Abstract
The objective of this study was to determine the safety and feasibility of performing transendocardial electromechanical mapping and mesenchymal precursor stem cell injections after left ventricular assist device (LVAD) implantation in a sheep model of acute myocardial infarction.
Six sheep were assigned to either an acute or chronic group. Then we created an acute myocardial infarction in each by occluding the distal left anterior descending coronary artery with a balloon for 90 minutes. All the sheep underwent LVAD implantation 30 days later. On the same day, sheep in the acute group underwent transendocardial cell injections and were euthanized. Sheep in the chronic group received cell injections 2 weeks after LVAD implantation and were euthanized 30 days later.
The presence of the LVAD or the use of chest-closure wires did not interfere with electromechanical mapping. Furthermore, no adverse events were observed during electromechanical mapping or the stem cell injections. In all sheep, the LVAD flow rate was approximately 4 L/min during mapping and the injections, and no adjustments were required. Histologic analysis confirmed that the mesenchymal precursor stem cells were successfully delivered. No differences were observed between the acute and chronic groups.
In conclusion, our study showed that transendocardial electromechanical mapping and stem cell injections are safe and feasible in the presence of an LVAD. Surgically implanted metal devices, including the LVAD, steel chest-closure wire, and skin staples, were compatible with the electromechanical mapping system.
Key words: Electromechanical mapping, heart failure, left ventricular assist device, mesenchymal stem cell
Treatment options for patients with end-stage heart failure are limited. Even with the best medical therapy, half of the patients with end-stage heart failure die within 5 years.1 Because the scarcity of donor organs limits the number of heart transplants that can be performed, left ventricular assist devices (LVADs) have been developed for use as a bridge to transplantation or destination therapy. These devices have shown great potential, and, in some patients, LVAD therapy has even led to ventricular recovery, obviating the need for transplantation or destination therapy. Another treatment option that has shown promise in patients with end-stage heart failure is stem cell therapy. Studies of cardiac cell therapy have shown that transplanted stem cells can differentiate into multiple cell types and have favorable effects on revascularization and cardiac remodeling in the injured heart.2–7 By combining stem cell and LVAD therapy, it may be possible to further improve the survival rate of patients with heart failure. We have previously shown that 3-dimensional (3-D) NOGA mapping (NOGA® XP Cardiac Navigation System, Biosense Webster, a Johnson & Johnson company; Diamond Bar, Calif) can be used to target viable ischemic myocardium for transendocardial injections of stem cells8 and that both NOGA mapping and the injection procedures are safe and feasible.9,10 In the present study, we assessed the safety and feasibility of using NOGA mapping for targeted delivery of stem cells via the transendocardial route in animals with an LVAD in place. We believe that this novel synergistic approach of combining cell and LVAD therapy could potentially be an important means of treating patients with heart failure.
Materials and Methods
Our study comprised 6 Suffolk crossbreed sheep weighing 40 to 100 kg. The animals were cared for in compliance with the “Principles of Laboratory Animal Care,” formulated by the National Society for Medical Research, and with the “Guide for the Care and Use of Laboratory Animals,” provided by the National Institutes of Health (NIH Publication No. 85-23, revised 2011). All protocols related to this study were in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of the Texas Heart Institute at St. Luke's Episcopal Hospital. The sheep were assigned to either the acute group (n=2; immediate cell injections) or the chronic group (n=4; delayed cell injections) (Fig. 1) to assess the feasibility of performing percutaneous transendocardial mapping and delivery of cells with an LVAD in place in both the acute and chronic settings. On day 0, acute myocardial infarction (AMI) was induced in all 6 sheep by balloon occlusion of the distal left anterior descending coronary artery for 90 minutes. After a 30-day recovery period, all sheep were anesthetized, and a transthoracic echocardiogram was performed to assess the adequacy of the AMI model. While the sheep were still anesthetized, a HeartMate II LVAD (Thoratec Corporation; Pleasanton, Calif) was implanted through a left 5th intercostal space thoracotomy. In the acute group, immediately after LVAD implantation, electromechanical mapping was performed by using the NOGA system. Then, sheep mesenchymal precursor stem cells (MPCs) (Mesoblast Ltd.; New York, NY) were delivered by 15 transendocardial injections of 0.2 mL each, for a total dose of 225 million cells. Injections were performed using the MyoStar® catheter (Cordis Corporation, a Johnson & Johnson Company; Miami Lakes, Fla) (Fig. 2). After the cell injections, the sheep were humanely euthanized. The organs were then removed for histopathologic analysis. In the chronic group, 45 days after AMI (15 days after LVAD implantation), the sheep were anesthetized and underwent electromechanical mapping, followed by MPC injections performed as described for the acute group. On day 75, the sheep in the chronic group were humanely euthanized, and the organs were removed for histopathologic analysis.
