Abstract
Introduction
Vitamin D (VD) is part of a complex steroid hormone system long known to be involved in bone metabolism. Recently, VD has been implicated physiologic processes as diverse as vascular health, immune function, metabolism and placental function. This review summarizes the current evidence for the role of VD in perinatal health.
Methods
A systematic review of articles published in PubMed between May 2010 and October 2011 was undertaken using key words for VD and pregnancy. Seventy-eight studies were reviewed.
Findings
The biologic evidence regarding a role for VD in reproductive outcomes is strong, and rates of VD deficiency may be high among pregnant women. However, no consensus exists regarding optimum VD levels in pregnancy or standard measurement of VD deficiency. Clinical studies establishing an association between VD levels and adverse pregnancy outcomes such as preeclampsia, gestational diabetes, low birthweight, preterm labor, cesarean delivery and infectious diseases have conflicting results. This is likely due to a paucity of randomized trials, heterogeneity of populations studied, and low sample size with poor adjustment for confounding among observational studies.
Conclusion
Further research should focus on defining optimum 25(OH)D levels in pregnancy as well as among various subgroups of the population. Randomized trials are needed to determine if VD supplementation can improve pregnancy outcomes. Currently, ACOG and IOM recommend 600 IU of daily VD supplementation during pregnancy to support maternal and fetal bone metabolism.
Keywords: Vitamin D, Pregnancy, Vitamin supplementation
Introduction
Vitamin D (VD) deficiency in the form of rickets was first described in the 17th century. [1] The “vitamin”—actually a fat-soluble steroid hormone—was not discovered until the early 20th century. A successful public health campaign, centered on cod liver oil supplementation and fortification of dairy products, was thought to have reduced VD deficiency in developed countries. [2] Recently, the prevalence of rickets has increased sparking a new interest in VD deficiency. In addition, studies of VD physiology suggest that effects of VD deficiency could be much broader than rickets including cardiovascular disease, cancers, diabetes, and pregnancy complications.
The naturally occurring form of VD in humans is cholecalciferol or Vitamin D3. [3, 4] It can be ingested in the diet (animal products) or produced in the skin when UV light interacts with a cholesterol derivative. Vitamin D2 or ergocalciferol is derived from plant sterols and is the form contained in most VD supplements. Both D2 and D3 travel in the blood bound to VD Binding Protein and must be hydroxylated to become active. D3 and D2 metabolites are thought to have equal physiologic activity but D3 levels may increase more quickly after supplementation. Current assays may perform differently when measuring D2 or D3 metabolites.
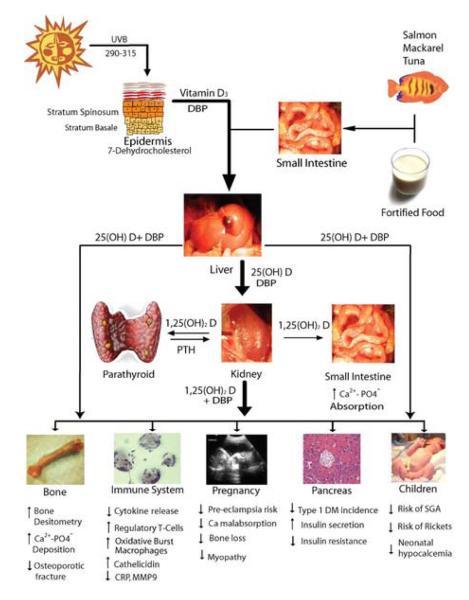
25(OH)D is first hydroxylated in the liver. The resulting metabolite, 25(OH)D, is very stable and is therefore most commonly used to measure VD status. The second hydroxylation to the active form 1,25(OH)D occurs mostly in the kidneys in a process tightly regulated by calcium, phosphorus and parathyroid hormone levels. [5] After the second hydroxylation, 1,25(OH)D binds to the VD Receptor (VDR). VDR is a transcription factor whose products are involved in a wide array of activities including bone metabolism, cellular growth and differentiation, glucose metabolism and immune function. Both the enzyme responsible for VD activation (1αhydroxyase) and its receptor have been located in peripheral tissues such as the placenta suggesting a farther-reaching role for VD than bone metabolism alone. (See Figure 1). [6]
Figure 1.
