Abstract
Aims
This study evaluated the efficacy of a low-cost, prize reinforcement contingency management (CM) intervention for reducing cocaine use.
Setting
Community-based treatment centers.
Participants and design
Cocaine-abusing out-patients (n = 120) were assigned randomly to one of three 12-week conditions: standard treatment, standard treatment plus CM with an expected maximum of $80 of reinforcement or standard treatment plus CM with an expected maximum of $240 of reinforcement.
Intervention
In the CM conditions, patients earned the opportunity to win prizes for submitting negative urine samples and completing goal-related activities.
Measurements
Drug use was measured at intake and throughout a 3-month treatment period.
Findings
Patients in the $240 CM condition achieved more abstinence than patients in the standard condition. Patients who initiated treatment with positive urinalysis results were most responsive to the CM intervention, with the $240 CM condition engendering the best effects in this subgroup. In contrast, patients who initiated treatment with negative urinalysis results generally remained abstinent during treatment, regardless of treatment assignment. On average, patients in the two CM conditions earned $36 and $68 in prizes.
Conclusions
This study suggests that prize reinforcement CM may be suitable for community-based settings, and beneficial effects may be magnitude-dependent in more severe patients.
Keywords: cocaine, contingency management, substance abuse treatment
INTRODUCTION
Cocaine use is a significant public health concern. In 1999, an estimated 1.2 million Americans (0.5%) were current cocaine users (Substance Abuse & Mental Health Service Administration 2000). Cocaine use is associated with some of our most serious social problems, including the spread of infectious diseases, crime and violence (Inciardi & Pottieger 1998; Flynn et al. 1999).
Contingency management (CM) is a promising intervention for treating drug dependence (DeRubeis & Crits-Cristoph 1998). CM treatments rearrange the environment to detect drug use readily and to promote participation in activities that are inconsistent with drug use. These treatments provide tangible reinforcers upon objective evidence of drug abstinence or participation in non-drug-related activities (Higgins et al. 1994; Budney & Higgins 1998; Petry 2000). In most CM studies of cocaine dependence, reinforcers have been provided in the form of vouchers, exchangeable for retail goods and services (Higgins et al. 1991, 1993, 1994, 2000a).
Provision of vouchers for submission of negative urine samples reduces cocaine use. Higgins et al. (1994) found that 55% of cocaine-dependent out-patients who received behavioral therapy plus vouchers for negative urine samples achieved at least 2 months of continuous abstinence in a 24-week trial. Only 15% of patients receiving behavioral therapy alone maintained this period of abstinence. A follow-up study demonstrated that the beneficial effects were related to contingent reinforcement, rather than simply the availability of vouchers. Higgins et al. (2000a) assigned 70 patients randomly to a condition in which they received vouchers contingent upon abstinence or a yoked control condition, in which they received vouchers regardless of sample results. Over one-third of patients in the contingent group maintained 3 or more months of continuous cocaine abstinence in a 24-week trial versus only about 10% of patients in the yoked condition.
While voucher reinforcement procedures are efficacious (Petry 2000), some issues associated with these techniques have hindered their implementation in community programs. Primary among these is cost. In the studies by Higgins and colleagues (1993, 1994, 2000a), for example, patients could earn about $1000-worth of vouchers, and average earnings were around $450–600. Carroll et al. (2002) found that CM enhanced compliance with naltrexone in detoxified opioid-dependent patients, but a higher magnitude voucher condition ($1152) did not improve outcomes relative to a lower magnitude voucher condition ($562). In contrast, other studies in cigarette smokers demonstrate direct relationships between magnitude of reinforcement and abstinence (Stitzer & Bigelow 1983, 1984; Stitzer et al. 1986). Among cocaine-using methadone patients, Silverman et al. (1999) found that patients who did not respond to the standard voucher amount of $1000 were more likely to abstain if the voucher amount increased to $3000. The cost of such treatments, however, may be prohibitive in clinical settings.