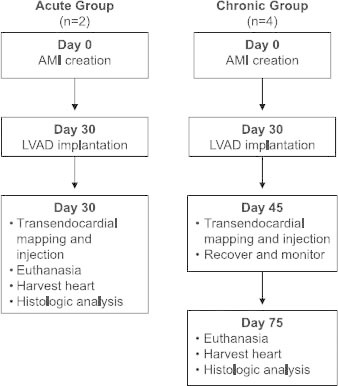
Fig. 1 Flowchart depicting the study design from AMI creation to study endpoint.
AMI = acute myocardial infarction; LVAD = left ventricular assist device
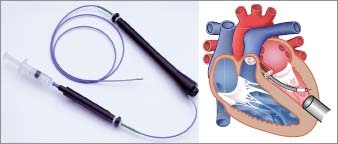
Fig. 2 A) MyoStar injection catheter with close-up view of attached syringe, and B) illustration showing the catheter traversing the aortic valve and the transendocardial extension of the needle during stem cell delivery.
Left Ventricular Electromechanical Mapping Using the NOGA System
The NOGA navigation system comprises a triangular location pad with 3 coils that generate ultralow magnetic field energy, a stationary reference catheter with a miniature magnetic field sensor located on the body surface, a navigation sensor mapping catheter with a deflectable tip and electrodes that provide endocardial signals, and a workstation for information processing and 3-D left ventricular (LV) reconstruction. Sequential sampling of the LV cavity was used to construct the maps. Each sample consisted of a map point that contained regional voltage and contractility information. The femoral artery was exposed through a left femoral cutdown, and an 8F sheath was inserted into the femoral artery. Based on the size of the left ventricle of each animal, we selected either a D-type or F-type NOGASTAR Mapping Catheter (Cordis Corporation). Using fluoroscopic guidance, we advanced the 7F catheter into the descending aorta, then around the aortic arch, and (fully deflected) across the aortic valve into the left ventricle. The deflection of the catheter was relaxed, and the tip was oriented to the LV apex. The initial map points were taken at the border of the LVAD inflow cannula. Subsequent points were taken until all 17 endocardial segments had been uniformly sampled with at least 3 points in each.
Cell Preparation and Labeling
Sheep MPCs were thawed, washed in phosphate buffered saline (PBS), and resuspended in PBS containing 50 mg/mL diamidino-2 phenylindole dihydrochloride (DAPI). The cells were then placed on a rocker and incubated for 30 minutes at room temperature. Afterwards, the cells were washed 3 times in PBS and resuspended at a concentration of 75 × 106 cells/mL in PBS.
Histologic Analysis
Upon excision, the 3 hearts designated for histologic analysis (2 from the acute group and 1 from the chronic group) were rinsed in saline solution and sectioned from the apex to the base into 1- to 1.5-cm–thick transverse slices. Each slice was subsequently divided into segments according to the guidelines on standardized LV myocardial segmentation and nomenclature provided by the American Heart Association.11 Segments of interest that contained targeted injection sites were identified by correlation with each animal's respective NOGA map. These segments were sliced transversely into 2-mm–thick sections. These sections were then fixed in 10% neutral buffered formalin or placed in embedding medium (Tissue-Tek® O.C.T. Compound, Sakura Finetek USA, Inc.; Torrance, Calif) and fresh-frozen at −80 °C. Formalin-fixed samples were subsequently processed and paraffin-embedded. Histologic sections from formalin-fixed and paraffin-embedded tissues were cut 5 mm thick and stained with hematoxylin and eosin (H & E) and Masson's trichrome. The presence of DAPI-labeled MPCs in frozen sections was determined by using fluorescence microscopy.
