Abstract
Isolated left ventricular noncompaction is a rare form of cardiomyopathy characterized by prominent left ventricular trabeculations and intertrabecular recesses. The typical clinical manifestations are severe systolic and diastolic dysfunction, conduction abnormalities, and cardiac embolic events theorized to result from thrombus formation within the intertrabecular recesses. Evidence-based recommendations for preventing thromboembolic events in isolated left ventricular noncompaction have not been established. We report the case of a woman who, at 10 years of age, had been diagnosed with hypertrophic cardiomyopathy without systolic dysfunction. At age 30, she presented with left hemiparesis consequent to a large right-hemispheric ischemic stroke, and she was diagnosed with isolated left ventricular noncompaction. In addition to discussing the patient's case, we review the medical literature that pertains to isolated left ventricular noncompaction.
Key words: Cardiomyopathies/complications/diagnosis/pathology; diagnosis, differential; diagnostic imaging; isolated noncompaction of the ventricular myocardium/complications/diagnosis/ultrasonography; myocardium/pathology; stroke/etiology; thromboembolism/etiology; treatment outcome; ventricular dysfunction, left/complications/diagnosis
Isolated left ventricular noncompaction (ILVNC), an uncommon form of cardiomyopathy, is characterized by prominent trabeculations and intertrabecular recesses that are caused by the arrested development of myocardial compaction during embryogenesis. The term ILVNC refers to noncompaction of the ventricular myocardium in the absence of any congenital cardiac anomalies. The condition commonly manifests itself with severe systolic and diastolic dysfunction, conduction abnormalities, and, more rarely, embolic cardiac events.1–4 There is no consensus in regard to the diagnostic criteria, which are based on echocardiographic findings.1,3–7 Evidence-based recommendations for the prevention of thromboembolic events in ILVNC have not been established. We present the case of a young woman who, as a child, had been diagnosed with hypertrophic cardiomyopathy (HCM) without systolic dysfunction. Twenty years thereafter, she presented with left hemiparesis consequent to a large right-hemispheric embolic stroke, and she was diagnosed with ILVNC. In addition to the patient's case, we review the medical literature relevant to ILVNC.
Case Report
A 30-year-old woman presented with left-sided weakness and slurred speech. She had been diagnosed with HCM at the age of 10 years and had an implantable cardioverter-defibrillator (ICD) for primary prevention. She had no other known cardiovascular risk factor, including any affected first-degree family members. Her only medications were a β-blocker and oral contraceptives. Physical examination yielded no evidence of heart failure. Palpation of the chest revealed a left ventricular (LV) heave. Cardiac auscultation revealed an S4 and no murmur. Neurologic examination yielded a right-sided gaze preference with a left-side visual-field defect, a facial droop, mild dysarthria, a weak left-shoulder shrug, and left upper- and lower-extremity muscle strength of 0/5 (on the Muscle Strength Grading System from the Medical Research Council) in all muscle groups. Left-sided reflexes, including bicipital, brachioradialis, and patellar, were 3/4 on the Deep Tendon Reflexes Grading System; the Babinski reflex was positive. Laboratory tests yielded a normal platelet count and coagulation profile. Noncontrast computed tomograms showed no evidence of intracranial bleeding. Computed tomographic angiograms showed a focal thrombus within the right M1 segment of the middle cerebral artery, reconstitution of the distal branches, a subtle hypodensity of the right lentiform nucleus with decreased blood flow and blood volumes representing a small area of acute infarction, and a large surrounding area of penumbra/salvageable tissue involving the entire right middle cerebral artery territory (Fig. 1). The patient was diagnosed with an ischemic right-caudate lentiform infarct, compatible with a cardiac embolic stroke. Her electrocardiogram upon hospital admission showed sinus rhythm with a left bundle branch block and occasional premature ventricular contractions. A transthoracic echocardiogram (TTE) revealed a thickened LV with more than 3 trabeculations protruding from the posterolateral wall; these moved synchronously with the myocardium (Fig. 2). Intertrabecular recesses were evident, and blood flow into them was apparent on color-flow Doppler echocardiograms (Fig. 3). Systolic function was moderately reduced (LV ejection fraction, 0.40). Interrogation of the ICD disclosed no arrhythmias or device therapies, and no arrhythmias were detected on monitoring throughout the patient's hospitalization. The cerebrovascular event was thought to be a consequence of ILVNC and moderately reduced LV function. The patient was started on anticoagulation with warfarin. Six months later, TTE showed no significant changes, and the patient had residual upper-extremity hemiparesis. A TTE obtained 28 months after her presentation revealed a substantial decline in LV systolic function (ejection fraction, 0.20) and marked noncompaction of the posterolateral walls that extended to the inferior and anterior walls. To our knowledge, after the diagnosis of ILVNC, no first-degree relatives underwent screening examinations.

