How did the study come about?
The earliest reports of high incidence of oesophageal cancer (OC) in the northern parts of Iran date back to the early 1970s.1,2 A population-based cancer registry was established in 1969 as a joint effort between Tehran University and the International Agency for Research on Cancer (IARC). This registry confirmed the high incidence of OC in the eastern portion of the Caspian Sea littoral, in the area that is now known as Golestan Province. The highest incidence rates were reported from the semi-desert plain settled mainly by people of Turkmen ethnicity in Gonbad and Kalaleh counties, with estimated incidence rates of 109/105 among men and 174/105 among women (adjusted to the 1970 World Standard Population).3 The registry also showed low incidence of OC in the nearby Gilan province, 300 km to the west of Golestan, with incidence rates of 15/105 and 5.5/105 among men and women, respectively. A series of studies were conducted in the region in the 1970s, but they were not conclusive in explaining the very high rates. However, they pointed to several factors, including: (i) a diet deficient in fruits and vegetables;4 (ii) low socio-economic status; (iii) thermal injury from consumption of very hot tea;5 and (iv) carcinogen exposure from lifestyle factors including opium consumption.6–8 The high incidence of OC in Golestan was confirmed by a recent screening study.9
Aetiological hypotheses related to diet and life style can be best addressed in prospective cohort studies, in which measurement error can be reduced and recall bias is minimal.10 From 2002 to 2003, a pilot study of 1057 subjects was conducted by the Digestive Disease Research Center (DDRC) of Tehran University of Medical Sciences in collaboration with IARC and the US National Cancer Institute (NCI) to evaluate the logistics of establishing a prospective study in Golestan. The aims of the pilot study were to assess the response rate of the study population, to develop valid and reliable methods for assessing nutritional, anthropometric and life-style factors, to develop follow-up methods to ascertain mortality and cancer incidence among the enrolled subjects, and to establish efficient procedures for collecting and storing biological samples. Results of the pilot study confirmed the feasibility of conducting a prospective cohort study in Golestan.11,12 Subsequently, the Golestan Cohort Study (GCS) was launched in January 2004. This study is part of a series of investigations into the etiology of upper gastrointestinal cancers in this area, collectively named Gastric and Oesophageal Malignancies in Northern Iran (GEMINI). The study protocol and the informed consent used for this study were approved by the ethical review committees of DDRC, IARC and NCI. In June 2008, the accrual goal of 50 000 subjects was reached and enrollment was closed.
What does the study cover?
The primary aims of the GCS are:
To identify risk factors for OC by a comprehensive assessment of ethnicity, occupational history, socio-economic status, past medical history, family history of cancers, gastrointestinal symptoms and signs, tobacco, opium and alcohol use, oral health, anthropometric characteristics, physical activity and tea drinking habits, including tea temperature. Nutritional patterns are also evaluated using a food frequency questionnaire (FFQ) specifically developed for this population and validated during the pilot study.12 The FFQ covers 116 food items, including bread and cereals, meat and dairy products, oils, sweets, legumes, vegetables, fruits and condiments, as well as cooking methods.
To establish biospecimen banks for blood, urine, hair and nail samples to be used in molecular and genetic studies of cross-sectional or nested case–control design.
To provide a model for population-based studies in a country in economic and social transition based on collaboration between local health workers, local health authorities, national research centres, national government and international research institutions.
Who is in the sample?
The study population is a sample of the Golestan population, aged 40–75 years. The primary goal was to establish a cohort of 50 000 healthy individuals, with equal numbers of men and women, 20% from urban areas and 80% of Turkmen ethnicity. We enrolled the urban participants from Gonbad City, the second largest city of Golestan, with 126 797 inhabitants (28 102 aged 40–75), and the rural participants from villages in Gonbad, Kalaleh and Aq-Qala counties (Figure 1), with 347 683 inhabitants (53 121 aged 40–75).
Figure 1.