Vitamin D metabolism and tissue actions. 25(OH)D, 25-hydroxy vitamin D; Ca2+, calcium; CRP, C-reactive protein; DBP, vitamin D binding protein; DM, diabetes mellitus; MMP9, matrix metalloproteinase 9; PO4, phosphate; PTH, parathyroid hormone; SGA, small for gestational age; UVB, ultraviolet B.
During pregnancy, serum levels of 1,25(OH)D increase up to 2-fold starting at 10-12 weeks of gestation and reaching a maximum in the third trimester. [4] It is unclear whether 25(OH)D levels increase during pregnancy. [7] However, given an increase in the active form of VD, pregnant women likely have a higher cellular exposure to VD during the second and third trimesters suggesting a role for VD in obstetric well being. Perinatal outcomes hypothesized to be related to VD include preeclampsia, gestational diabetes, low birth weight, preterm delivery, cesarean section, and infectious disease. This review will summarize scientific literature published in the last 18 months regarding the potential role of VD in improving perinatal outcomes.
Methods
Medline was searched with key words related to VD and pregnancy. We limited the search to English language articles published between May 1, 2010 and October 31, 2011. Inclusion criteria were human or animal pregnant subjects, perinatal outcomes, and in vitro, animal, case control, cohort, systematic review or clinical trial study design. Articles reporting on infant, childhood, or long-term maternal outcomes associated with maternal VD status were excluded.
The Medline search resulted in 250 citations. After abstract review, 137 were excluded due to lack of pregnant subjects, perinatal outcomes, or original research. After full text review, 38 additional articles were excluded that were not original research. The remaining 75 articles were reviewed. The reference sections of the articles were hand searched for additional citations; three such studies were reviewed. Key articles published prior to May 2010 were reviewed in order to provide context.
Vitamin D physiology in pregnancy
VDR and 1αhydroxylase are active in reproductive tissues, but their roles in those tissues are still being elucidated. In pregnant mice, VDR was expressed differentially throughout pregnancy in placental, decidual, and ovarian follicular tissue supporting the hypothesis that VD is integrally involved in the physiologic changes of pregnancy. [8] Enquobahrie et. al. compared gene expression profiles of pregnant women with low levels of VD (<25.5ng/ml) to those with higher levels (>31.7ng/ml). Over 300 genes showed significant differences in expression of at least 1.5 fold. [9] Affected genes were known to be involved in a variety of functions such as angiogenesis, carbohydrate metabolism, and immune function.
One mechanism whereby VD may affect pregnancy outcomes is modulation of the immune response. 1,25(OH)D and 25(OH)D treatments of human trophoblast cells significantly altered the innate immune response. [10, 11] In VDR or 1αhydroxylase knockout mice, trophoblast cells had an increased inflammatory response to bacterial components compared with wild type cells. [12] Treatment of wild type cells with 25(OH)D significantly suppressed the inflammatory response compared to that of untreated cells. In human syncytiotrophoblastic cells, high doses of VD decreased markers of oxidative stress. [13]
Identifying Vitamin D Deficiency
Measuring VD deficiency in pregnant women is complicated by a lack of agreement among researchers in three areas: whether 25(OH)D levels are the most accurate markers of deficiency, what level of 25(OH)D should be considered optimal in pregnant women, and which test of VD deficiency is the most accurate.