In recent studies (Petry et al. 2000; Petry, Martin & Finocche 2001; Petry & Martin 2002) we have described the efficacy of a lower-cost reinforcement procedure. Rather than earning vouchers, patients earn a chance to draw from an urn and win prizes ranging from $1 to $100 in value. This schedule was chosen because it contains aspects of a fixed ratio schedule that escalates with increasing abstinence (Higgins et al. 1994; Roll, Higgins & Badger 1996) and at the same time, it reduces the overall expense of reinforcers by utilizing an intermittent schedule of tangible rewards. It also provides access to large magnitude reinforcers that are known to be associated with behavioral change (Silverman et al. 1999; Robles et al. 2000). Average maximal earnings are arranged to be $240, about one-quarter of voucher studies (Higgins et al. 1994, 2000a; Silverman et al. 1996).
Patients in our initial study (Petry et al. 2000) were 42 alcohol-dependent veterans. They were reinforced for providing alcohol-free breath samples and completing goal-related activities. By the end of the 8-week treatment, 69% in the CM group were abstinent from alcohol, compared with 39% of those in the standard group. Even though alcohol was targeted as the substance upon which drawings were contingent, decreases in illicit drug use were also noted among patients in the CM condition. However, retention differed between groups, so whether beneficial results were related to contingent reinforcement or greater participation in treatment itself could not be ascertained.
Another study (Petry & Martin 2002) evaluated the efficacy of this procedure in 42 cocaine-abusing metha-done patients. In this study, reinforcement was contingent upon providing opioid- or cocaine-negative samples, with bonus draws provided when abstinence for both drugs occurred simultaneously. Patients assigned to the CM condition submitted significantly more cocaine and opioid-free samples than those assigned to standard methadone treatment, and the longest duration of continuous abstinence from both drugs in the CM group was 4.5 weeks, compared with 2.7 weeks among patients in the standard treatment condition. The average cost of prizes won per patient was $137.
One purpose of this study was to evaluate the efficacy of this lower-cost prize CM approach in reducing cocaine use in non-methadone-maintained cocaine users. Given that two studies (Petry et al. 2000; Petry & Martin 2002) demonstrated beneficial effects of this CM procedure when the average expected maximum earnings were $240, this study also sought to determine whether the costs of the prizes could be decreased further and still produce beneficial effects. Therefore, two CM conditions were investigated—one that provided an expected average maximum of $240 and another that had a similar probability of winning prizes, but with an expected average maximum winning of $80.
Even if low-cost CM procedures are efficacious in reducing drug use, some patients may benefit more from these procedures than others. Identifying characteristics that are associated with outcomes is an important issue in deciding how limited resources should be allocated. One characteristic that is consistently associated with poor response to treatment is severity of drug use at intake (Carroll et al. 1993; McLellan et al. 1994; Alterman et al. 1997; Preston et al. 1998). Thus, this study also evaluated potential interaction effects between response to treatment conditions and results of urine samples submitted at intake. For example, larger magnitude reinforcement may be necessary to enhance outcomes among patients who initiate treatment while actively using cocaine. On the other hand, patients who are able to achieve a brief period of abstinence prior to starting treatment (about 50% of patients, Higgins et al. 1994) may do equally well in low- and high-magnitude CM conditions, or even in non-CM conditions.
Both abstinence from drugs and compliance with goal-related activities were reinforced, as in previous studies (Petry et al. 2000, 2001a,b). Because about 50% of patients fail to earn reinforcement when abstinence alone is reinforced (e.g. Stitzer et al. 1992; Silverman et al. 1996; Iguchi et al. 1997), reinforcement of activity completion increases the probability that patients will experience the reinforcers. Hence, the reinforcers may be more likely to modify behavior. To increase generalization of the findings, the study was implemented in existing, non-research community-based treatment programs.