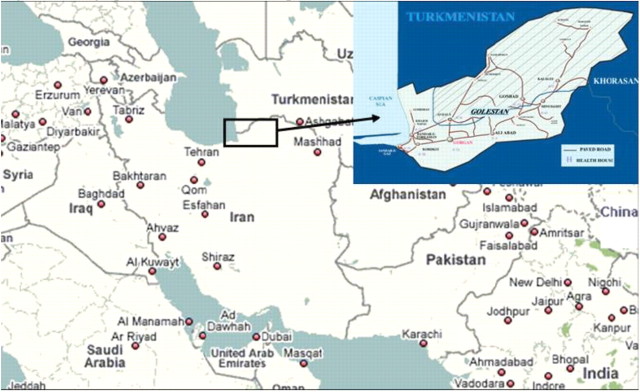
Geographic location of Golestan province in northern Iran. The dashed area approximately corresponds to the GCS field
A total of 16 599 urban inhabitants older than 40 years were selected randomly from five areas of Gonbad City by systematic clustering based on household number. The selected inhabitants were contacted at home by specially trained health workers and invited to visit the Golestan Cohort Study Center, a research centre specifically established for this project in Gonbad, to participate in the study. A total of 10 032 urban participants were enrolled from Gonbad, with participation rates of ∼70% for women and 50% for men.
In rural areas, recruitment took advantage of the network of health houses, primary health care centres present in each group of villages, which are typically staffed by two auxiliary health personnel (locally called the Behvarz). The Behvarz are in charge of vaccination programs, family planning, reporting births, deaths and major communicable diseases, and initial primary care treatment. All residents of all villages in the study catchment area who were eligible for this study were invited to participate. Temporary recruitment centres were established in the health houses of 198 selected villages, and the Behvarz accompanied the GCS research team to contact the selected subjects at their homes. The invitation group thoroughly explained the purpose and procedures of the study to the eligible subjects and invited them to participate in the study. If an eligible subject did not fully understand the procedures, he/she was invited to visit the study centre and observe all steps of the study in person. A total of 40 013 participants were enrolled from 326 villages, with participation rates of 84% for women and 70% for men.
Exclusion criteria were: (i) unwillingness to participate at any stage of the study for any reason; (ii) being a temporary resident; and (iii) having a current or previous diagnosis of an upper gastrointestinal (UGI) cancer. The only data recorded about non-participants were their gender and place of residence (urban or rural). Before interview, a written informed consent was obtained from each participant.
Each subject was interviewed by a trained general physician and a trained nutritionist, either in the local language (Turkmen) or in the national formal language (Persian), depending on the participant's preference. Two structured questionnaires were administered: a life-style questionnaire and an FFQ. Following the questionnaires and a limited physical examination, samples of blood (10 ml), urine (4.5 ml), hair (3 cm from the base of scalp) and nails (trimmings from all 10 toenails) were collected by a trained technician. In the urban area, all biological samples were immediately processed in the central laboratory at the Golestan Cohort Study Center. In the rural areas, blood and urine samples were kept in refrigerators (+4°C), until they were transferred in cooling boxes to the central laboratory; the maximum duration between blood collection and final processing was 8 h. The blood samples were centrifuged and aliquoted in 500 μl straws (eight straws of plasma, four straws of buffy coat and two straws of red blood cells) and stored at −80°C. Urine samples were stored at −20°C, and hair and nail samples were stored at room temperature. Half of the frozen blood samples were subsequently transferred on dry ice to DDRC in Tehran, and then shipped at regular intervals to IARC in Lyon, France, where they are stored in nitrogen vapour (approximately −135°C).
All participants received a personal GCS identification card at the time of enrollment, which allows them to come to Atrak Clinic if they experience any gastrointestinal symptoms. Atrak Clinic is a specialized gastrointestinal clinic established by DDRC in the main hospital in Gonbad,13 and provides free services for the GCS participants.
Table 1 shows demographic characteristics of the cohort participants and non-participants. Compared with participants, the non-participants were more likely to be men and to live in urban areas. The distribution of participants by place of residence and ethnicity is close to the initial goal; however, because of a higher response rate, the number of women in the cohort (n = 28 804) is higher than that of men (n = 21 241).
Table 1.
