Abstract
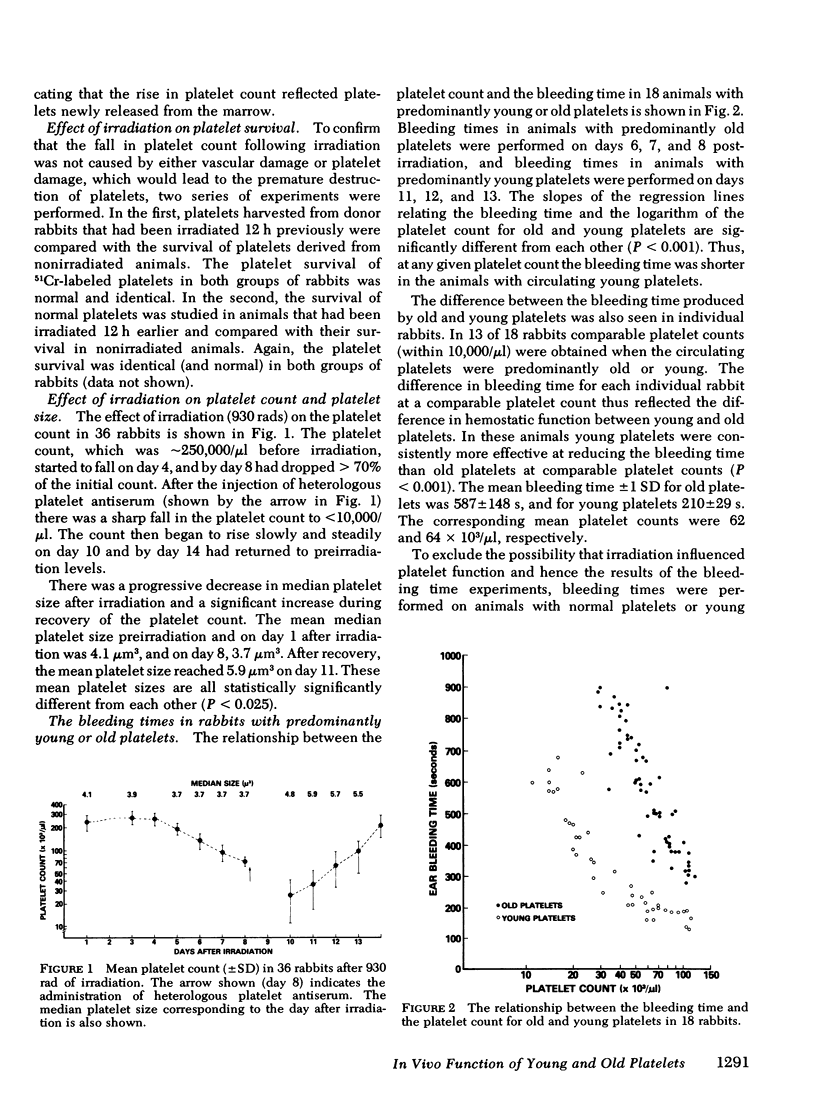
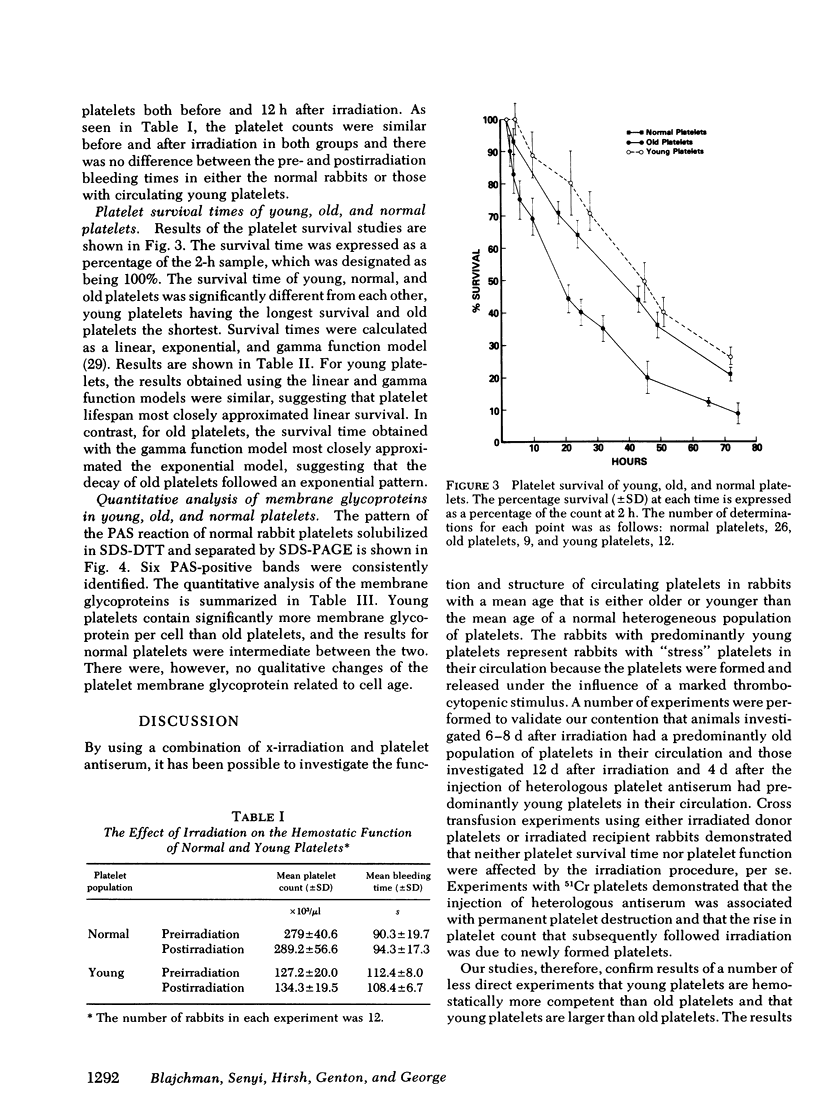
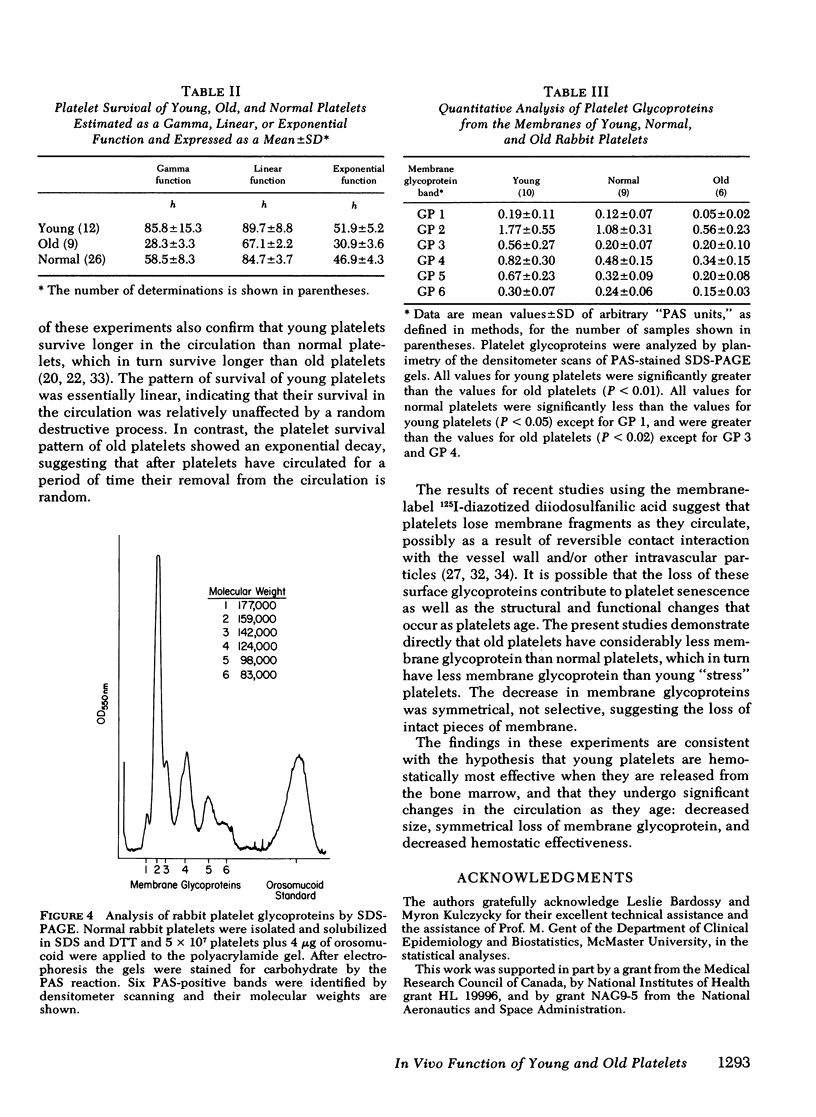
Although in vitro studies have demonstrated functional differences between young and old platelets, in vivo differences have not been precisely established. Therefore the in vivo hemostatic function of young and old platelets and the survival time have been examined in rabbits. The hemostatic function was measured by performing serial ear bleeding times in irradiation-induced thrombocytopenic rabbits. After irradiation with 930 rad the platelet count gradually diminished reaching a nadir (∼20 × 103/μl) at 10 d. The platelets present in the circulation, 7-10 d after irradiation, were considered old platelets, and the platelets present after recovery, 11-14 d postirradiation, young platelets. The measurement of platelet size was consistent with the hypothesis that platelets become smaller with age: the mean size was 3.84 μm3 for old platelets and 5.86 μm3 for young platelets. Regression analysis of the relationship between the bleeding time and the platelet count in 18 rabbits showed a significantly different slope for rabbits with predominantly old platelets compared with rabbits with predominantly young platelets (P < 0.001). Young platelets were more effective giving much shorter bleeding times than old platelets at comparable platelet counts. Survival times of young and old platelets were measured using platelets harvested on day 8 postirradiation (old platelets) and day 12 postirradiation (young platelets) that were labeled and then reinjected into normal recipient animals. The mean platelet survival time, calculated by gamma function, of old platelets was 28.8 h; of young platelets, 87.4 h; and of normally circulating heterogeneous platelets, (normal platelets) 53.0 h. Notably, the survival of old platelets was found to be exponential, and of young platelets, linear. Analysis of the membrane glycoproteins in young, old and normal platelets indicated that there was no qualitative difference amongst the young, normal, and old platelets. The relative relationship among all the glycoprotein peaks was equal and the only changes observed were