Abstract
In recent years, our understanding of the physiologic mechanisms of transient takotsubo cardiomyopathy has improved because of the growing use of emergent heart catheterization in patients who present with severe ischemic syndromes. However, even this procedure has revealed only that, in most patients with takotsubo syndrome, the sudden onset of ventricular dysfunction is not due to fixed coronary artery occlusions. We present a case of transient takotsubo cardiomyopathy with an exceptional feature—uneven impairment of both right and left ventricular function, or biventricular takotsubo—and we discuss a novel, comprehensive theory that we have devised to explain the pathophysiology of this syndrome's many manifestations.
Key words: Acetylcholine/diagnostic use; angina pectoris, variant; coronary endothelial dysfunction; coronary vasospasm/physiopathology; takotsubo cardiomyopathy/classification/diagnosis/physiopathology; ventricular dysfunction, left/etiology/diagnosis; ventricular dysfunction, right/etiology/diagnosis
WEBSITE FEATURE
Only during the last 2 decades have Japanese authors1,2 specifically categorized transient takotsubo cardiomyopathy (TTC) as an entity in itself. Before that time, TTC was often called “acute myocardial infarction with normal coronary arteries.”3 Its prevalence is probably as low today as in the remote past. However, acute coronary artery syndromes are now studied aggressively with emergent heart catheterization, which documents better than any previous means the transience of the myopathy and the presence of apparently normal coronary arteries. These circumstances have stimulated the quest to generate a pathophysiologic concept broad enough to encompass the full clinical spectrum of TCC.
Apical ballooning (resulting in a systolic takotsubo or “octopus trap”) is the most frequent and emblematic feature of TTC. The use of this term has successfully promoted awareness of the disease in the cardiology community at large, but it has also impeded clinicians' understanding of the breadth of this entity's clinical manifestations. Our persistently inadequate knowledge of the nature and spectrum of TTC seems to warrant an update on the subject.
Here, we present a case of right ventricular (in union with left ventricular) TTC. In addition, we discuss a pathophysiologic theory that our group has recently proposed, which might explain the newly discovered and broad spectrum of TTC clinical manifestations.
Case Report
In November 2011, an 82-year-old woman presented at our hospital with a 3-hour history of resting chest pain and shortness of breath. In addition, she had been having lower-extremity edema and progressive shortness of breath for the past year, as well as unexplained, intermittent right-sided chest pain during the previous 2 weeks.
She had a history of hypertension and hyperlipidemia. One year before the current admission, an accidental fall had caused a left femoral bone fracture, which was surgically replaced by a hip prosthesis. Postoperative prosthetic infection had necessitated revision of the hip replacement and caused major emotional distress. The patient's chronic anxiety and pain, together with her need for intravenous antibiotics, had necessitated prolonged hospitalization.
At the current admission, the patient was not in acute distress. She had a non-tender, distended abdomen and mild lower-extremity edema. Before admission, her medications included aspirin, atenolol, gabapentin, triamterene, omeprazole, and fenofibrate. Bilateral pleural effusion was found on chest radiography. An electrocardiogram showed mild ST-segment elevation on leads II, III, aVF, V3, and V4 (Fig. 1).
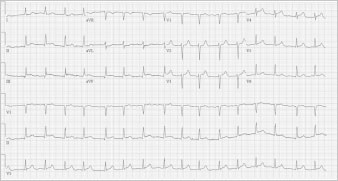
Fig. 1 Electrocardiogram obtained on admission shows ST-segment elevation in leads II, III, aVF, V3, and V4.
The patient was emergently taken to the catheterization laboratory. Selective angiography showed no coronary obstruction; left ventricular angiography revealed an ejection fraction of 0.62 and severe localized apical hypokinesia. Right-sided heart catheterization showed pulmonary hypertension (peak pulmonary artery pressure, 45–50 mmHg) and a mean right atrial pressure of 15 mmHg. The mean pulmonary capillary wedge pressure was 15 mmHg. Cardiac output was 4.3 L/min, the cardiac index was 2.43 L/min/m2, and oxygen saturations were 57% in the pulmonary artery and 92% in the femoral artery.