Demographic characteristics of the 50 045 participants and 18 308 non-participants in the GCS (2004–08)
| Place of residencea |
Ethnicitya |
||||
|---|---|---|---|---|---|
| Total | Urban | Rural | Turkmen | Non-Turkmen | |
| Participants | |||||
| All | 50 045 | 20.0 | 80.0 | 74.4 | 25.6 |
| Men (years) | 21 241 | 18.5 | 81.5 | 75.6 | 24.4 |
| ≤45 | 5394 | 19.4 | 80.6 | 76.4 | 23.6 |
| 46–55 | 7973 | 17.0 | 83.0 | 77.0 | 23.0 |
| 56+ | 7874 | 19.4 | 80.6 | 73.6 | 26.4 |
| Women (years) | 28 804 | 21.2 | 78.8 | 73.6 | 26.4 |
| ≤45 | 8877 | 19.7 | 80.3 | 74.3 | 25.7 |
| 46–55 | 11 532 | 20.8 | 79.2 | 74.0 | 26.0 |
| 56+ | 8395 | 23.3 | 76.7 | 72.3 | 27.7 |
| Non-participants | |||||
| All | 18 308 | 35.9 | 64.1 | – | – |
| Men | 11 361 | 34.7 | 65.3 | – | – |
| Women | 6947 | 37.8 | 62.2 | – | – |
aThese data are row percentages.
How are the subjects being followed up?
Follow-up procedures
All participants are being followed up actively every 12 months. Each cohort member was also instructed at the time of enrollment to contact the GCS team in case of certain conditions like hospitalization or development of a new major disease. These contacts are registered and subsequently followed up. The databases of Atrak Clinic and of the Golestan Cancer Registry are also reviewed monthly to look for cancer cases among the study subjects. The follow-up is expected to continue for a minimum of 10 years.
Ninety-eight percent of participants have a private telephone line. At the time of baseline registration, the participants were questioned about their home and mobile phone numbers. They were also asked to provide two other phone numbers of family members, neighbours or close friends. The participants are first contacted by telephone, if they have a number. If a study participant is not accessible after seven attempts (on different days during 2 consecutive weeks), the GCS team calls other phone numbers available for that participant. If this approach is still unsuccessful, in urban areas the GCS team visits the participant at their home, and in the rural areas, the team contacts the Behvarz and asks them to complete a case review questionnaire.
The GCS team completes a case review questionnaire during each phone call or home visit and records the vital status of the participant and any occurrence of disease or hospital admissions that have taken place since the previous follow-up contact. The participants are also questioned about any plans to change their place of residence in near future. In case a death, cancer or UGI endoscopy is reported, the GCS team visits the participant's home and the medical centres in which any major diagnostic or therapeutic procedures were done. The team collects all clinical reports, pathology reports and hospital records, and any tumor samples that are available. For deceased participants, a verbal autopsy14,15 is also performed.
Up to August 2008, the GCS team contacted 41 008 [10 780 (26.3%) urban and 30 228 (73.7%) rural] participants through 74 922 phone calls or home visits. A total of 19 556 participants were followed once, 12 160 participants twice, 6122 participants three times and 3170 participants four times. The success rate of the follow-up during the first 4 years has been 99.8%.
Case confirmation and outcomes
Three types of outcomes are assessed in the GCS: death (from any cause), occurrence of any cancer (of any site) and occurrence of a UGI cancer (oesophageal or gastric cancer). Two external internists independently review all available clinical documents and allocate a disease code and a date of occurrence to each outcome. The two disease codes are compared, and if they are different, a third, more senior internist reviews the data and makes the final decision on the code. UGI cancer are the most important outcomes of the study, so they are further reviewed and verified by an International Endpoint Review Committee (IERC) composed of experts from DDRC, IARC and NCI.
What has the GCS found?
The GCS is currently too young to provide prospective results regarding the aetiology of OC in Golestan. Nevertheless, we have analysed and present data on baseline demographic characteristics and habits of the study population, cancer incidence and cause-specific mortality, distribution of some cancer risk factors and evidence for internal validity and repeatability of the collected data.
Baseline demographic characteristics and habits
Table 2 shows some of the baseline characteristics of the GCS population. About 50% of men and 85% of women had no formal education. The highest attained educational level was lower in older subjects and among women, compared with younger subjects and men, respectively. The GCS confirms previous findings of a low prevalence of tobacco smoking, nass (a kind of smokeless tobacco) chewing and alcohol drinking in this population, particularly among women.4,11 Among men, ∼60% had never smoked tobacco, and 83 and 92% had never used nass or alcohol, respectively. Among women, the rates of tobacco smoking and consumption of nass and alcohol were negligible. Twenty-two percent of men and 7% of women were current opium users.
Table 2.