Fig. 1 Noncontrast-enhanced computed tomograms. A) An axial view of the patient's head on admission shows an area of infarct involving the right caudate nucleus, lentiform nucleus, and posterior limb of the internal capsule (arrows). B) A coronal view shows the infarct site (arrow). C) A 3-dimensional reconstruction image of the circle of Willis (arrow) shows the long segment of stenosis and focal thrombus within the right M1 branch of the right middle cerebral artery (MCA) (arrow), with reconstitution of the distal branches. D) An inferior view of the circle of Willis (MCA) (arrow) shows the site of origin of the right MCA (arrow), which appears to be occluded.
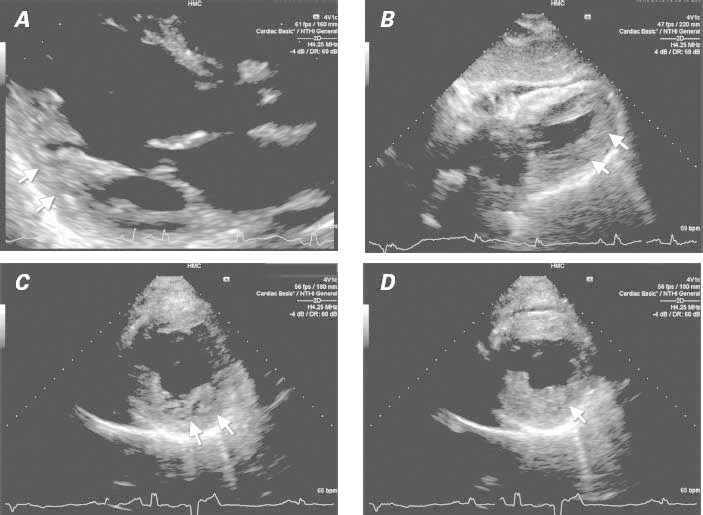
Fig. 2 Transthoracic echocardiogram shows thickened, noncompacted myocardial layers with prominent and excessive trabeculations (arrows) in A) parasternal long-axis view during diastole, B) subcostal view during systole, and parasternal short-axis views during C) diastole and D) systole.
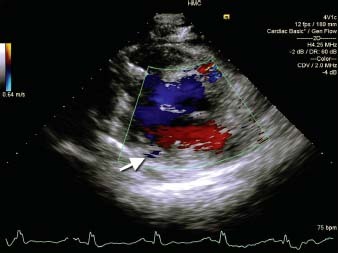
Fig. 3 Two-dimensional color-flow Doppler transthoracic echocardiogram (parasternal short-axis view) of the left ventricle shows blood flow into the intertrabecular recesses (arrow).