METHODS
Participants
Participants were 120 patients initiating intensive outpatient treatment at BlueRidge Center or Alcohol and Drug Recovery Centers, Inc. in Hartford, CT. The two programs are located within a 0.3-mile radius, serve the same general patient population and have a similar treatment philosophy and format. Study inclusion criteria were age 18 years and older, evidence of recent cocaine use via urinalysis testing or self-report and English-speaking. The only exclusion criteria were severe dementia (<21 on the Mini Mental Status Examination; Folstein & Folstein 1975), Diagnostic and Statistical Manual (DSM) diagnosis of current opioid dependence or active, uncontrolled psychosis or bipolar disorder, or pathological gambling. This latter exclusionary criterion was used because of the similarity between gambling and the prize reinforcement system. Although we did not mandate that study patients meet DSM criteria for a diagnosis of cocaine dependence, the vast majority (85%) were dependent. Study criteria intentionally were not restrictive to increase generalization of findings to the larger population of individuals entering outpatient treatment facilities (Rounsaville, Petry & Carroll, in press), and our sample of study patients did not differ from patients with cocaine problems seen in other community-based settings (c.f., Carroll et al. 1999; McLellan et al. 1994). Patients provided written informed consent, as approved by the University of Connecticut Health Center Institutional Review Board.
Patients were recruited to the study via intake workers’ suggestions of new patients who self-reported cocaine use as a problem and who agreed to be contacted by study staff. We are unaware of any patients who refused contact with study staff, and only 9 patients who were approached for the study were unwilling to participate. Of these, one expressed concern about confidentiality, and the remainder stated that they did not have enough time. Among patients who expressed interest and signed the consent, 95% completed the intake and were randomly assigned to a condition. Non-participants never completed the intake due to failure to return to the clinic after the initial appointment (n = 5). One patient was excluded due to non-stabilized bipolar disorder.
Study evaluations were conducted within 48 hrs of initiation of treatment at the clinic, and consisted of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders for substance use disorders (First et al. 1996) and the Addiction Severity Index (ASI) (McLellan et al. 1985). The ASI measures severity of problems in seven domains, with higher scores indicative of more severe problems. A gambling section was included (Lesieur & Blume 1991; Petry 2003) to monitor potential changes in gambling in response to the intervention.
Four and 12 weeks after study initiation, patients were contacted for follow-up assessments with the ASI. They received $15 for completing the week 4 evaluation and $35 for the week 12 evaluation. Completion rates did not differ by group, and were 82% for week 4 and 78% for week 12 evaluations. Only 14 patients (range 4–6 patients/group) failed to participate in both the follow-up assessments. Thus, some postintake data were available on 88% of patients. No patients refused participation in the evaluations, and missing assessments were due to inability to contact patients.
Randomization
Patients were randomized to one of three conditions: standard treatment plus more intensive urine monitoring (henceforth referred to as ‘standard treatment’), or that same treatment with CM with an expected average maximal earning of $80 in prizes, or standard treatment with CM with an expected average maximal earning of $240. Minimum likelihood allocation (Aiken 1982), which employs probabilistic balancing techniques, was used to randomize patients. This study used a similar method for randomizing patients to that used by the Project MATCH Research Group 1998). The purpose of randomization was to achieve a balance between the treatment groups on baseline characteristics that may influence outcome. Groups were allocated on: gender, race, age, employment status (full or part-time versus unemployed), whether or not an in-patient alcohol detoxification immediately preceded entry to the out-patient program and psychiatric scores on the Addiction Severity Index. Separate randomization programs were used in each clinic to ensure balance on these variables at each clinic.
Treatment conditions
Standard treatment
Standard treatment consisted of group counseling sessions held 3–5 days weekly during the initial 3–4 weeks of treatment. Thereafter, group counseling sessions diminished in frequency over time, from 2 to 3 days per week in weeks 4–6, down to a minimum of 1 day per week in the latter 6 weeks of treatment. For the purposes of this study, patients were followed for 12 weeks. Sessions included 12-Step oriented treatment, cognitive– behavioral therapy, health education and AIDS prevention and life skills training. Groups were led by recovering individuals as well as bachelor- and master-level counselors. Typically, clinics requested urine testing at intake, and thereafter one to four times monthly during treatment. Patients were not discharged from these clinics on the basis of urinalysis results, and the study did not influence standard procedures of urine testing.