Education and habits of the 50 045 participants in the GCS (2004–08), by age and sexa
| Men, by age (years) |
Women, by age (years) |
||||||
|---|---|---|---|---|---|---|---|
| ≤45 | 46–55 | 56+ | ≤45 | 46–55 | 56+ | Total | |
| Highest educational level | |||||||
| University/college | 6.8 | 5.4 | 1.7 | 0.9 | 0.4 | 0.1 | 2.1 |
| 9–12 years at school | 23.8 | 11.7 | 4.2 | 4.3 | 1.8 | 0.3 | 6.3 |
| 6–8 years | 13.1 | 8.8 | 4.8 | 2.7 | 1.4 | 0.6 | 4.5 |
| 1–5 years | 31.3 | 30.7 | 17.9 | 19.2 | 8.3 | 3.0 | 16.9 |
| No schooling | 25.0 | 43.4 | 71.4 | 72.9 | 88.1 | 96.0 | 70.2 |
| Tobacco smoking | |||||||
| Current smoker | 27.8 | 20.0 | 16.1 | 1.4 | 1.9 | 2.4 | 10.5 |
| Ex-smoker | 12.1 | 16.5 | 21.2 | 0.4 | 0.7 | 1.6 | 7.7 |
| Never smoker | 60.1 | 59.5 | 62.7 | 98.2 | 97.4 | 96.0 | 81.8 |
| Nass chewing | |||||||
| Current | 9.4 | 12.0 | 17.6 | 0.4 | 0.8 | 1.8 | 6.2 |
| Ex-chewer | 1.1 | 2.6 | 5.1 | 0.0 | 0.1 | 0.2 | 1.4 |
| Never chewer | 89.5 | 85.4 | 77.3 | 99.6 | 99.1 | 98.0 | 92.4 |
| Alcohol drinking | |||||||
| Current | 2.9 | 1.8 | 0.7 | 0.0 | 0.0 | 0.0 | 0.7 |
| Ex-drinker | 5.0 | 6.6 | 7.0 | 0.0 | 0.0 | 0.0 | 2.7 |
| Never drinker | 92.1 | 91.6 | 92.3 | 100.0 | 100.0 | 100.0 | 96.6 |
| Opium consumption | |||||||
| Current | 22.9 | 23.0 | 22.2 | 4.7 | 7.2 | 9.3 | 13.7 |
| Ex-user | 3.8 | 6.4 | 7.6 | 0.6 | 0.8 | 2.1 | 3.3 |
| Never user | 73.3 | 70.6 | 70.2 | 94.7 | 92.0 | 88.6 | 83.0 |
aAll data are column percentages.
Cancer incidence and cause-specific mortality
Until August 2008, the total number of cancers identified in the cohort was 243 (Table 3), which corresponds to a crude incidence rate of 324 per 100 000 person-years. OC was the most common cancer, comprising nearly 25% of all cancer cases. All OC cases had endoscopy reports and were histologically confirmed. Of the 60 OC cases, 37 were diagnosed in Atrak Clinic. The age-standardized incidence rate of OC (per 100 000 person-years) was 82.6 among men and 95.7 among women. Other major cancers were stomach, breast, leukaemia, lung, colorectal and pancreatic cancer. The total number of deaths was 743, for a crude total mortality rate of 992 per 100 000 person-years (Table 4). Cardiac diseases were the most common cause of death, followed by cancer and stroke, in both urban and rural areas. A total of 2397 GCS participants were referred to Atrak clinic by August 2008; 202 underwent endoscopy, and OC was diagnosed in 37.
Table 3.
The most common cancers diagnosed among the participants of the GCS during the first 4 years of follow up (2004–08)
| Cancer | Number (%) |
|---|---|
| Oesophagus | 60 (24.7) |
| Stomach | 29 (11.9) |
| Breast | 15 (6.2) |
| Leukaemia | 13 (5.4) |
| Lung | 12 (4.9) |
| Colorectal | 10 (4.1) |
| Pancreas | 10 (4.1) |
| Lymphoma | 8 (3.3) |
| Ovary | 7 (2.9) |
| Other | 79 (32.5) |
| Total | 243 (100) |
Table 4.