Discussion
Isolated LV noncompaction is being recognized more often in the differential diagnosis of patients with heart failure. This rare form of cardiomyopathy is characterized by prominent trabeculations and intertrabecular recesses that communicate with the ventricular cavity but not the coronary circulation.1,2 The condition is thought to be caused by arrested development of the myocardial compaction between weeks 5 and 8 of embryogenesis. By definition, congenital heart disease is absent in patients with ILVNC.1–3 The condition chiefly affects the LV, but it can be biventricular.2,3,8–10 The most frequently affected myocardial segments are the apex and the distal and middle segments of the inferior and lateral walls.1,2,4 Affected segments have a 2-layered structure: a thin compacted pericardial layer, and a thicker noncompacted endocardial layer with deep recesses—the basis of the LV noncompaction definition. Diagnosis can be difficult,11,12 because prominent trabeculations can be found in up to 68% of healthy hearts13 and can be observed in hearts with muscular hypertrophy secondary to dilated, valvular, or hypertensive cardiomyopathy. Among patients with normal heart variants, the anatomic distribution of the trabeculations can be a useful criterion, because they course from the free wall to the ventricular septum in 85% of ILVNC cases.13 The similarities between ILVNC and other cardiac diseases, together with insufficient awareness among clinicians, can lead to a delay in the condition's diagnosis. In one series, the diagnosis of ILVNC was initially missed in 89% of the cases: 42% of the incorrect original diagnoses were of dilated cardiomyopathy and 15% were of hypertrophic cardiomyopathy.3
Isolated LV noncompaction is typically diagnosed with use of TTE and, with increasing frequency, cardiac magnetic resonance (CMR).14 Additional detail of the deep intertrabecular recesses can be seen upon the use of contrast echocardiography,15,16 3-dimensional echocardiography,16 transesophageal echocardiography, multidetector computed tomography,17 or LV angiography.2,3 Several sets of echocardiographic definitions have been proposed for the diagnosis of ILVNC (Table I), all of which focus on the morphology of the condition; however, they vary substantially in their approach. One study18 determined poor correlation between the 3 most frequently used criteria (those established by Chin,1 Jenni,5 and Stöllberger7 and their respective colleagues). Despite the lack of consensus about echocardiographic definitions, ILVNC is often diagnosed in accordance with Jenni's criteria (as in our patient) or modifications thereof.4,5 The high resolution and multiplanar imaging of CMR are advantageous.14 Although the use of CMR entails a modified version of existing echocardiographic criteria with its same limitations, direct comparison with TTE is not always possible, because the detection of ILVNC with CMR—unlike that with TTE—is performed during diastole.19
TABLE I. Echocardiographic Criteria

The cause of ILVNC is unknown. Familial occurrence has been established in several cases.1-4,6,8,9,20,21 Genetically, there is an autosomal and X-linked inheritance.18,20,22 Analysis supports a heterogeneous genetic linkage of ILVNC to mutations in several genes: the α-dystrobrevin (DTNA) gene on the 18q12.1-q12.2 chromosome region, which encodes for a cytoskeletal protein component of the dystrophin-associated glycoprotein complex (DAPC)23; the cypher/ZASP gene that encodes for a cardiac- and skeletal-muscle Z-line protein that is expressed in the cytoplasm associated with myofibrillar myopathy when deficient24; the LMNA gene on the 1q12.1-q23 chromosome, which encodes for the lamins A and C; components of the nuclear lamina, where other diseases such as dilated cardiomyopathy, limb-girdle muscular dystrophy, Emery-Dreifuss muscular dystrophy, and partial lipodystrophy have been confined25; the G4.5 gene on the Xq28 chromosomal region, where other myopathies such as Barth syndrome, Becker's muscular dystrophy, and myotubular myopathy have been located,22,26 suggesting the possibility that ILVNC occurs as part of a systemic myopathic process; and the sarcomeric protein genes comprising the β-myosin heavy chain (MYH7), α-cardiac actin (ACTC) and the troponin T type 2 (TNNT2) genes. These encode for the thick and thin filaments of the cardiac sarcomere, mutations of which might be a common cause of ILVNC.27 Male subjects predominate among published cases (Table II),1-4,21,28–32 presumably consequent to the underlying pattern of inheritance. Isolated LV noncompaction might also be an acquired pathologic condition, on the basis of the potential development of this condition during adulthood.2,18,22,31,33 Nevertheless, most cases of acquired ILVNC are associated with neuromuscular disorders.6,20,22 Our patient had no evidence of facial dysmorphic or other myopathic features. Different hypotheses attempt to explain the development of acquired ILVNC; however, they remain speculative, and the cause and pathogenesis of the condition remains unclear. The World Health Organization catalogues this condition as an unclassified cardiomyopathy,34 although more recently the American Heart Association has considered it to be a distinct cardiomyopathy.35