25(OH)D is the clinical and research gold standard measure of VD status. However, given the complexity of the VD system, it is unclear whether 25(OH)D levels have the same clinical implications in all women or throughout all stages of pregnancy. For example, although 25(OH)D levels were lower among black women than Caucasian women in one study, their VD Binding Protein levels were also lower resulting in equivalent levels of calculated free 1,25(OH)D. [14] Some researchers suggest measuring Parathyroid Hormone levels as a biologic marker of VD deficiency. However, Parathyroid Hormone levels have been inconsistently associated with 25(OH)D levels pregnant women. [15-19] This may be due to a threshold effect: Parathyroid Hormone levels are only increased when 25(OH)D is very low (e.g. 20ng/L). However, such a threshold has not been identified. [19]
According to the 2010 Institute of Medicine (IOM) Report, 12ng/L (30nmol/L) of 25(OH)D is the point below which “persons are at risk for bone deficiency”. “Some but not all are potentially at risk” with levels below 20ng/L (50nmol/L). [20] However, the 2011 ACOG Practice Bulletin “Vitamin D: Screening and Supplementation” defines deficiency as 25(OH)D levels less than 20ng/L (50nmol/L). [21] These cut points and others are used to define VD deficiency in different studies; therefore levels of “VD deficiency” in various populations cannot be directly compared.
Finally, there are multiple commercially available 25(OH)D assays, which do not yield identical results. This makes comparing data from multiple studies more complicated. [22, 23] Historically 25(OH)D has been measured with protein binding immunoassays, but liquid chromatography with tandem mass spectroscopy may be the most accurate technology. [24]
Vitamin D deficiency and risk factors in pregnancy
Using the IOM cut points, 7% of U.S. pregnant or lactating women in a large national database were at risk for VD deficiency (<12ng/L) and an additional 21% were at risk for inadequacy in 2006 (<20ng/L). [25] These levels have not changed since 2001. Using the 20ng/L cut point, however, studies from around the world show high percentages of VD deficiency during pregnancy: 100% of Somali immigrants in Sweden, 98% of Omani women, 96% of urban Indian women, 89% of urban Japanese women, 69% of urban Chinese women, 54.7% of UK women in the first trimester, 50% of Baltimore teens, 46% of laboring women from Pakistan, 41% of South Carolina women, 41% of U.S. women in a national database, 35-46% of Australian women, 24% of Western Canadian women, and 7% of North Carolina women. [7, 15, 16, 22, 26-37]
Non-modifiable risk factors consistently associated with deficiency are winter season and darker skin pigmentation. Modifiable risk factors include less sun exposure (geography or clothing-related), less dietary intake of foods high in VD or VD supplements, and higher BMI. Unfortunately, not all studies control for the same risk factors or measure risk factors in a standard way, therefore it is difficult to compare results directly between populations.
Treating Vitamin D deficiency in pregnancy
Sun exposure is measured by a variety of methods: latitude, season, skin coverage with clothing, skin pigmentation, and ethnicity. However, in all studies, more sun exposure is significantly associated with increased VD levels. Sun exposure may be more strongly associated with VD levels than oral VD intake. [22] There are no trials of sun exposure to increase VD levels in pregnancy.
VD supplementation likely increases 25(OH)D levels. Supplemented women had less VD deficiency in 3 large well-controlled cohorts (N=1539), a similar trend in one poorer quality study (N=201) and no difference in one study with an overall low rate of supplementation (N=559). [7, 16, 22, 27, 28] RCTs of VD supplementation have consistently shown success in raising 25(OH)D levels in pregnant women and neonates albeit with varying doses of 25(OH)D. [38] Only one negative trial is reported in the literature, and the dose was 400IU. All other doses were higher, ranging from 800-1000 IU/day to 100,000-200,000 IU given as one-time doses. Despite increases with supplementation, 25(OH)D levels remained low in most studies. A recent randomized, double-blinded placebo controlled trial occurred in pregnant South Carolina women. The treatment arms were 2000 IU VD daily plus standard prenatal vitamins and 4000 IU VD daily plus standard prenatal vitamins compared with a placebo arm consisting of a placebo pill plus standard prenatal vitamins. The mean VD level measured at 36 weeks was 79 nmol/L for the control group, 105 for the 2000 IU group, and 119 for the 4000 IU group. This difference was statistically significant (p<0.0001); however, the levels were higher than most other trials even in the placebo group. Unfortunately, almost 30% of the participants were excluded from the analysis based on “poor adherence”. Although the intervention decreased “VD deficiency”, no difference was found in perinatal outcomes measured. In its 2011 report, the IOM recommended 600 IU per day of 25(OH)D for pregnant women specifically to support bone metabolism and no more than 4000 IU per day to avoid hypercalcemia. [20] ACOG endorses these recommendations and proposes 1000-2000 IU per day of 25(OH)D when deficiency is identified (<20ng/ml). [21]
Dietary intake may be another avenue to increase VD levels. VD deficiency is not as high as expected in countries at higher latitudes (less sun exposure) such as northern Europe, the U.S. and Canada. [39] This finding may be explained by the higher rates of cod liver oil supplementation and fortified dairy products in these countries. Women with a diet higher in dairy, poultry and eggs had less VD deficiency in Pakistan. [26] U.S. women with lower VD dietary intake had more deficiency. [17, 27, 28] All data regarding diet are self-reported. There are no clinical trials regarding increasing VD status through dietary modification.