The most common causes of mortality in the Golestan Cohort Study during the first 4 years of follow up (2004–08)
| Cause of Death | Number (%) |
|---|---|
| Cardiac disease | 235 (31.6) |
| Cancer | 164 (22.1) |
| Stroke | 120 (16.2) |
| Vehicle accident | 33 (4.4) |
| Other | 153 (20.6) |
| Pending | 15 (2.0) |
| Unknown | 23 (3.1) |
| Total | 743 (100) |
Distribution of some cancer risk factors
Several sub-studies were conducted within the pilot study of the GCS. Exposure to polycyclic aromatic hydrocarbons (PAHs), estimated by measuring a stable urinary metabolite, was high in the great majority of the participants, most of whom were non-smokers.16 Median serum selenium was 155 µg/l, which suggests that the population of Golestan receives adequate selenium and selenium deficiency is not a risk factor for OC in this region.17 Contamination with carcinogenic mycotoxins was not found in a limited number of raw rice, sorghum and wheat samples that were collected from the region.11 Symptoms of gastro-oesophageal reflux disease were common among pilot study participants, and 31% experienced these symptoms at least once a week.18 Approximately 4.3% of the pilot study participants were positive for hepatitis-B surface antigen (HBsAg),19 and we have developed a plan to enroll them in a separate cohort of HBV carriers. In data obtained from twelve 24-h dietary recalls, rural residents reported significantly lower intake of several food groups and nutrients, and intake of some vitamins was lower than the recommended values among rural dwellers and women.20 The prevalence of the gluten-sensitive enteropathy was ∼1%, so this disease is unlikely to have a major impact on the incidence of OC in Golestan.21 Average body mass index (BMI) in a subset of GCS participants was shown to be high; the prevalences of overweight (BMI ≥25) and obesity (BMI ≥30) were 63.5 and 28.4%, respectively.22
Internal validity and repeatability
The pilot study interviewed 1057 study subjects, and a repeat interview was performed on 131 subjects 2 months after the first interview. The kappa statistics for agreement were above 0.7 for most variables, including tobacco, nass, opium and alcohol consumption, as well as for most self-reported gastro-oesophageal symptoms.11 Two different methods were examined for estimating the temperature at which tea was usually consumed, and the method with the higher repeatability (kappa statistics = 0.71) was selected for use in the actual cohort.11 The validity of the questionnaire data about opium use was assessed in 150 subjects by comparing their questionnaire responses with the presence of codeine or morphine in their urine; the questionnaire responses had a sensitivity of 0.93 and a specificity of 0.89 for identifying subjects with these urinary opium metabolites.23 There was also a good agreement between self-reported current tobacco smoking or nass use and positive urinary cotinine.11,23 To validate the study FFQ, twelve 24-h recall questionnaires (one every month) and four FFQs (one in each season) were administered to 131 participants during 1 year. There was good correlation between FFQ and recall data on food group and nutrient intakes, and there was acceptable correlation between FFQ data and biomarker measurements.12
To examine the repeatability of the data collected in the actual cohort, we repeated the entire enrollment process, including interviews and sample collections, in 698 cohort participants from rural areas. The mean interval between the first and second enrollments was 45 months. Representative results are presented in Table 5; they show very good agreement between data collected at the two interviews.
Table 5.
Distribution of selected variables in 698 participants of the GCS who were interviewed twice, ∼4 years aparta
| Percentb |
|||
|---|---|---|---|
| Characteristic | First interview | Second interview | Kappa statistics |
| Ethnicity | 1.00 | ||
| Turkmen | 97.4 | 97.4 | |
| Non-Turkmen | 2.6 | 2.6 | |
| Highest educational level | 0.87c | ||
| University/college | 1.3 | 1.6 | |
| 9–12 years at school | 3.9 | 2.7 | |
| 6–8 years | 3.0 | 2.3 | |
| 1–5 years | 12.9 | 12.2 | |
| No schooling | 78.9 | 81.2 | |
| Tobacco smoking | 0.85 | ||
| Ever smoker | 11.3 | 11.9 | |
| Never smoker | 88.7 | 88.1 | |
| Nass chewing | 0.78 | ||
| Ever chewer | 4.7 | 5.0 | |
| Never chewer | 95.3 | 95.0 | |
| Opium consumption | 0.76 | ||
| Ever user | 11.8 | 14.6 | |
| Never user | 88.2 | 85.4 | |
aThe mean interval between the two interviews was 45 months.
bAll data are column percentages.
cWeighted kappa statistic.
What are the main strengths and weaknesses?
Strengths and advantages
Establishing the first large, population-based prospective study in Western or Central Asia which has detailed exposure assessments, biological samples and virtually complete follow-up.
Building capacity in terms of training researchers locally and at DDRC, and creating a research infrastructure, including Atrak Clinic.