TABLE II. Case Series Reporting Patients' Characteristics, Risk of Thromboembolic Events, and Recommendations in Regard to Anticoagulation for Primary or Secondary Prevention

The prevalence of adult ILVNC in different case series has ranged from 0.01% to 0.27%.2,7,30 More recent studies have suggested a higher prevalence (0.25%–5%),36,37 presumably because of increased awareness, family screening of first-degree relatives of affected individuals, overly sensitive diagnostic criteria, or even the lack of specificity of currently available criteria.32 The presentation and age of onset of ILVNC are highly variable (Table II).1-4,9,21,28–32,38 One patient was first diagnosed with ILVNC at age 90 years.39 Heart-failure symptoms are the usual reason for presentation,4,9,21,30,40 hospital admission,4 and echocardiographic investigation (in 53%–68% of patients).1,4,30,40 Other reasons are chest pain (in 19%–25%),30,40,41 syncope or palpitations (9%–22%),30,40,41 and stroke or embolism (3%–5%).30,42 Among patients with heart failure, ILVNC accounts for 3% of adult cases.43 Up to 59% of patients are asymptomatic, and diagnosis is made after familial referral (59%), findings of electrocardiographic abnormalities (23%), and detection of cardiac murmurs (6%).21 The clinical spectrum can range from subclinical or asymptomatic presentation2,3,21 to life-threatening arrhythmias and sudden cardiac death.1,4,28,43–46 In early reports, prognoses were poor, with high morbidity rates (35%–38%)1,4 from thromboembolism, ventricular arrhythmias, and severe systolic dysfunction1-4,42,47 that led to heart transplantation4,43 or caused death.1,2,4,48 However, later studies indicated better prognoses than had been suspected, especially in patients without symptoms3,6,21,49: freedom from transplantation and death was approximately 75% and 85% at 3 and 5 years, respectively.21 Some have suggested that ILVNC exists as a spectrum of diseases, which might explain the discrepancy in prognoses attributed to the condition.4,21
Since ILVNC was first described,1 it has been associated with cardiac embolic complications. Some authors thought that these resulted merely from thrombus formation within the intertrabecular recesses, suggesting that ILVNC by itself would constitute a significant risk factor for stroke and peripheral embolism.1,2,4,28,42,50,51 Other authors have reported that additional cardiovascular or coagulation abnormalities that increase the risk for systemic embolism—such as patent foramen ovale, atrial fibrillation,48 atrial flutter,44,52 LV systolic dysfunction,8,28,29,31,34,44,53–55 antiphospholipid syndrome,4 elevated factor VIII levels,55 sepsis,29 or essential thrombocythemia34—are required for the development of cardiac embolic phenomena.29,32 Additional case series have yielded no cardiac embolic events3 or increased risk of stroke in association with ILVNC.31,32 There have been fewer reported cases of peripheral cardiac embolic complications, including 2 cases of embolism to the superior mesenteric artery4,56 and one of coronary artery embolism.57 Detailed clinical data of individual patients were not provided in the first report,4 which included 8 patients with ILVNC; the patients in the other 2 cases had severe LV dysfunction56,57 and mildly elevated homocysteine levels.56 Whether ILVNC itself has a direct association with the development of cardiac embolic complications is an open question.
As a child, our patient was diagnosed with HCM and preserved ventricular function. As an adult, she presented with clinical and radiologic evidence of a cardiac embolic stroke and fulfilled all of the echocardiographic criteria proposed by Chin1 and Jenni5 (Table I) for the diagnosis of ILVNC. Her stroke was attributed to ILVNC. With the exception of ILVNC, newly diagnosed mild LV systolic dysfunction, and the taking of oral contraceptives, our patient had no risk factor for thrombus formation. Her ILVNC could have been present for many years; however, her first cardiac embolic event developed at age 30, coincidental with the deterioration of her LV function. These findings, along with a few other reports,53 support the general consideration that LV dysfunction warrants the initiation of prophylactic anticoagulation in patients with ILVNC.