Immunohistochemical Analysis
The following antibodies were used for antigen detection in the frozen tissue: anti-α-sarcomeric actinin (Sigma-Aldrich; St. Louis, Mo), anti-α-smooth muscle actin (Sigma-Aldrich), and anti-von Willebrand factor (Dako North America, Inc.; Carpinteria, Calif). All antibodies were diluted in PBS with 0.02% bovine serum albumin. Frozen sections were cut into 4-μm–thick sections, placed on positively charged slides, and allowed to air dry for 2 hours at room temperature. The sections were then fixed in 4 °C acetone for 2 minutes, air dried at room temperature for 15 minutes, and rinsed in PBS (pH 7.5) for 5 minutes. Next, the sections were placed in 1.5% normal horse serum (Vector Laboratories, Inc.; Burlingame, Calif) for 10 minutes. The horse serum was gently tapped off, and the sections were incubated with the primary antibodies for 1 hour at room temperature. They were then rinsed with PBS for 5 minutes and incubated in a fluorescein solution in a dark chamber for 10 minutes at room temperature. Fluorescein horse anti-mouse IgG antibody (Vector Laboratories, Inc.) was used as a secondary antibody for the monoclonal antibodies against α-sarcomeric actinin and α-smooth muscle actin. Fluorescein goat anti-rabbit IgG antibody (Vector Laboratories, Inc.) was used as a secondary antibody for the polyclonal antibody against von Willebrand factor. The paraffin-embedded sections were immunostained with anti-Ki-67 (Dako North America). The designated histologic sections were deparaffinized and pretreated with 10 mM citrate buffer (pH=6) for 15 minutes in a microwave oven and then cooled for 20 minutes. The sections were incubated with the primary antibody for 1 hour at room temperature and subsequently developed by using a Mouse IgG VECTASTAIN Elite ABC kit (Vector Laboratories, Inc.).
Results
Five of the 6 sheep survived AMI induction; one animal in the chronic group died during the AMI procedure. All sheep were found to have an LV ejection fraction <0.55, as shown on the 30-day echocardiogram, and remained in the study. An LVAD was implanted in each of the sheep without complications. All sheep underwent successful NOGA mapping and cell injections at the prespecified timepoints. There were no complications, such as stroke, cardiac muscle perforation, or pericardial infusion/tamponade, during the mapping or MPC injections. No deaths were associated with either procedure. All areas of the LV cavity were easily accessible with the NOGA catheter system (Fig. 3). Mapping and cell injections were actually facilitated by the presence of the inflow cannula, which provided a radiographic anatomical reference for visualization at all times. The endocardial surfaces around the LVAD inflow cannula and the inflow cannula itself were easily mapped. There were no difficulties with manipulation of the NOGA catheter, and the flow into the cannula did not interfere with catheter movement. Unipolar voltage and linear local shortening maps appeared to provide reliable and accurate data. The presence of the LVAD or other metal components, such as the sternal wires or skin staples, did not interfere with the NOGA system. The LVAD flow rate was assessed throughout the NOGA procedure and averaged 4 L/min. Both hearts from the sheep in the acute study had transmural, anteroseptal infarcts that showed signs of healing. In both hearts, there was evidence of MPC delivery in the segments corresponding to the injection sites in the respective NOGA maps. In the H & E-stained sections, the injected MPCs were generally observed as clusters of cells with large, rounded nuclei that accumulated along tissue planes or within needle tracts or that pooled at perivascular sites (Fig. 4A–C). The MPC clusters were found mostly at the infarct border, either in the adjacent normal myocardium or within the healing infarct itself. In these acute cases, the injected MPCs showed preserved morphology. Immunohistochemical staining for Ki-67 showed proliferative activity within the MPC clusters (approximately 10% to 15%) (Fig. 4D). We did not observe any evidence of ventricular transmural perforation resulting from cell delivery; however, injection sites that displayed relatively larger clusters were often associated with mild focal hemorrhage. Frozen sections from sheep in both groups were examined by using fluorescence microscopy. In the acute group, these sections showed DAPI-labeled MPCs in a distribution pattern that matched the description above (that is, in clusters or within needle tracts and accumulations) but also revealed a degree of fine dissemination of the injected cells into the adjacent tissue. Immunohistochemical staining of these sections to detect markers of mature cardiac tissue components (sarcomeric α-actinin, α-smooth muscle actin, and von Willebrand factor) showed infiltration and dissemination of the injected DAPI-labeled MPCs within the myocardial tissue (interstitial localization) (Fig. 4E). In the heart from the chronic group, we saw a large transmural organizing infarct in the anterior septum. In the H & E-stained sections, no obvious MPC clusters were observed. However, fluorescence microscopy of the frozen sections revealed an area of DAPI-positive cells within the organizing infarct scar present in the lower anterior wall, suggesting that the location was a former injection site (Fig. 4F).