Study urine testing
All patients in this study received standard treatment as described above. They also met with a bachelor-level research assistant between 1 and 3 days per week to provide a staff-observed urine sample that was screened for opioids and cocaine using Ontrak Teststick (Roche, Somersville, NJ, USA) and a breath sample (blood alcohol concentration: BAC) that was screened for alcohol using an Alcosensor IV Altometer (Intoximeters, St Louis, MO, USA). The days on which patients provided urine and breath samples were selected from among the days on which patients were scheduled to attend group therapy sessions. During the initial 3 weeks of study participation, they provided samples on Mondays, Wednesdays and Fridays. During weeks 4–6 samples were collected twice per week, with at least 48 hours separating tests, e.g. Tuesday and Fridays or Mondays and Thursdays. During the final 6 weeks of study participation, one sample per week was collected. For patients who were scheduled to attend the clinic more than 1 day per week, 1 day was selected randomly for urine sample testing. Thus, a total of 21 samples was collected from patients who remained in treatment for the full 12 weeks (nine in weeks 1–3, six in weeks 4–6 and six in weeks 7–12).
If a patient failed to attend the clinic or refused to provide a sample on a scheduled testing day, samples were considered positive unless an excused absence was granted (e.g. for a court appearance or severe illness). In cases of missed or refused samples, samples were collected on the next day whenever possible. In total, only one patient on one occasion refused to provide a sample, and missing samples were more likely to be the consequence of clinic nonattendance than refusal.
Contingency management treatment
Patients assigned to both of the CM conditions received the same standard treatment and urine sample monitoring as described above. Each time patients in a CM group provided a urine sample negative for cocaine, they earned one draw from a bowl (see below). Samples were also required to be negative for opioids and BACs negative for alcohol (< 0.003 g/day) to be eligible for draws. This stipulation was provided based on feedback from the clinic directors and staff to not reinforce patients who were using these other substances. Throughout the study, only six samples were positive for alcohol and five for opioids. Due to the low occurrence of positive samples for these other drugs samples will be referred to as simply ‘cocaine-negative’ throughout the paper, although ‘cocaine-negative’ means that samples tested negative for alcohol and opioids as well. If patients provided negative samples for a consecutive week they earned five bonus draws. In total, patients could earn 81 draws if they provided negative samples throughout the 12 weeks.
Patients who failed to provide a sample on a scheduled testing day, or provided a positive sample, earned no draws that day. After a week of negative samples, they were again eligible for bonus draws. Each day, patients were informed of how many draws they earned that day and how many they could earn if the next sample was negative.
Patients in the CM groups also earned draws for completing steps related to their treatment goals. Each week patients signed an ‘activities contract’, with the research assistant indicating three specific activities related to long-term treatment goals (see Petry, Tedford & Martin 2001). For example, if a goal was to become active in Alcoholics Anonymous (AA) the patient may have agreed to attend three AA meetings in the upcoming week. If a goal was to improve relationships with family members specific activities may have included: writing a letter to an adult child or taking children to a community event or the movies. If the goal was to improve health, the activities may have included setting up a doctor’s appointment or attending an appointment. The activity and acceptable proof of verification was listed on the contract. Verification consisted of receipts or signed pamphlets, with names and telephone numbers (see Petry et al. 2001b).
Patients earned one draw for each of the three activities they completed weekly. If they completed all three activities in a week they earned four bonus draws. In total, they could earn up to 84 draws if they completed all their activities throughout the 12 weeks. Research assistants managed all aspects of the activity contracts, although patients were encouraged to discuss goals and activities with their counselors as well.
Prize bowls
Patients assigned to both CM conditions could earn the same number of draws for the same behaviors (abstinence and activity completion). For both conditions, the bowls contained 250 slips of paper. Half the slips were non-winning and said ‘good job’, but did not result in a prize. The other half were ‘winning’ slips. The difference between conditions was related to the magnitudes of the two most frequently won prizes.
For the $80 condition there were three types of winning slips: mini, medium and jumbo. Of the winning slips, 109 (43.6%) were mini prizes: patient’s choice of candy bars, sodas, batteries, stamps, toiletries and other items worth about $0.33 in value. Fifteen (6%) were medium prizes, worth about $5 in value. Popular prizes were: coffee mugs, make-up items, AA books, picture frames, T-shirts, make-up and costume jewelry. One of the winning slips (0.4%) was a jumbo prize worth up to $100. The jumbo prize consisted of the patient’s choice of a large radio, television set, VCR or Playstation.