The goal in treating ILVNC is to prevent the development or recurrence of comorbidities. Arrhythmias are usually treated with β-blockers, calcium-channel blockers, or antiarrhythmic drugs. Studies have shown that the implantation of ICDs in patients with ILVNC was effective for secondary and even primary prevention of sudden cardiac death.49,50 Prophylactic anticoagulative therapy is debated. Some authors recommend oral anticoagulation for primary prevention in all patients with confirmed diagnoses of ILVNC,43,45 regardless of LV size and function1,4,45,46,48 or whether thrombi are seen on TTE.2,25 Oral anticoagulation can be considered in patients with other conditions associated with an increased risk of cardiac embolic phenomena, such as coexisting atrial or ventricular arrhythmias, confirmed LV functional impairment, or right or left atrial or ventricular thrombi.26,27 Some authors recommend antiplatelet therapy in all patients diagnosed with ILVNC,9 although its role in primary prevention of thromboembolic events in ILVNC has not been well studied.58 Evidence-based recommendations for preventing thromboembolic events in ILVNC have not been established.
As suggested in our patient's case, the development of systolic dysfunction in ILVNC could warrant prophylactic anticoagulation to prevent cardiac embolic stroke. An ILVNC registry or larger patient cohort is needed to determine the role and timing of anticoagulative therapy.
Footnotes
Address for reprints: Giselle A. Baquero, MD, 500 University Dr., P.O. Box 850 (Mail Code H047), Hershey, PA 17033-0850
E-mail: gbaquero@hmc.psu.edu
References
- 1.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990;82(2):507–13. [DOI] [PubMed]
- 2.Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997;72(1):26–31. [DOI] [PubMed]
- 3.Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999;34(1):233–40. [DOI] [PubMed]
- 4.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000;36(2):493–500. [DOI] [PubMed]
- 5.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86(6):666–71. [DOI] [PMC free article] [PubMed]
- 6.Belanger AR, Miller MA, Donthireddi UR, Najovits AJ, Goldman ME. New classification scheme of left ventricular noncompaction and correlation with ventricular performance. Am J Cardiol 2008;102(1):92–6. [DOI] [PubMed]
- 7.Stollberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr 2004;17(1):91–100. [DOI] [PubMed]
- 8.Vijayvergiya R, Jha A, Panda SN, Grover A. Biventricular non-compaction–the rare cause of stroke in a young boy. Int J Cardiol 2008;129(3):e84–5. [DOI] [PubMed]
- 9.Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, Belmont JW, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation 2003;108(21):2672–8. [DOI] [PubMed]
- 10.Frischknecht BS, Attenhofer Jost CH, Oechslin EN, Seifert B, Hoigne P, Roos M, Jenni R. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr 2005;18(8):865–72. [DOI] [PubMed]
- 11.Perez-David E, Garcia-Fernandez MA, Gomez-Anta I, de Diego JJ, Garcia-Robles JA, Lafuente J. Isolated noncompaction of the ventricular myocardium: infrequent because of missed diagnosis? J Am Soc Echocardiogr 2007;20(4):439.e1–4. [DOI] [PubMed]
- 12.Stollberger C, Finsterer J, Sodeck GH, Grassberger M, Zimpfer D. Stroke from noncompaction overlooked by echocardiography. Int J Cardiol 2011;148(3):357–8. [DOI] [PubMed]
- 13.Boyd MT, Seward JB, Tajik AJ, Edwards WD. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two-dimensional echocardiography. J Am Coll Cardiol 1987;9(2):323–6. [DOI] [PubMed]
- 14.Yun H, Zeng MS, Jin H, Yang S. Isolated noncompaction of ventricular myocardium: a magnetic resonance imaging study of 11 patients. Korean J Radiol 2011;12(6):686–92. [DOI] [PMC free article] [PubMed]
- 15.Buss SJ, Mereles D, Katus HA, Hardt SE. Left ventricular non-compaction cardiomyopathy mimicking an infiltrative cardiac disease. Int J Cardiol 2011;147(3):e41–3. [DOI] [PubMed]