In the first 12 hours after her admission, the patient's troponin I level peaked at 7.12 ng/mL and her creatine kinase–MB fraction at 19.7 ng/mL. Her B-type natriuretic peptide level was 194 pg/mL on admission and 311 pg/mL the next day. Transthoracic echocardiography showed normal overall left ventricular systolic function (ejection fraction, 0.55) and wall thickness. The apical portions of the septum and left ventricular free wall were thin and akinetic. Her global right ventricular systolic function was mildly reduced, but there was localized apical akinesia of the free wall (Fig. 2) and severe tricuspid insufficiency.
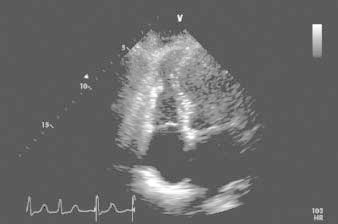
Fig. 2 Transthoracic echocardiogram (4-chamber view), obtained shortly after admission. The associated video shows distal right ventricular free-wall akinesia/dyskinesia, lower septal and apical left ventricular dyskinesia, and cavity dilation.
Real-time motion image is available at www.texasheart.org/journal.
After a favorable clinical recovery on medical therapy, the patient was discharged on day 5 with instructions to take clopidogrel, pantoprazole, rosuvastatin, metoprolol, valsartan, and warfarin. She was eventually re-evaluated as an outpatient at our takotsubo clinic, where she reported persistent fatigue upon minimal exertion, but no more lower-extremity edema. At that time, she was advised to undergo additional acetylcholine testing of her endothelial function to rule out recurrent TTC as the cause of her right-sided heart failure over the past several months and to rule out residual liability to coronary spasm; this spasm was considered a probable cause of her TTC, which was localized in both the right and the left ventricular apical portions. Acetylcholine challenge (100 μg into the left coronary artery, and 50 μg into the right coronary artery) caused mild but definitely abnormal spasm in only some epicardial arteries (mainly the distal left anterior descending and distal right coronary arteries). In addition, echocardiographic monitoring showed worsening of the patient's baseline residual bilateral apical hypokinesia during the test (Fig. 3).
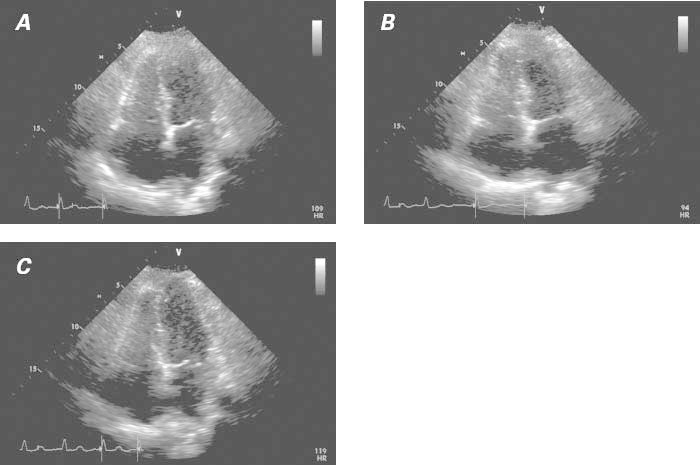
Fig. 3 Transthoracic echocardiograms (4-chamber view), obtained 15 days after onset of takotsubo syndrome. A) Baseline image. The associated video shows moderate improvement of right and left ventricular dysfunction with respect to its state at admission (see Fig. 2). B) Image obtained after intracoronary acetylcholine infusion (100 μg in the left coronary artery). In the associated video, mild worsening of the dysfunction detected at baseline is apparent. C) Image obtained after intracoronary nitroglycerin infusion. The associated video shows definite improvement in ventricular function.
Real-time motion images are available at www.texasheart.org/journal.
After 200 μg of intracoronary nitroglycerin was administered, there was a definite improvement in wall motion at the affected levels. The patient's chest pain quickly disappeared after catheterization. This result, obtained 15 days after presentation, was interpreted as evidence of residual, albeit resolving, endothelial dysfunction. Medical therapy was upgraded to more specific vasodilator treatment and included calcium antagonists, L-arginine, and nitrates. At the 1-month follow-up, echocardiography confirmed the total recovery of normal right and left ventricular function. We concluded that this was a case of biventricular TTC with unusual, uneven impairment of right and left ventricular function.