quantitative, with young platelets having significantly more membrane glycoprotein per cell than old platelets and normal platelets. Normal platelets had intermediate concentrations of each glycoprotein. These results demonstrate that young platelets are hemostatically more effective in vivo than old platelets. The data are compatible with the hypothesis that platelets age in the circulation by losing membrane fragments and then after becoming senescent, are removed from the circulation by a random process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRECHER G., CRONKITE E. P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950 Dec;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- Blajchman M. A., Senyi A. F., Hirsh J., Surya Y., Buchanan M., Mustard J. F. Shortening of the bleeding time in rabbits by hydrocortisone caused by inhibition of prostacyclin generation by the vessel wall. J Clin Invest. 1979 May;63(5):1026–1035. doi: 10.1172/JCI109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Hoveke T. P., Rafelson M. E., Jr Studies on human platelets. II. Protein synthetic activity of various platelet populations. Biochim Biophys Acta. 1968 May 21;157(3):660–663. [PubMed] [Google Scholar]
- Charmatz A., Karpatkin S. Heterogeneity of rabbit platelets. I. Employment of an albumin density gradient for separation of a young platelet population identified with se75-selenomethionine. Thromb Diath Haemorrh. 1974 Jun 30;31(3):485–492. [PubMed] [Google Scholar]
- Corash L. Platelet heterogeneity. Psychopharmacol Bull. 1980 Jan;16(1):65–67. [PubMed] [Google Scholar]
- Corash L., Shafer B., Perlow M. Heterogeneity of human whole blood platelet subpopulations. II. Use of a subhuman primate model to analyze the relationship between density and platelet age. Blood. 1978 Oct;52(4):726–734. [PubMed] [Google Scholar]
- Corash L., Tan H., Gralnick H. R. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977 Jan;49(1):71–87. [PubMed] [Google Scholar]
- DETWILER T. C., ODELL T. T., Jr, MacDONALD T. P. Platelet size, ATP content, and clot retraction in relation to platelet age. Am J Physiol. 1962 Jul;203:107–110. doi: 10.1152/ajplegacy.1962.203.1.107. [DOI] [PubMed] [Google Scholar]