Vitamin D and pregnancy outcomes
A 2010 systematic review of first trimester 25(OH)D levels and adverse pregnancy outcomes concluded that evidence regarding the association between VD levels and pregnancy complications such as preeclampsia and diabetes is inconclusive but warrants further investigation. [40] The five studies reviewed were nested case control studies and only a minority controlled for dietary quality or intake of VD. This is perplexing and could result in confounding; dietary quality is likely associated with adverse pregnancy outcomes as well as VD deficiency. Recent observational studies investigating the associations between 25(OH)D levels in pregnant women and perinatal outcomes are summarized below. The only recent randomized trial of VD supplementation found no change in birthweight, gestational age at delivery or cesarean delivery among supplemented women despite increased circulating levels of 25(OH)D.
Gestational Hypertension/Preeclampsia
VD and its receptor are active in the human placenta and immune modulation suggesting a causative role for VD deficiency and preeclampsia. This hypothesis has been supported by a cohort study of women exposed and unexposed to VD supplementation, one RCT of VD supplements, and one RCT of fish oil supplements. [41-43] Women exposed to VD developed less preeclampsia. A 2011 systematic review found that hypertension in pregnancy is more common in winter or rainy seasons when VD deficiency is also higher. [44] Higher VD levels have been consistently associated with lower preeclampsia incidence and lower blood pressure when measured at diagnosis or delivery. [45-47] However, it is unclear which comes first. Therefore, several groups have sought to determine wither 25(OH)D levels measured prior to the diagnosis of preeclampsia are associated with the diagnosis. [14, 48-51] [52] The findings of these mostly case control studies are mixed. The two cohort studies found no association. There are likely several reasons this inconsistency: 1) small sample sizes leading to insufficient power, 2) different control groups (some studies excluded “complicated pregnancies”), 3) racial/ethnic and socioeconomic heterogeneity of populations studied, 4) different methods of measuring 25(OH)D concentration, 5) different cut points for VD deficiency, and 6) incomplete adjustment for confounding by VD dietary intake and sun exposure. Overall, the findings suggest that 25(OH)D levels in early pregnancy may be associated with later preeclampsia only in specific subgroups: women with very low levels of 25(OH)D (<13.2 ng/L) or African American women.
Gestational Diabetes
In non-pregnant adults, VD deficiency has been associated with higher levels of Type 2 Diabetes. Studies investigating the relationship between VD levels and gestational diabetes (GDM) have mixed results. Three recent case control studies from India, the UK and North Carolina aimed to determine whether early pregnancy 25(OH)D levels were associated with a later diagnosis of GDM. [53-55] Results were mixed. In one, women with GDM had 2.7 times the odds (CI 1.0-7.0) of VD deficiency in the first trimester as those without. A second showed no association between low VD levels and a GDM diagnosis but did find increased fasting glucose and hemoglobin a1c at 28 weeks. The third found no association between diagnosis of GDM and first trimester VD deficiency but had very wide confidence intervals. None of the studies were adjusted for dietary factors or sun exposure.