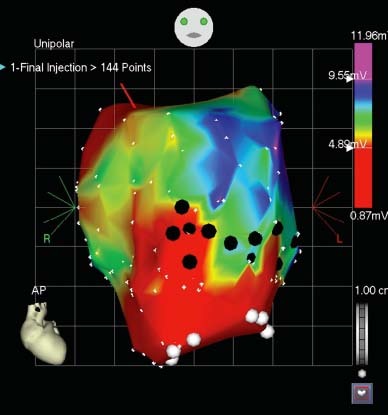
Fig. 3 Representative unipolar view of a NOGA electromechanical map showing the left ventricle after cell injections. Large white dots indicate the inflow tract of the LVAD. Black dots indicate the injection sites. Note that the injections were made in the border zone between the scar of the pre-induced myocardial infarct and the viable zone.
AP = anteroposterior
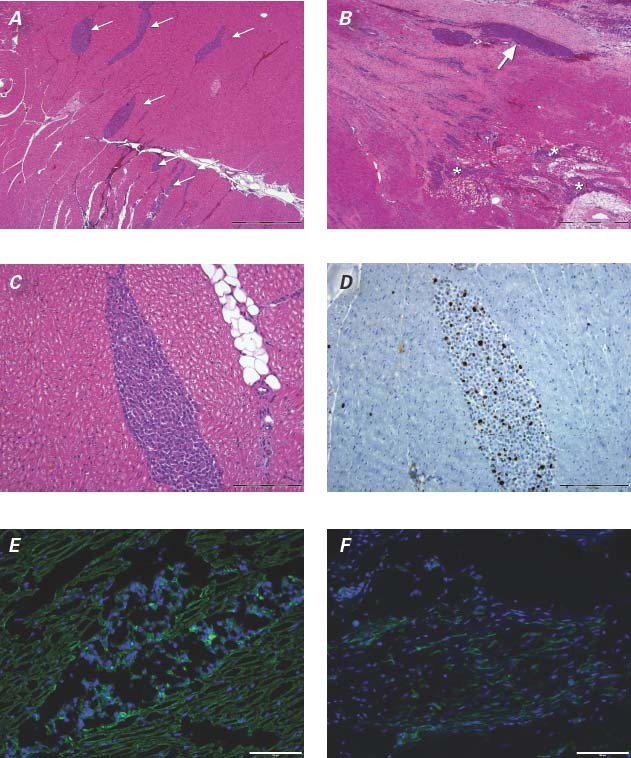
Fig. 4 The distribution pattern of mesenchymal precursor stem cells (MPCs) after NOGA-guided delivery. Photomicrographs of histologic sections from sheep in the acute group showing A) multiple MPC clusters (arrows) in the myocardium (H & E, orig. ×4) and B) a large, linear MPC cluster at the myocardial infarct border (arrow). A more disseminated, infiltrative pattern is observed at the bot-tom right of the section (asterisks) (H & E, orig. ×4). C) Higher power magnification of an MPC cluster in an H & E-stained section (orig. ×20) from a sheep in the acute group and D) its corresponding Ki-67-stained section (orig. ×20). E) DAPI-positive MPCs seen (in blue) in a cluster formation in the center of the image and infiltrating the interstitium of the adjacent myocardium in a sheep in the acute group (α-actinin [green], orig. ×20). F) DAPI-positive MPCs observed (in blue) within the organizing infarct of a sheep in the chronic group (α-smooth muscle actin [green], orig. ×20).