Conducting high-quality follow up with negligible loss to follow up and detailed information on cancer occurrence and causes of death.
Applying international standards for long-term bio-banking of biological samples.
Repeating measurements (interviews and bio-sampling) in 698 GCS participants and documenting the reproducibility of the results.
Explaining the study methods to people with no formal education by asking them to visit the study centre and observing the procedures before obtaining informed consent;24 a method which could be used in other areas with low literacy.
In addition, the GCS is a main core and logistical supporter for multiple ongoing studies of gastrointestinal, hepatic, metabolic, nephrologic and cardiac diseases, including studies of PAH exposure, a prospective study of viral hepatitis, and a clinical trial of the effect of a poly-pill in preventing cardiovascular events.
Weaknesses
Lack of a systematic update of exposure information is one weakness of the study. However, since the majority of the oesophageal cancer cases will be diagnosed or treated at Atrak Clinic, exposure information can be updated at those visits for these subjects. If further funds become available, updating the exposure data on all or a subset of the study participants will be feasible. This could help in analysing socioeconomic, food pattern and life-style changes among the participants over several years, and could reduce exposure misclassification.
International funds may be needed for long-term follow up and conduct of nested case–control analyses.
Can I get hold of the data? Where can I find out more?
Information about the study design, updated interim analyses, ongoing sub-studies and relevant publications are available at www.ddrc.ac.ir. Specific proposals for national and international collaborations are welcomed. Initial proposals, which include the aim of the proposed study, the required data and a time-table, should be submitted to R.M. (malek@ams.ac.ir) or P.B. (boffetta@iarc.fr). The proposals will be discussed within the steering committee, which includes the principal investigators of the study and, if necessary, other experts according to the proposal's theme.
Funding
Tehran University/Medical Sciences (81/15 to R.M., Principal Investigator); Cambridge University (Cancer Research UK, C20/A5860 to B.P., N.D., Principal Investigators); the intramural research program of the NCI, National Institutes of Health; the IARC.
Acknowledgements
Many individuals have contributed to this study. We wish to thank the study participants for their cooperation over many years and the Behvarz working in the study areas for their help. We also would like to thank Paul Brennan, Pierre Hainaut, Elodie Caboux, Francois Deloche and Mitra Saadatian-Elahi from IARC, and Goharshad Goglani, Karim Aghcheli, Masoud Sotoudeh, Ali Yoonessi, Mohsen Sadat-Safavi, Ramin Shakeri, Mohammad R Akbari and Arash Etemadi from DDRC for their collaboration. We also would like to express our special thanks to general physicians, nurses and nutritionists in the enrollment teams for their collaboration and assistance. We received special support from the Social Security Organization of Iran Golestan Branch. We also enjoyed the close collaboration of Golestan health deputies and the Chief of the Gonbad health district. We thank the Center for Nutrition and Health, National Institute of Public Health and the Environment, Bilthoven, The Netherlands, and the Cancer Research Center of Hawaii, Honolulu, Hawaii, USA, for providing straw filling machines (used for filling the blood storage straws after the blood samples were processed). This study was conducted as a collaboration between DDRC/TUMS (Principal Investigator: R.M.), IARC (Principal Investigator: P.B.), NCI (Principal Investigator: S.M.D.), and Golestan University of Medical Sciences, Gorgan, Iran.
Conflict of interest: None declared.