Birth Weight
Vitamin D is known to be involved in bone metabolism, therefore VD deficiency has been hypothesized to be associated with low birth weight. Study results have been mixed both for the association between low birthweight and VD status at delivery and between low birth weight and first trimester 25(OH)D levels. [28, 56-59] The largest cohort study (3730 Amsterdam women) found the odds of birthing a baby small for gestational age was higher among women with severe VD deficiency in early pregnancy (<12ng/L). [60] No studies fully controlled for sun exposure or dietary factors. Taken together, these data suggest that if an association between VD status and infant birth weight exists, it may vary according to subgroups such as severe VD deficiency or race. A potential mechanism for the latter was suggested by Swamy et. al. [61] In a study of VDR haplotypes among a cohort of 615 pregnant women, 8 polymorphisms in black women were significantly associated with infant birthweight, and no polymorphisms in white women were associated with birthweight.
Preterm Delivery
Given the known association between VD deficiency and increased markers of inflammation, some have suggested a role for VD in prevention of preterm birth. Only a handful of studies have investigated this question. In a recent North Carolina study, 40 cases who suffered a preterm birth without evidence of chorioamnionitis were matched by age and race with three controls. [36] No association between first trimester 25(OH)D deficiency (<50nmol/L) and spontaneous preterm birth was found. The power was low given low incidence of preterm birth and VD deficiency in this upper class population. A Japanese retrospective study found lower mean 25(OH)D among women in the 3rd trimester that had been hospitalized for preterm labor earlier in the pregnancy but delivered at term compared with those who had not been. [37]
Cesarean delivery
Severe VD deficiency and rickets causes pelvic deformities, which have been known for many years to increase the risk of obstructed labor. In 2009, a Boston study of 300 women found women with VD levels <37.5ng/L had four times the odds of a cesarean delivery than those with higher levels. [62] A Northern California study found no change in mean VD levels between women who underwent cesarean delivery and those who did not after adjustment for confounders. [28] Neither study was adjusted for dietary factors although the California study adjusted for sun exposure. The RCT of VD supplementation in South Carolina, found no difference in Cesarean section rates between treatment and control groups. [18]
Infectious Diseases
VD is involved in immune function. Several groups have investigated associations between VD levels and infectious diseases during pregnancy. In a North Carolina case control study (Cases=117 Controls=118), women less than 26 weeks of gestation with periodontal disease had 2 times the odds of VD deficiency. In a US national cohort study (N=3523), there was no association between Bacterial Vaginosis and VD status in non-pregnant women; however, in pregnant women, VD deficiency (<30ng/L) was independently associated with Bacterial Vaginosis (OR 2.9, CI 1.1-7.3). [63] HIV mortality, disease progression and anemia were associated with VD deficiency (<32ng/L) in a cohort of HIV positive Tanzanian pregnant women. [64] None of the studies adjusted for dietary factors or sun exposure.
Conclusion
Biologic data suggests a role for VD in women’s reproductive health. However, results from studies investigating the link between 25(OH)D levels and adverse pregnancy outcomes are contradictory. This is likely due to small sample sizes, inadequate control for confounding, significant heterogeneity in populations studied, and significant heterogeneity in exposure measurement. [41] A standard definition of VD deficiency in pregnancy must be determined. A pooled analysis of observational studies could then be considered. VD is likely involved in reproductive health; however, it is unlikely to be the cure for pregnancy complications that have multifactorial etiologies. Until we have clear evidence for optimal levels during pregnancy, IOM guidelines recommending 600IU of daily VD supplementation for pregnant women should be followed.
Key Points.
Vitamin D is involved in reproductive health.
Optimal Vitamin D levels in pregnancy are unknown.
No conclusive evidence links lower levels of 25(OH)D with adverse pregnancy outcomes.
Pregnant women should receive 600IU of Vitamin D supplementation daily.
Acknowledgments
Dr. Urrutia was supported by Award Number T32HD040672 from the National Institute Of Child Health And Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Child Health And Human Development or the National Institutes of Health.