- 16.Baez-Escudero J, Pillai M, Nambi V, Dokainish H. Comprehensive contrast and 3-dimensional echocardiographic imaging of left ventricular noncompaction cardiomyopathy. Eur J Echocardiogr 2008;9(1):156–7. [DOI] [PubMed]
- 17.Bladt O, Vanhoenacker R, Bevernage C, Leyman P. Isolated noncompaction of ventricular myocardium. Diagnosis with multidetector computed tomography. JBR-BTR 2008;91(4): 153–4. [PubMed]
- 18.Finsterer J, Stollberger C, Schubert B. Acquired left ventricular hypertrabeculation/noncompaction in mitochondriopathy. Cardiology 2004;102(4):228–30. [DOI] [PubMed]
- 19.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46(1):101–5. [DOI] [PubMed]
- 20.Bleyl SB, Mumford BR, Thompson V, Carey JC, Pysher TJ, Chin TK, Ward K. Neonatal, lethal noncompaction of the left ventricular myocardium is allelic with Barth syndrome. Am J Hum Genet 1997;61(4):868–72. [DOI] [PMC free article] [PubMed]
- 21.Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, Perugini E, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 2007;93(1):65–71. [DOI] [PMC free article] [PubMed]
- 22.Bleyl SB, Mumford BR, Brown-Harrison MC, Pagotto LT, Carey JC, Pysher TJ, et al. Xq28-linked noncompaction of the left ventricular myocardium: prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet 1997;72(3):257–65. [PubMed]
- 23.Kenton AB, Sanchez X, Coveler KJ, Makar KA, Jimenez S, Ichida F, et al. Isolated left ventricular noncompaction is rarely caused by mutations in G4.5, alpha-dystrobrevin and FK Binding Protein-12. Mol Genet Metab 2004;82(2):162–6. [DOI] [PubMed]
- 24.Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol 2003;42(11):2014–27. [DOI] [PubMed]
- 25.Monserrat L, Hermida-Prieto M, Fernandez X, Rodriguez I, Dumont C, Cazon L, et al. Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur Heart J 2007;28(16):1953–61. [DOI] [PubMed]
- 26.Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, Miyawaki T, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 2001;103 (9):1256–63. [DOI] [PubMed]
- 27.Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 2008;117(22):2893–901. [DOI] [PubMed]
- 28.Tong KL, Ding ZP. Isolated non-compaction of ventricular myocardium: a report of three cases. Ann Acad Med Singapore 2001;30(5):539–41. [PubMed]
- 29.Stollberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction and stroke or embolism. Cardiology 2005; 103(2):68–72. [DOI] [PubMed]
- 30.Stollberger C, Winkler-Dworak M, Blazek G, Finsterer J. Cardiologic and neurologic findings in left ventricular hypertrabeculation/noncompaction relating to echocardiographic indication. Int J Cardiol 2007;119(1):28–32. [DOI] [PubMed]
- 31.Finsterer J, Stollberger C, Molzer G, Winkler-Dworak M, Blazek G. Cerebrovascular events in left ventricular hypertrabeculation/noncompaction with and without myopathy. Int J Cardiol 2008;130(3):344–8. [DOI] [PubMed]
- 32.Fazio G, Corrado G, Zachara E, Rapezzi C, Sulafa AK, Sutera L, et al. Anticoagulant drugs in noncompaction: a mandatory therapy? J Cardiovasc Med (Hagerstown) 2008;9(11):1095–7. [DOI] [PubMed]
- 33.Stollberger C, Finsterer J, Blazek G, Bittner RE. Left ventricular non-compaction in a patient with Becker's muscular dystrophy. Heart 1996;76(4):380. [DOI] [PMC free article] [PubMed]
- 34.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93(5):841–2. [DOI] [PubMed]
- 35.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113(14):1807–16. [DOI] [PubMed]
- 36.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 2008; 29(1):89–95. [DOI] [PubMed]
- 37.Stollberger C, Finsterer J. Cardiologic and neurologic findings in left ventricular hypertrabeculation/non-compaction related to wall thickness, size and systolic function. Eur J Heart Fail 2005;7(1):95–7. [DOI] [PubMed]