Identical probabilities of winning were used in the bowl for the $240 condition. Again, 250 slips were present, and half were winning. In this bowl, 109 (43.6%) slips could be exchanged for ‘small’ prizes, worth up to $1: choice of $1 gift certificates to fast-food restaurants, socks, make-up or jewelry. Fifteen (6%) were ‘large’ prizes worth up to $20: Walkmans, watches, backpacks, toasters, coffee makers, clothing items and gift certificates to theaters and restaurants. As in the $80 condition, one slip (0.4%) was a jumbo prize—patient’s choice of the same jumbo items described above.
Prizes of all magnitudes were kept on site in a lockable cabinet, which was restocked about twice monthly. A research grant paid for all the prizes. Throughout the study, patients in the contingent conditions were encouraged to make suggestions for prizes in all the categories for which they were eligible. Slips were returned to the bowls following each drawing so that probabilities remained constant.
Data analysis
First, to evaluate baseline differences across groups, ANOVAs were used for continuous variables and χ2 tests for nominal variables. Non-normally distributed data (e.g. income) were transformed using log transformations prior to statistical testing.
Primary analyses for full sample
For all analyses intent-to-treat analyses were used, including all patients assigned randomly to a condition. Because no differences in outcome measures were noted between patients treated at the two clinics, analyses are shown for the full sample. As in other CM studies (Higgins et al. 1994; Petry & Martin 2002), the main dependent variable was longest duration of continuous abstinence. A week of abstinence was defined as at least a 7-day period during which all urinalysis results tested negative for cocaine (as well as for alcohol and opioids). Thus, during the early weeks in the study, more samples would need to be negative to constitute a week of abstinence (Monday, Wednesday, Friday, Monday). In the latter weeks of the study when only one sample/week was tested, samples collected on, for example, a Tuesday of week 10 and a Tuesday of week 11 would need to test negative to constitute a week of abstinence. Unexcused, missed or positive samples broke a period of abstinence. Kruskal–Wallis tests compared differences in continuous abstinence between groups from the date of intake to the end of the 12-week study period. Post hoc Mann–Whitney U-tests then compared each group to every other if significant effects were noted across groups.
Other abstinence and retention variables
Because the primary measure is influenced by time in treatment and the total number of samples submitted, secondary outcomes were also evaluated. In other words, patients who failed to attend the clinic or who withdrew from treatment early will have achieved shorter periods of abstinence based on the above criteria, even though they may not have relapsed. Therefore, percentages of negative samples were derived for each patient by dividing the number of negative samples by number of samples submitted. Kruskal–Wallis tests, followed by post hoc Mann–Whitney U-tests, evaluated differences across groups for weeks in treatment, number of samples submitted and percentage of negative samples. SPSS for Windows was used for these analyses, and P-values <0.05 were considered significant.
ASI scores
Longitudinal analyses of ASI scores were conducted using hierarchical linear models (HLM; Gibbons et al. 1993). Time, group and group × time effects were analyzed using MIXREG software (Hedeker 1993).
Predictors of outcomes
We also assessed the impact of initiating treatment with a positive versus a negative urinalysis result on outcomes. First, patients initiating treatment with cocaine-negative samples were compared to those initiating treatment with cocaine-positive samples on other indices of drug use. For the primary outcome measure of continuous abstinence, group differences for initially cocaine-positive patients and initially cocaine-negative patients were evaluated independently using similar analyses to those described above. Secondary analyses evaluated the effect of treatment condition on weeks in treatment, total number of urine samples submitted and percentage of negative samples, again independently for the initially positive and initially negative patients. The intake urine sample from one patient was lost prior to testing due to staff error, and thus 119 patients were included in these analyses.
RESULTS
Analysis of full sample
Baseline characteristics
Table 1 shows characteristics of patients. The groups did not differ in any demographics or psychosocial problems (Table 2) at study initiation.
Table 1.