The authors wish to thank Kate McGraw, MA, MLS, University of North Carolina, Health Services Library, for her assistance with the literature search.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrett H, McElduff A. Vitamin D and pregnancy: An old problem revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(4):527–39. doi: 10.1016/j.beem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Weick MT. A history of rickets in the United States. Am J Clin Nutr. 1967;20(11):1234–41. doi: 10.1093/ajcn/20.11.1234. [DOI] [PubMed] [Google Scholar]
- 3.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brannon PM, Picciano MF. Vitamin d in pregnancy and lactation in humans. Annu Rev Nutr. 2011;31:89–115. doi: 10.1146/annurev.nutr.012809.104807. [DOI] [PubMed] [Google Scholar]
- 5.Christakos S, et al. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39(2):243–53. doi: 10.1016/j.ecl.2010.02.002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan ML, et al. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429, e1–9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Ginde AA, et al. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436, e1–8. doi: 10.1016/j.ajog.2009.11.036. NHANES 2001-2006 survey of VD deficiency among U.S. women in a nationally representative sample. Well-controlled study which compares levels between pregnant and non-pregnant women. Does not control for dietary quality or sun exposure.
- *8.Shahbazi M, et al. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta. 2011;32(9):657–64. doi: 10.1016/j.placenta.2011.06.013. Mouse study which shows evidence of varying VD levels in reproductive tissues during gestation giving evidence of a role for VD in the physiology of reproduction.
- *9.Enquobahrie DA, et al. Global maternal early pregnancy peripheral blood mRNA and miRNA expression profiles according to plasma 25-hydroxyvitamin D concentrations. J Matern Fetal Neonatal Med. 2011;24(8):1002–12. doi: 10.3109/14767058.2010.538454. Genetic evidence of hundreds of genes induced differentially in pregnant women depending on VD levels.
- *10.Liu N, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80(3):398–406. doi: 10.1095/biolreprod.108.073577. Provides evidence that VD may be involved in regulating the innate immune response in the human trophoblast.
- *11.Evans KN, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75(6):816–22. doi: 10.1095/biolreprod.106.054056. Provides evidence that VD may be involved in regulating the innate immune response in the human trophoblast.
- 12.Liu NQ, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186(10):5968–74. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 13.Halhali A, et al. Effects of calcitriol on calbindins gene expression and lipid peroxidation in human placenta. J Steroid Biochem Mol Biol. 2010;121(1-2):448–51. doi: 10.1016/j.jsbmb.2010.03.008. [DOI] [PubMed] [Google Scholar]
- **14.Powe CE, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56(4):758–63. doi: 10.1161/HYPERTENSIONAHA.110.158238. This high quality case-control study involved excellent control for potential confounding factors, a power calculation and an appropriate control group. In addition, the measurement of VD levels was sophisticated lending a better understanding of the complex VD steroid system. Powe et. al. found no evidence that VD levels in the first trimester were associated with subsequent preeclampsia.
- 15.Marwaha RK, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011:1–7. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton SA, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. Int J Endocrinol. 2010;2010:917428. doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Essley BV, et al. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with PTH and calcitriol in pregnant adolescents. J Bone Miner Res. 2011 doi: 10.1002/jbmr.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Hollis BW, et al. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. This fair-quality randomized controlled trial in South Carolina showed higher than expected baseline VD levels, increased levels of 25(OH)D in response to supplementation, and no change in pregnancy outcomes measured. The study was flawed in that it was not intention to treat. The analysis excluded up to 30% of the participants who were not compliant.
- 19.Haddow JE, et al. The relationship between PTH and 25-hydroxy vitamin D early in pregnancy. Clin Endocrinol (Oxf) 2011;75(3):309–14. doi: 10.1111/j.1365-2265.2011.04066.x. [DOI] [PubMed] [Google Scholar]
- **20.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2011. IOM guidelines for VD supplementation with a special section devoted to pregnancy. Found no evidence that VD deficiency is related to any outcome besides bone health. Recommends VD deficiency be defined as less than 12ng/L. Recommends routine supplementation of 600IU 25(OH)D daily in pregnancy to promote bone health only.