- Friedhoff A. J., Miller J. C., Karpatkin S. Heterogeneity of human platelets. VII. Platelet monoamine oxidase activity in normals and patients with autoimmune thrombocytopenic purpura and reactive thrombocytosis: its relationship to platelet protein density. Blood. 1978 Feb;51(2):317–323. [PubMed] [Google Scholar]
- George J. N., Lewis P. C., Sears D. A. Studies on platelet plasma membranes. II. Characterization of surface proteins of rabbit platelets in vitro and during circulation in vivo using diazotized (125i)-diiodosulfanilic acid as a label. J Lab Clin Med. 1976 Aug;88(2):247–260. [PubMed] [Google Scholar]
- George J. N., Lewis P. C. Studies on platelet plasma membranes. III. Membrane glycoprotein loss from circulating platelets in rabbits: inhibition by aspirin-dipyridamole and acceleration by thrombin. J Lab Clin Med. 1978 Feb;91(2):301–306. [PubMed] [Google Scholar]
- George J. N. Studies on platelet plasma membranes. IV. Quantitative analysis of platelet membrane glycoproteins by (125I)-diazotized diiodosulfanilic acid labeling and SDS-polyacrylamide gel electrophoresis. J Lab Clin Med. 1978 Sep;92(3):430–446. [PubMed] [Google Scholar]
- Ginsburg A. D., Aster R. H. Changes associated with platelet aging. Thromb Diath Haemorrh. 1972 Jul 31;27(3):407–415. [PubMed] [Google Scholar]
- Ginsburg A. D., Aster R. H. Kinetic studies with 51-chromium-labeled platelet cohorts in rats. J Lab Clin Med. 1969 Jul;74(1):138–144. [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972 Jul 27;287(4):155–159. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Glynn M. F., Mustard J. F. The effect of platelet age on platelet adherence to collagen. J Clin Invest. 1968 Mar;47(3):466–473. doi: 10.1172/JCI105743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S., Charmatz A. Hetrogeneity of human platelets. 3. Glycogen metabolism in platelets of different sizes. Br J Haematol. 1970 Aug;19(2):135–143. doi: 10.1111/j.1365-2141.1970.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1073–1082. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of rabbit platelets. VI. Further resolution of changes in platelet density, volume, and radioactivity following cohort labelling with 75Se-selenomethione. Br J Haematol. 