Bar = 1 mm in A and B, 200 μm in C and D, 100 μm in E and F; DAPI = 4′,6-diamidino-2 phenylindole dihydrochloride
Discussion
In this preclinical study, we have shown for the first time the feasibility of using the NOGA catheter to navigate the LV cavity in the presence of an LVAD. The LVAD flow was stable throughout the procedure, and the mapping procedure delineated the LVAD inflow cannula and the surrounding myocardium. Moreover, identification of the underlying tissue and administration of targeted stem cell injections were easily accomplished. The presence of the LVAD did not interfere with either the mapping or the injection procedures. Furthermore, our histologic analysis demonstrated that the stem cells were delivered successfully into the targeted area in the presence of an LVAD. Electromechanical NOGA mapping can be used to map the LV cavity for the purpose of 3-D navigation and characterization of underlying tissue to permit targeting of transendocardial injections. On the basis of unipolar voltage assessment, the NOGA system is able to differentiate between viable and non-viable myocardium, enabling delivery of cells into viable tissue, infarcted myocardium, or the infarct border zone.12 NOGA mapping has previously been performed successfully in patients who have undergone major cardiac surgery; in these cases, there was no mention of surgically implanted materials interfering with the NOGA mapping system.4,5 The present study shows that transendocardial injections performed with the MyoStar catheter can be targeted to viable or non-viable tissue or the infarct border zone of LV myocardium with an LVAD inflow cannula in place. Our group has previously shown that transendocardial cell delivery is superior to intracoronary delivery in regard to cell retention and functional outcome in a canine model of ischemia.13 Therefore, using a transendocardial cell delivery route may be an optimal delivery method in LVAD patients.13,14 Furthermore, injecting cells by the percutaneous/transendocardial route may allow better timing of cell delivery, in that cell therapy can be administered postoperatively—after the patient has undergone LVAD implantation, has been discharged, is ambulatory, and is on a stable medical regimen. Our findings suggest the safety of NOGA mapping and transendocardial injections in the presence of an LVAD. No deaths or complications were associated with either procedure. The only death, which occurred in a sheep in the chronic group, was secondary to the AMI procedure. This death was not unexpected, because there is a 27% mortality rate associated with the AMI model in these animals.15 Our study is limited by its small sample size. This limitation would be a major concern if the objective of our study had been to assess efficacy. However, our sample size was adequate to demonstrate the feasibility and safety of both mapping and cell delivery to a specific area of the heart in the presence of an LVAD.
Conclusion
This study indicates that the use of NOGA mapping and transendocardial stem cell delivery is feasible and safe when performed in the presence of a surgically implanted LVAD in both the acute and chronic settings. The combination of cell therapy and LVAD support may hold promise for treating patients with end-stage heart failure.
Footnotes
Address for reprints: Emerson C. Perin, MD, PhD, 6770 Bertner Ave., MC 2-255, Houston, TX 77030
E-mail: eperin@mac.com
References
- 1.American College of Cardiology, American Heart Association. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure)[published erratum appears in J Am Cardiol 2006;47(7):1503–5]. J Am Coll Cardiol 2005;46(6): e1–82. [DOI] [PubMed]
- 2.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308(22):2369–79. [DOI] [PMC free article] [PubMed]
- 3.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res 2011;109(4):428–36. [DOI] [PMC free article] [PubMed]
- 4.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am Heart J 2011;161(6):1078–87.e3. [DOI] [PubMed]
- 5.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 2012;307(16):1717–26. [DOI] [PMC free article] [PubMed]
- 6.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005; 111(2):150–6. [DOI] [PubMed]
- 7.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002; 105(1):93–8. [DOI] [PubMed]
- 8.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107(18):2294–302. [DOI] [PubMed]
- 9.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation 2004;110(11 Suppl 1):II213–8. [DOI] [PubMed]
- 10.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, et al. Safety of catheter-based intramyocardial autologous bone marrow cells implantation for therapeutic angiogenesis. Am J Cardiol 2006;98(1):60–2. [DOI] [PubMed]
- 11.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105(4):539–42. [DOI] [PubMed]
- 12.Perin EC, Silva GV, Sarmento-Leite R, Vaughn WK, Fish RD, Ferguson JJ 3rd. Left ventricular electromechanical mapping: preliminary evidence of electromechanical changes after successful coronary intervention. Am Heart J 2002;144 (4):693–701. [DOI] [PubMed]
- 13.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol 2008;44(3):486–95. [DOI] [PubMed]
- 14.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction [published erratum appears in J Nucl Med 2006;47(9):1399]. J Nucl Med 2006;47(8): 1295–301. [PubMed]
- 15.Cheng Y, Yi G, Conditt GB, Sheehy A, Kolodgie FD, Tellez A, et al. Catheter-based endomyocardial delivery of mesenchymal precursor cells using 3D echo guidance improves cardiac function in a chronic myocardial injury ovine model. Cell Transplant 2012 Oct 25. [Epub ahead of print]. [DOI] [PubMed]