- **21.ACOG Committee Opinion No. 495: Vitamin D: Screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197–8. doi: 10.1097/AOG.0b013e318227f06b. ACOG guidelines for VD supplementation in pregnancy. Does not recommend routine screening. Recommends 600IU daily in pregnancy. Physicians may consider screening women thought to be at high risk for deficiency. Physicians may recommend treating pregnant women with deficiency (<20ng/L) with 1000-2000 IU 25(OH)D daily.
- *22.Perampalam S, et al. Vitamin D status and its predictive factors in pregnancy in 2 Australian populations. Aust N Z J Obstet Gynaecol. 2011;51(4):353–9. doi: 10.1111/j.1479-828X.2011.01313.x. Shows the inaccuracy of directly comparing 25(OH)D levels measured by different assays.
- 23.Roth HJ, et al. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45(Pt 2):153–9. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 24.Schleicher RL, et al. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta. 2011;412(17-18):1594–9. doi: 10.1016/j.cca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- **25.Looker AC, et al. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011;(59):1–8. Using national data and the IOM cut point for deficiency, this group found a low prevalence of VD deficiency or insufficiency among pregnant women in the United States.
- 26.Karim SA, Nusrat U, Aziz S. Vitamin D deficiency in pregnant women and their newborns as seen at a tertiary-care center in Karachi, Pakistan. Int J Gynaecol Obstet. 2011;112(1):59–62. doi: 10.1016/j.ijgo.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Li W, et al. Suboptimal vitamin D levels in pregnant women despite supplement use. Can J Public Health. 2011;102(4):308–12. doi: 10.1007/BF03404056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Dror DK, et al. Association of modifiable and nonmodifiable factors with vitamin D status in pregnant women and neonates in Oakland, CA. J Am Diet Assoc. 2011;111(1):111–6. doi: 10.1016/j.jada.2010.10.002. This study attempted to control for dietary and sun exposure to VD in order to understand risk factors associated with VD deficiency in a diverse US population. They found that inner arm skin pigmentation was more associated with VD deficiency than stated race/ethnicity and that VD levels increased with supplementation. They found no association between VD levels and low birth weight or cesarean section.
- 29.Yu CK, et al. Normal range of maternal serum vitamin d at 11-13 weeks’ gestation. Fetal Diagn Ther. 2011;30(2):94–9. doi: 10.1159/000324340. [DOI] [PubMed] [Google Scholar]
- 30.Tao M, et al. Vitamin D status of pregnant women in Shanghai, China. J Matern Fetal Neonatal Med. 2011 doi: 10.3109/14767058.2011.569613. [DOI] [PubMed] [Google Scholar]
- 31.Teale GR, Cunningham CE. Vitamin D deficiency is common among pregnant women in rural Victoria. Aust N Z J Obstet Gynaecol. 2010;50(3):259–61. doi: 10.1111/j.1479-828X.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnson DD, et al. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 33.Saaf M, et al. Severe vitamin D deficiency in pregnant women of Somali origin living in Sweden. Acta Paediatr. 2011;100(4):612–4. doi: 10.1111/j.1651-2227.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 34.Al Kalbani M, et al. Vitamin D Status in Pregnant Omanis: A disturbingly high proportion of patients with low vitamin D stores. Sultan Qaboos Univ Med J. 2011;11(1):52–5. [PMC free article] [PubMed] [Google Scholar]
- *35.Agarwal N, Arya SC. Vitamin D levels in pregnant women and newborns at a private tertiary care hospital in Delhi, India. Int J Gynaecol Obstet. 2011;113(3):240–1. doi: 10.1016/j.ijgo.2011.01.005. A large, well-controlled study from India that showed that VD deficiency increased in the second and third trimesters while parathyroid hormone levels increased.