Demographic characteristics and baseline variables for patients assigned to the three treatment conditions. Values represent means and standard deviations unless indicated.
| Variable | Standard treatment |
$80 Contingency management |
$240 Contingency management |
Statistical test and P value |
|---|---|---|---|---|
| n | 37 | 45 | 38 | |
| Clinic | χ2(2) = 0.13, P = 0.94 | |||
| A (n = 77) | 64.9% | 62.2% | 65.8% | |
| B (n = 43) | 35.1% | 37.8% | 34.2% | |
| Male | 45.9% | 37.8% | 50.0% | χ2(2) = 1.32, P = 0.52 |
| Age | 33.6 ± 6.7 | 34.6 ± 7.1 | 35.7 ± 9.4 | F2,117 = 0.73, P = 0.48 |
| Race/ethnicity | χ2(6) = 3.34, P = 0.76 | |||
| African American | 62.2% | 68.9% | 60.5% | |
| Caucasian | 24.3% | 22.2% | 23.7% | |
| Hispanic | 13.5% | 6.7% | 10.5% | |
| Other | 0.0% | 2.2% | 5.3% | |
| Years of education | 11.4 ± 1.6 | 11.5 ± 1.5 | 11.8 ± 2.2 | F2,117 = 0.71, P = 0.50 |
| Employed full-time | 32.4% | 20.0% | 34.2% | χ2(2) = 2.49, P = 0.29 |
| Past year legal income | $9950 ± 11 960 | $9520 ± 11 450 | $12 600 ± 14 520 | F2,117 = 0.72, P = 0.49 |
| Never been married | 54.1% | 57.8% | 47.4% | χ2(2) = 0.91, P = 0.64 |
| Any intravenous drug use | 8.1% | 4.4% | 18.4% | χ2(2) = 4.68, P = 0.10 |
| HIV positive | 8.3% | 9.5% | 8.1% | χ2(2) = 0.03, P = 0.98 |
| Years of regular cocaine use | 11.0 ± 6.6 | 9.8 ± 6.5 | 11.9 ± 6.6 | F2,107 = 1.01, P = 0.37 |
| Mean cocaine treatments | 3.8 ± 6.8 | 2.9 ± 7.3 | 2.7 ± 2.5 | F2,117 = 0.37, P = 0.61 |
| Met DSM criteria for cocaine dependence | 81.1% | 84.4% | 94.7% | χ2(2) = 3.33, P = 0.19 |
| Met DSM criteria for alcohol abuse/dependence | 48.6% | 64.4% | 65.8% | χ2(2) = 2.89, P = 0.24 |
| Transferred from in-patient detoxification program | 18.9% | 22.2% | 28.9% | χ2(2) = 1.10, P = 0.58 |
| Past month spent on cocaine | $310 ± 747 | $444 ± 845 | $305 ± 408 | F2,117 = 0.72, P = 0.49 |
| Past month spent on alcohol | $33 ± 100 | $41 ± 73 | $38 ± 96 | F2,117 = 0.69, P = 0.50 |
| Cocaine-positive at intake | 35.1% | 43.2% | 35.1% | χ2(2) = 0.78, P = 0.69 |
Table 2.
Addiction Severity Index composite scores; values represent means and standard deviations.
| Variable | Intake | 1 month | 3 month | Effect of time Z score (P-value) |
|---|---|---|---|---|
| Medical | 1.86 (0.06) | |||
| Standard | 0.21 ± 0.28 | 0.14 ± 0.25 | 0.35 ± 0.39 | |
| $80 CM | 0.20 ± 0.31 | 0.26 ± 0.37 | 0.24 ± 0.36 | |