1978 Jul;39(3):459–469. doi: 10.1111/j.1365-2141.1978.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Karpatkin S., Khan Q., Freedman M. Heterogeneity of platelet function. Correlation with platelet volume. Am J Med. 1978 Apr;64(4):542–546. doi: 10.1016/0002-9343(78)90571-5. [DOI] [PubMed] [Google Scholar]
- Karpatkin S., Strick N. Heterogeneity of human platelets. V. Differences in glycolytic and related enzymes with possible relation to platelet age. J Clin Invest. 1972 May;51(5):1235–1243. doi: 10.1172/JCI106918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD T. P., ODELL T. T., Jr, GOSSLEE D. G. PLATELET SIZE IN RELATION TO PLATELET AGE. Proc Soc Exp Biol Med. 1964 Mar;115:684–689. doi: 10.3181/00379727-115-29006. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Costa J. L., Shafer B., Corash L. Monoamine oxidase activity in different density gradient fractions of human platelets. Psychopharmacology (Berl) 1978 Oct 31;59(2):193–197. doi: 10.1007/BF00427757. [DOI] [PubMed] [Google Scholar]
- Murphy E. A., Francis M. E. The estimation of blood platelet survival. II. The multiple hit model. Thromb Diath Haemorrh. 1971;25(1):53–80. [PubMed] [Google Scholar]
- Okuma M., Steiner M., Baldini M. Lipid peroxidation in aging platelets. Blood. 1969 Nov;34(5):712–716. [PubMed] [Google Scholar]
- Penington D. G., Lee N. L., Roxburgh A. E., McGready J. R. Platelet density and size: the interpretation of heterogeneity. Br J Haematol. 1976 Nov;34(3):365–376. doi: 10.1111/j.1365-2141.1976.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Penington D. G., Streatfield K., Roxburgh A. E. Megakaryocytes and the heterogeneity of circulating platelets. Br J Haematol. 1976 Dec;34(4):639–653. doi: 10.1111/j.1365-2141.1976.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Shulman N. R., Watkins S. P., Jr, Itscoitz S. B., Students A. B. Evidence that the spleen retains the youngest and hemostatically most effective platelets. Trans Assoc Am Physicians. 1968;81:302–313. [PubMed] [Google Scholar]
- Steiner M., Baldini M. Protein synthesis in aging blood platelets. Blood. 1969 Apr;33(4):628–633. [PubMed] [Google Scholar]