References
- 1.Mahboubi E. Epidemiologic study of esophageal carcinoma in Iran. Int Surg. 1971;56:68–71. [PubMed] [Google Scholar]
- 2.Kmet J, Mahboubi E. Esophageal cancer in the Caspian littoral of Iran: initial studies. Science. 1972;175:846–53. doi: 10.1126/science.175.4024.846. [DOI] [PubMed] [Google Scholar]
- 3.Mahboubi E, Kmet J, Cook PJ, Day NE, Ghadirian P, Salmasizadeh S. Oesophageal cancer studies in the Caspian Littoral of Iran: the Caspian cancer registry. Br J Cancer. 1973;28:197–214. doi: 10.1038/bjc.1973.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook-Mozaffari PJ, Azordegan F, Day NE, Ressicaud A, Sabai C, Aramesh B. Oesophageal cancer studies in the Caspian Littoral of Iran: results of a case-control study. Br J Cancer. 1979;39:293–309. doi: 10.1038/bjc.1979.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghadirian P. Thermal irritation and esophageal cancer in northern Iran. Cancer. 1987;60:1909–14. doi: 10.1002/1097-0142(19871015)60:8<1909::aid-cncr2820600840>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Joint Iran-IARC Study Group. Esophageal cancer studies in the Caspian littoral of Iran: results of population studies—a prodrome. Joint Iran-International Agency for Research on Cancer Study Group. J Natl Cancer Inst. 1977;59:1127–38. [PubMed] [Google Scholar]
- 7.Dowlatshahi K, Miller RJ. Role of opium in esophageal cancer: a hypothesis. Cancer Res. 1985;45:1906–7. [PubMed] [Google Scholar]
- 8.Ghadirian P, Stein GF, Gorodetzky C, et al. Oesophageal cancer studies in the Caspian littoral of Iran: some residual results, including opium use as a risk factor. Int J Cancer. 1985;35:593–97. doi: 10.1002/ijc.2910350505. [DOI] [PubMed] [Google Scholar]
- 9.Saidi F, Sepehr A, Fahimi S, et al. Oesophageal cancer among the Turkomans of northeast Iran. Br J Cancer. 2000;83:1249–54. doi: 10.1054/bjoc.2000.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White E, Hunt JR, Casso D. Exposure measurement in cohort studies: the challenges of prospective data collection. Epidemiol Rev. 1998;20:43–56. doi: 10.1093/oxfordjournals.epirev.a017971. [DOI] [PubMed] [Google Scholar]
- 11.Pourshams A, Saadatian-Elahi M, Nouraie M, et al. Golestan cohort study of oesophageal cancer: feasibility and first results. Br J Cancer. 2005;92:176–81. doi: 10.1038/sj.bjc.6602249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malekshah AF, Kimiagar M, Saadatian-Elahi M, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr. 2006;60:971–77. doi: 10.1038/sj.ejcn.1602407. [DOI] [PubMed] [Google Scholar]
- 13.Islami F, Kamangar F, Aghcheli K, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–6. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anker M, Black RE, Coldham C, et al. A Standard Verbal Autopsy Method for Investigating Causes of Deaths in Infants and Children. Geneva: World Health Organization; 1999. [Google Scholar]
- 15.Gajalakshmi V, Peto R, Kanaka S, Balasubramanian S. Verbal autopsy of 48 000 adult deaths attributable to medical causes in Chennai (formerly Madras), India. BMC Public Health. 2002;2:7. doi: 10.1186/1471-2458-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamangar F, Strickland PT, Pourshams A, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25:425–28. [PubMed] [Google Scholar]
- 17.Nouarie M, Pourshams A, Kamangar F, et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroenterol. 2004;10:2544–46. doi: 10.3748/wjg.v10.i17.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pourshams A, Rahmani AR, Hatami K. Gastroesophageal reflux disease in Iran. Govaresh. 2005;10:48–53. [Google Scholar]
- 19.Pourshams A, Nasiri J, Mohammadkhani A, Nasrollahzadeh D. Hepatitis B in Gonbad-Kavoos: prevalence, risk factors and intrafamilial spreading. Govaresh. 2004;9:222–25. [Google Scholar]
- 20.Islami F, Malekshah AF, Kimiagar M, et al. Patterns of food and nutrient consumption in northern Iran, a high-risk area for esophageal cancer. Nutr Cancer. 2009 doi: 10.1080/01635580902803735. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoshnia M, Pourshams A, Mohammadkhani A, Tavangar SM, Shahbazkhani B, Malekzadeh R. Celiac Disease in Gonbad-Kavoos. Govaresh. 2005;10:131–33. [Google Scholar]
- 22.Bahrami H, Sadatsafavi M, Pourshams A, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6:158. doi: 10.1186/1471-2458-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abnet CC, Saadatian-Elahi M, Pourshams A, et al. Reliability and validity of opiate use self-report in a population at high risk for esophageal cancer in Golestan, Iran. Cancer Epidemiol Biomarkers Prev. 2004;13:1068–70. [PubMed] [Google Scholar]
- 24.Malekzadeh R, Pourshams A, Aletaha N, Jafari E, Goglani G. How to take consent from illiterate people; GCS experience. Iran: Tehran; 2008. The Second International Congress of Medical Ethic in Iran, Abstract Book; pp. 245–47. [Google Scholar]


