxic Pathol. 2001;53:275–290. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- 20.Harris DL, Niaz MS, Morrow JD, Washington MK, Ramesh A. Diet as a modifier of benzo(a)pyrene-induced colon tumors in ApcMin mice. In: Obayashi Y, Isobe T, Subramanian A, Suzuki S, Tanabe S, editors. Interdisciplinary Studies on Environmental Chemistry-Environmental Research in Asia-Pacific. Tokyo: TerraPub Publishers; 2009. pp. 227–238. [Google Scholar]
- 21.Walker SA, Addai AB, Mathis M, Ramesh A. Effect of dietary fat on metabolism and DNA adduct formation after acute oral exposure of F-344 rats to fluoranthene. J Nutr Biochem. 2007;18:236–249. doi: 10.1016/j.jnutbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Tavakoli L, Yamini Y, Ebrahimzadeh H, Shariati S. Homogeneous liquid-liquid extraction for preconcentration of polycyclic aromatic hydrocarbons using a water/methanol/chloroform ternary component system. J Chromatogr A. 2008;1196-1197:133–138. doi: 10.1016/j.chroma.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Liu X, Zhao Z, Huang C, Zhang M, Wang H, Wang X. Homogeneous liquid-liquid extraction combined with high performance liquid chromato-graphy-fluorescence detection for determination of polycyclic aromatic hydrocarbons in vegetables. J Sep Sci. 2009;32:2051–2053. doi: 10.1002/jssc.200900019. [DOI] [PubMed] [Google Scholar]
- 24.Hennion MC. Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J Chromatogr A. 1999;856:3–54. doi: 10.1016/s0021-9673(99)00832-8. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan RJ, Taylor A, Watson ID, Whelpton R: Fundamentals of analytical toxicology. John Wiley & Sons, Ltd., England, pp 505.
- 26.Pan H, Cao Y. Optimization of pretreatment procedures for analysis of polycyclic aromatic hydrocarbons in char coal–grilled pork. Anal Lett. 2010;43:97–109. [Google Scholar]
- 27.Arruda AF, Goicoechea HC, Santos M, Campiglia AD, Oliveiri AC. Solid-liquid extraction room temperature phopshorimetry and pattern recognition for screening polycyclic aromatic hydrocarbons and polychlorinated biphenyls in water samples. Environ Sci Technol. 2003;37:1385–1391. [Google Scholar]
- 28.Buesen R, Mock M, Seidel A, Jacob J, Lampen A. Interaction between metabolism and transport of benzo[a]pyrene and its metabolites in enterocytes. Toxicol Appl Pharmacol. 2002;183:168–178. doi: 10.1006/taap.2002.9484. [DOI] [PubMed] [Google Scholar]
- 29.MacLeod MC, Lew L. A rapid, spectrophotometric assay for the integrity of diol epoxides. Carcinogenesis. 1988;9:2133–2135. doi: 10.1093/carcin/9.11.2133. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham MJ, Kurian P, Milo GE. Metabolism and binding of benzo[a]pyrene in randomly-proliferating, confluent and S-phase human skin fibroblasts. Cell Biol Toxicol. 1989;5:155–168. doi: 10.1007/BF00122650. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Gelhaus SL, Mangal D, Harvey RG, Blair IA, Penning TM. Metabolism of benzo[a]pyrene in human broncho-alveolar H358 cells using liquid chromatography-mass spectrometry. Chem Res Toxicol. 2007;20:1331–1341. doi: 10.1021/tx700107z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Shimada T, Guengerich FP. Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol. 2006;19:288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- 34.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbons. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 19–96. [Google Scholar]
- 35.Cavalieri EL, Rogan EG. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25:677–688. doi: 10.3109/00498259509061885. [DOI] [PubMed] [Google Scholar]
- 36.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Yang H, Ramesh A, Roberts LJ, 2nd, Zhou L, Lin X, Zhao Y, Guo Z. Overexpression of Cu/Zn-superoxide dismutase and/or catalase accelerates benzo(a)pyrene detoxification by upregulation of the aryl hydrocarbon receptor in mouse endothelial cells. Free Radic Biol Med. 2009;47:1221–1229. doi: 10.1016/j.freeradbiomed.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Erratum