- 38.Captur G, Nihoyannopoulos P. Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int J Cardiol 2010;140(2):145–53. [DOI] [PubMed]
- 39.Cevik C, Stainback RF. Isolated left ventricular noncompaction in a 90-year-old man. Tex Heart Inst J 2012;39(2):255–7. [PMC free article] [PubMed]
- 40.Stollberger C, Blazek G, Wegner C, Winkler-Dworak M, Finsterer J. Neuromuscular and cardiac comorbidity determines survival in 140 patients with left ventricular hypertrabeculation/noncompaction. Int J Cardiol 2011;150(1):71–4. [DOI] [PubMed]
- 41.Yousef ZR, Foley PW, Khadjooi K, Chalil S, Sandman H, Mohammed NU, Leyva F. Left ventricular non-compaction: clinical features and cardiovascular magnetic resonance imaging. BMC Cardiovasc Disord 2009;9:37. [DOI] [PMC free article] [PubMed]
- 42.Conces DJ Jr, Ryan T, Tarver RD. Noncompaction of ventricular myocardium: CT appearance. AJR Am J Roentgenol 1991;156(4):717–8. [DOI] [PubMed]
- 43.Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, Attenhofer Jost CH. Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience. Cardiology 2009;112(2):158–64. [DOI] [PubMed]
- 44.Vatthyam RK, Bates JR, Waller BF. Acute cardiac and neurologic decompensation in a high school athlete. J Am Soc Echocardiogr 2009;22(12):1420.e1–3. [DOI] [PubMed]
- 45.Yasukawa K, Terai M, Honda A, Kohno Y. Isolated noncompaction of ventricular myocardium associated with fatal ventricular fibrillation. Pediatr Cardiol 2001;22(6):512–4. [DOI] [PubMed]
- 46.Seres L, Lopez J, Larrousse E, Moya A, Pereferrer D, Valle V. Isolated noncompaction left ventricular myocardium and polymorphic ventricular tachycardia. Clin Cardiol 2003;26 (1):46–8. [DOI] [PMC free article] [PubMed]
- 47.Jenni R, Rojas J, Oechslin E. Isolated noncompaction of the myocardium. N Engl J Med 1999;340(12):966–7. [DOI] [PubMed]
- 48.Conraads V, Paelinck B, Vorlat A, Goethals M, Jacobs W, Vrints C. Isolated non-compaction of the left ventricle: a rare indication for transplantation. J Heart Lung Transplant 2001; 20(8):904–7. [DOI] [PubMed]
- 49.Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J 2005;26 (2):187–92. [DOI] [PubMed]
- 50.Ker J, Van Der Merwe C. Isolated left ventricular non-compaction as a cause of thrombo-embolic stroke: a case report and review. Cardiovasc J S Afr 2006;17(3):146–7. [PubMed]
- 51.Oduncu V, Akgun T, Erkol A, Mutlu B. Biventricular noncompaction presenting with stroke. Eur J Cardiothorac Surg 2008;33(4):737. [DOI] [PubMed]
- 52.Celebi AS, Gulel O, Cicekcioglu H, Celebi OO, Ulusoy V. Isolated noncompaction of the left ventricular myocardium complicated by thromboembolic cerebrovascular accident in a patient with essential thrombocythemia. Int J Cardiol 2008; 128(1):e22–4. [DOI] [PubMed]
- 53.Nakajima M, Hirano T, Doi H, Uchino M. Stroke and ventricular dysfunction in a patient with isolated left ventricular noncompaction. Intern Med 2007;46(15):1251–4. [DOI] [PubMed]
- 54.Kobza R, Jenni R, Erne P, Oechslin E, Duru F. Implantable cardioverter-defibrillators in patients with left ventricular noncompaction. Pacing Clin Electrophysiol 2008;31(4):461–7. [DOI] [PubMed]
- 55.Kobza R, Steffel J, Erne P, Schoenenberger AW, Hurlimann D, Luscher TF, et al. Implantable cardioverter-defibrillator and cardiac resynchronization therapy in patients with left ventricular noncompaction. Heart Rhythm 2010;7(11):1545–9. [DOI] [PubMed]
- 56.Blessing E, Rottbauer W, Mereles D, Hosch W, Benz A, Friess H, et al. Isolated left ventricular noncompaction of the myocardium as a cause of embolic superior mesenteric artery occlusion. J Am Soc Echocardiogr 2005;18(6):693. [DOI] [PubMed]
- 57.Everett ME, Kirkpatrick JN, Lang RM. Noncompaction of the myocardium complicated by coronary artery embolism. J Am Soc Echocardiogr 2005;18(2):194–6. [DOI] [PubMed]
- 58.Finsterer J. Cardiogenetics, neurogenetics, and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol 2009;30(5):659–81. [DOI] [PubMed]