| $240 CM | 0.22 ± 0.33 | 0.19 ± 0.34 | 0.30 ± 0.35 | |
| Employment | −2.83 (0.001) | |||
| Standard | 0.67 ± 0.32 | 0.63 ± 0.29 | 0.60 ± 0.32 | |
| $80 CM | 0.72 ± 0.33 | 0.69 ± 0.35 | 0.96 ± 1.61 | |
| $240 CM | 0.63 ± 0.34 | 0.62 ± 0.32 | 0.61 ± 0.33 | |
| Drug | −8.29 (0.001) | |||
| Standard | 0.16 ± 0.08 | 0.10 ± 0.07 | 0.08 ± 0.07 | |
| $80 CM | 0.19 ± 0.10 | 0.10 ± 0.08 | 0.09 ± 0.07 | |
| $240 CM | 0.18 ± 0.09 | 0.13 ± 0.13 | 0.10 ± 0.07 | |
| Alcohol | −3.74 (0.001) | |||
| Standard | 0.19 ± 0.22 | 0.12 ± 0.15 | 0.08 ± 0.11 | |
| $80 CM | 0.24 ± 0.22 | 0.14 ± 0.14 | 0.13 ± 0.13 | |
| $240 CM | 0.22 ± 0.24 | 0.11 ± 0.14 | 0.14 ± 0.15 | |
| Gambling | 0.78 (0.43) | |||
| Standard | 0.01 ± 0.04 | 0.03 ± 0.06 | 0.01 ± 0.03 | |
| $80 CM | 0.03 ± 0.07 | 0.04 ± 0.08 | 0.04 ± 0.08 | |
| $240 CM | 0.01 ± 0.03 | 0.02 ± 0.05 | 0.02 ± 0.05 | |
| Legal | −2.87 (0.001) | |||
| Standard | 0.17 ± 0.23 | 0.13 ± 0.20 | 0.06 ± 0.13 | |
| $80 CM | 0.15 ± 0.23 | 0.05 ± 0.15 | 0.09 ± 0.20 | |
| $240 CM | 0.13 ± 0.18 | 0.12 ± 0.23 | 0.09 ± 0.22 | |
| Family/social | −2.42 (0.01) | |||
| Standard | 0.17 ± 0.20 | 0.15 ± 0.15 | 0.17 ± 0.24 | |
| $80 CM | 0.25 ± 0.23 | 0.16 ± 0.24 | 0.17 ± 0.24 | |
| $240 CM | 0.32 ± 0.27 | 0.27 ± 0.26 | 0.20 ± 0.22 | |
| Psychiatric | −3.44 (0.001) | |||
| Standard | 0.23 ± 0.25 | 0.18 ± 0.20 | 0.19 ± 0.20 | |
| $80 CM | 0.23 ± 0.22 | 0.13 ± 0.18 | 0.14 ± 0.19 | |
| $240 CM | 0.27 ± 0.25 | 0.18 ± 0.20 | 0.17 ± 0.17 |
Continuous abstinence
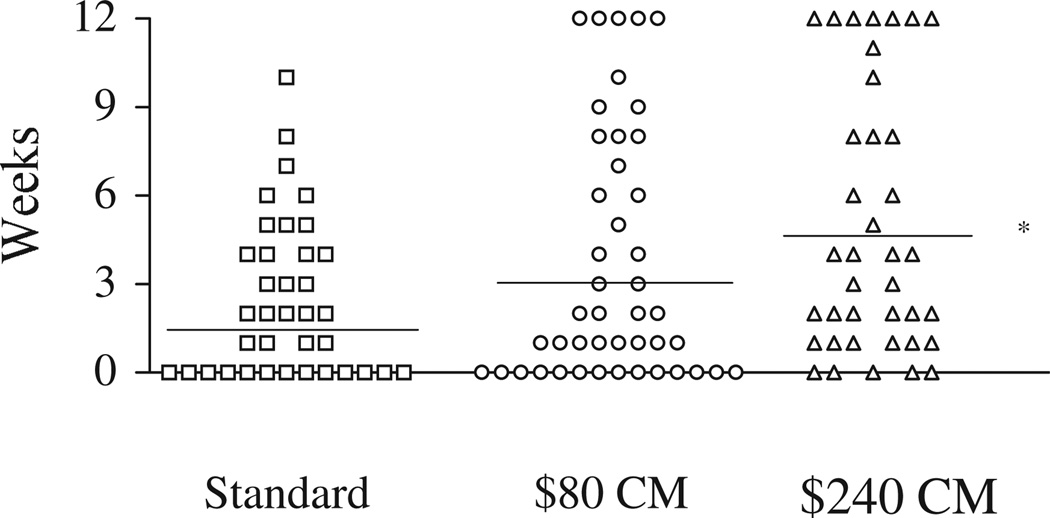
Figure 1 shows the longest duration of continuous abstinence that patients in the three treatment groups were able to achieve during the 12-week treatment period. Each symbol represents one patient, and horizontal lines represent mean weeks of continuous abstinence for the group. The groups differed with respect to longest duration of continuous abstinence, χ2(2) = 6.14, P < 0.05. Post hoc analyses comparing each group to every other group revealed that the $240 CM condition resulted in longer periods of abstinence than the standard condition, U = 476, P < 0.05, but the $80 condition fared no better than the standard condition (P= 0.40). A non-significant trend toward longer periods of consecutive abstinence was noted in the $240 condition compared to the $80 condition, U = 1710, P = 0.09.