Discussion
We believe that this case is one of TTC because it meets these 3 diagnostic-inclusion criteria: segmental onset of transient ventricular dysfunction; absence of critical, fixed coronary lesions that could have explained this dysfunction; and positivity of the acetylcholine test.4–6 The relatively mild elevation of cardiac enzyme levels, in view of the severity of myocardial impairment, also supported the TTC diagnosis. More important were the test results, during the month after the patient's initial presentation, that indicated recovery of normal right and left apical ventricular function. This patient probably started to manifest a similar condition (right-sided heart failure) before the event that brought her to our emergency room. Fluid retention with abdominal distention, pleural effusion, and lower-limb edema had started several months earlier and disappeared after treatment; the fluid retention was probably a manifestation of intermittent right ventricular failure caused by recurrent TTC. Pulmonary embolism had been ruled out. Undoubtedly, this patient had prolonged sepsis, as well as stress and anxiety (which can precipitate an episode of TTC) caused by her postoperative complications,5 but there was no clinical evidence (such as tachycardia or hypertension) of a catecholamine surge on admission. We underscore this last point because of the frequently repeated notion—not substantially supported by reliable evidence—that TTC is generically a “catecholamine cardiomyopathy.”7
This case of TTC had an atypical pattern of ventricular dysfunction8–10 that made some members of our team hesitant in their initial interpretation of the clinical presentation. (Small infarction was at first the prevailing diagnosis.) The electrocardiographic findings and clinical presentations of typical versus bilateral TTC do not seem to differ, but the incidence of pleural effusion has been reported as significantly higher in cases of bilateral TTC.8–10 In particular, the few series of TTC patients who have undergone early cardiovascular magnetic resonance suggest that there is right ventricular involvement in 26% of TTC cases.11 The need for a definite diagnosis (before the transient nature of the condition becomes clear) and for specific effective treatment (to improve the patient's symptoms and to prevent recurrence, but also to reach the diagnosis ex juvantibus) makes acetylcholine testing ideal in cases of TTC. Particularly useful is the ability of this test (when performed in the early phase of recovery) to reproduce the original pattern of ventricular dysfunction, which shows its probable mechanism of causation: severe but transient, distinctive coronary spasticity. Our group has suggested, on the basis of a short case series, that transient coronary spasm (totally reversible at its initial manifestation by selective nitroglycerin infusion) in different coronary territories is usually involved.4–6 We theorize, and we are starting a prospective multicenter trial to prove, that an early acetylcholine test of endothelial dysfunction (performed a few days after an episode of TTC activity, when the ventricular dysfunction has mostly resolved) can reveal different patterns of endothelial dysfunction that lead to coronary spasm in the related territories. This segmental spectrum of endothelial dysfunction relates to the different patterns of transient ventricular dysfunction (Fig. 4).4–6 In particular, it seems likely that more than one coronary artery or branch is generally involved at the epicardial and arteriolar levels.4
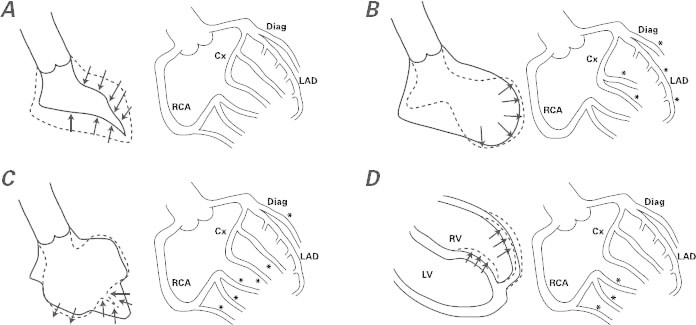
Fig. 4 Side-by-side diagrams illustrate ventricular patterns of regional dysfunction at the end of an endothelial function test performed when the left ventricular (LV) dysfunction has mostly resolved (usually 3–7 d after onset). Findings by LV angiography appear in A–C (at the left in each figure) and by cardiovascular magnetic resonance in D (again at left), and the corresponding coronary angiographic findings after acetylcholine testing appear in A–D (at the right in each figure), according to our group's theory and the early results of acetylcholine testing. A) Negative acetylcholine test, observed in patients with normal endothelial function. B) Apical ballooning seen in typical takotsubo syndrome, accompanied by severe spasm (asterisks) at most of the left-sided, epicardial branches on acetylcholine testing. (Small vessels in these territories are also affected.) C) The effect of acetylcholine infusion on the branches of the left coronary artery in a case of mid-ventricular takotsubo. The middle segments of the LV are dyskinetic at the same time that most branches, except for the left anterior descending coronary artery (LAD), are severely obstructed. D) Right ventricular (RV) apical dyskinesia and isolated right coronary artery (RCA) spasm at acetylcholine testing in a case of RV takotsubo. Usually, RV takotsubo occurs jointly with apical LV takotsubo, as in panel B.