- 36.Baker AM, et al. A nested case-control study of first-trimester maternal vitamin d status and risk for spontaneous preterm birth. Am J Perinatol. 2011;28(9):667–72. doi: 10.1055/s-0031-1276731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata M, et al. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. J Bone Miner Metab. 2011;29(5):615–20. doi: 10.1007/s00774-011-0264-x. [DOI] [PubMed] [Google Scholar]
- 38.Roth DE. Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. J Perinatol. 2011;31(7):449–59. doi: 10.1038/jp.2010.203. [DOI] [PubMed] [Google Scholar]
- *39.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1-2):297–300. doi: 10.1016/j.jsbmb.2010.02.021. An interesting ecologic study which shows VD deficiency to be lower than expected in some countries with low sun exposure. This suggests a role for dietary quality in improving VD levels.
- **40.Nassar N, et al. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2011.03.058. A systematic review of first-trimester VD levels and their impact on the diagnosis of gestational diabetes and preeclampsia. This review found inconclusive evidence to support an assocation.
- 41.Haugen M, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720–6. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 42.Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest. 1987;24(1):38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- 43.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr. 1990;64(3):599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 44.TePoel MR, Saftlas AF, Wallis AB. Association of seasonality with hypertension in pregnancy: a systematic review. J Reprod Immunol. 2011;89(2):140–52. doi: 10.1016/j.jri.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Robinson CJ, et al. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366, e1–6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hossain N, et al. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int J Gynaecol Obstet. 2011;112(3):229–33. doi: 10.1016/j.ijgo.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Ringrose JS, et al. Vitamin D and hypertension in pregnancy. Clin Invest Med. 2011;34(3):E147–54. doi: 10.25011/cim.v34i3.15187. [DOI] [PubMed] [Google Scholar]
- 48.Shand AW, et al. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117(13):1593–8. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 49.Azar M, et al. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study. Diabetes Care. 2011;34(6):1258–64. doi: 10.2337/dc10-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker AM, et al. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95(11):5105–9. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodnar LM, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517–22. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Alonso AM, et al. First-trimester maternal serum 25-hydroxyvitamin D(3) status and pregnancy outcome. Int J Gynaecol Obstet. 2011 doi: 10.1016/j.ijgo.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3(11):e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker AM, et al. First trimester maternal vitamin D status and risk for gestational diabetes mellitus: a nested case-control study. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makgoba M, et al. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care. 2011;34(5):1091–3. doi: 10.2337/dc10-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doi M, et al. Association between calcium in cord blood and newborn size in Bangladesh. Br J Nutr. 2011:1–10. doi: 10.1017/S0007114511001747. [DOI] [PubMed] [Google Scholar]
- 57.Robinson CJ, et al. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol. 2011;204(6):556, e1–4. doi: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez-Alonso AM, et al. First trimester serum levels of 25-hydroxyvitamin D, free beta-human chorionic gonadotropin, and pregnancy-associated plasma protein A in Spanish women. Gynecol Endocrinol. 2011 doi: 10.3109/09513590.2011.569799. [DOI] [PubMed] [Google Scholar]
- 59.Bodnar LM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140(5):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010;104(1):108–17. doi: 10.1017/S000711451000022X. A very large cohort study in the Netherlands which found that women with severe VD deficiency (<30nmol/L) bore infants who weighed 114 grams less on average than women with adequate VD (>50nmol/L) and had 2.4 times the Odds of small for gestational age babies.
- *61.Swamy GK, et al. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am J Med Genet A. 2011;155A(6):1264–71. doi: 10.1002/ajmg.a.33583. This study found polymorphisms in the VD receptor that was associated with smaller infant size in black women but not in white women.
- 62.Merewood A, et al. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–5. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hensel KJ, et al. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011;204(1):41, e1–9. doi: 10.1016/j.ajog.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Mehta S, et al. Vitamin D Status and its Association with Morbidity Including Wasting and Opportunistic Illnesses in HIV-Infected Women in Tanzania. AIDS Patient Care STDS. 2011;25(10):579–85. doi: 10.1089/apc.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]