*Site of severe spasm
Cx = circumflex coronary artery; Diag = diagonal coronary artery
The fact that some cases of TTC have patterns of ventricular dysfunction that change over the years12 reveals the complexity and broad spectrum of this unusual entity. Unfortunately, it remains unclear why the different patterns of transient endothelial dysfunction and myopathy occur, why some patients develop endothelial dysfunction, and why they generally seem to recover spontaneously. It is also not known why this dysfunction usually does not recur, even with the persistence of environmental and physiologic conditions that we tentatively call precipitating causes.4–6 Indeed, only 10% of TTC patients seem to have recurrent presentations.6,7
Acknowledgment
We thank James Philpot, ACE, for his assistance with the figures.
Supplementary Material
Footnotes
Address for reprints: Paolo Angelini, MD, 6624 Fannin St., Suite 2780, Houston, TX 77030
E-mail: PAngelini@leachmancardiology.com
References
- 1.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases [in Japanese]. J Cardiol 1991;21(2):203–14. [PubMed]
- 2.Sato H, Tateishi H, Uchida T. Takotsubo-like ventricular dysfunction due to multivessel coronary spasm. In: Kodama K, Haze K, Hori M, editors. Clinical aspects of myocardial injury: from ischemia to heart failure. Tokyo: Kagakuhyoronsha Press; 1990. p. 56–64.
- 3.Alpert JS. Myocardial infarction with angiographically normal coronary arteries. Arch Intern Med 1994;154(3):265–9. [PubMed]
- 4.Angelini P. Transient left ventricular apical ballooning: a unifying pathophysiologic theory at the edge of Prinzmetal angina. Catheter Cardiovasc Interv 2008;71(3):342–52. [DOI] [PubMed]
- 5.Angelini P. Takotsubo cardiomyopathy: what is behind the octopus trap? Tex Heart Inst J 2010;37(1):85–7. [PMC free article] [PubMed]
- 6.Angelini P. Midventricular variant of transient apical ballooning: a likely demonstration of its pathophysiologic mechanism. Mayo Clin Proc 2009;84(1):92–3. [DOI] [PMC free article] [PubMed]
- 7.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352(6):539–48. [DOI] [PubMed]
- 8.Mrdovic I, Kostic J, Perunicic J, Asanin M, Vasiljevic Z, Ostojic M. Right ventricular takotsubo cardiomyopathy. J Am Coll Cardiol 2010;55(16):1751. [DOI] [PubMed]
- 9.Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, et al. Right ventricular involvement in takotsubo cardiomyopathy. Eur Heart J 2006;27(20):2433–9. [DOI] [PubMed]
- 10.Burgdorf C, Hunold P, Radke PW, Schunkert H, Kurowski V. Isolated right ventricular stress-induced (“Tako-Tsubo”) cardiomyopathy. Clin Res Cardiol 2011;100(7):617–9. [DOI] [PubMed]
- 11.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306(3):277–86. [DOI] [PubMed]
- 12.Kaushik M, Alla VM, Madan R, Arouni AJ, Mohiuddin SM. Recurrent stress cardiomyopathy with variable regional involvement: insights into etiopathogenetic mechanisms. Circulation 2011;124(22):e556–7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