Figure 1.
The longest duration of consecutive abstinence throughout the 12-week study period. Each symbol represents one patient. The horizontal lines represent the group means. * P < 0.05; group differs from the standard condition
Retention, number of samples and percentage of negative samples
The mean (and standard deviation) of weeks in the study was 6.2±4.1, 6.2 ± 4.1 and 6.7 ± 4.1 for the standard, $80 CM and $240 CM conditions, respectively. A Kruskal-Wallis test revealed no differences in retention across groups, χ2(2) = 1.02, P > 0.60. Of patients in the standard condition 13.5% completed all 12 weeks of treatment, as did 20.0% of those in the $80 CM condition and 31.6% of those in the $240 CM condition (data not shown).
Because patients in the CM conditions may have had a greater incentive to submit specimens than patients in the standard condition, we evaluated whether differences existed in the total number of urine specimens submitted. No differences were noted, F1,117 = 1.02, P > 0.37, and mean number of samples per group was 8.9 ± 5.3, 8.7 ± 6.3 and 10.5 ± 6.6.
However, the groups did differ in the percentages of drug-free specimens submitted, F2,117 = 3.75, P < 0.05. Patients in the standard condition submitted an average of 62.3 ± 41.5% drug-free specimens, compared with 66.4 ± 40.8% in the $80 CM condition and 84.2 ± 26.8% in the $240 CM condition. Although the $80 CM condition did not differ from the standard condition [t(80) = 0.45, P >0.65], the $240 CM condition differed from both the standard [t(73) = 2.72, P < 0.01] and $80 CM conditions [t(81) = 2.30, P < 0.05].
ASI scores
Most ASI composite scores decreased over time, including employment, drug, alcohol, legal, family and psychiatric (see Table 2). However, there were no differences in rates of change over time by treatment group.
Analysis of subgroups based on initial urinalysis result
Baseline differences
We assessed the interactive effect of cocaine use severity on outcomes, using a positive cocaine urine sample at intake as a proxy of severity. To demonstrate that initial urinalysis result is associated with severity of drug use, we compared patients with a cocaine-positive specimen at intake (n = 46) with patients with a cocaine-negative specimen at intake (n = 73). Self-reported days of cocaine use in the month prior to treatment intake were 10.4 ± 9.1 for the cocaine-positive group versus 3.3 ± 6.7 for the cocaine-negative group, t(117) = 4.82, P < 0.001. Amounts spent on cocaine in the month before intake were $510 ± 101 versus $269 ± 708, t(117) = 3.49, P < 0.001 for the two groups. ASI drug scores also differed: 0.22 ± 0.09 versus 0.15 ± 0.08, t(117) = 4.56, P < 0.001. Thus, it appears that patients who initiate treatment with a cocaine-negative specimen have less severe cocaine problems than those who begin treatment with a cocaine-positive specimen.
Treatment outcomes
In terms of duration of continuous abstinence, no statistically significant differences were noted across the groups when only patients who provided a cocaine- negative sample at intake were included in the analyses, χ2(2) = 1.87, P = 0.39. The means were 3.5 ± 2.6, 5.4 ± 4.5 and 5.6 ± 4.7 weeks for those assigned to the standard, $80 and $240 conditions, respectively. However, for patients who submitted cocaine-positive samples at intake, significant group effects were noted in longest duration of continuous abstinence, χ2(2) = 9.64, P < 0.01, with means of 0.5 ± 1.2, 1.5 ± 2.7 and 3.6 ± 3.6 for the three respective groups. Initially cocaine-positive patients who were assigned to the standard condition did not differ from cocaine-positive patients assigned to the $80 CM condition, U= 103.5, P > 0.45, but patients in the $240 CM condition evidenced longer durations of abstinence than those in standard, U = 34.5, P < 0.01, and $80 CM conditions, U= 75.5, P < 0.05.
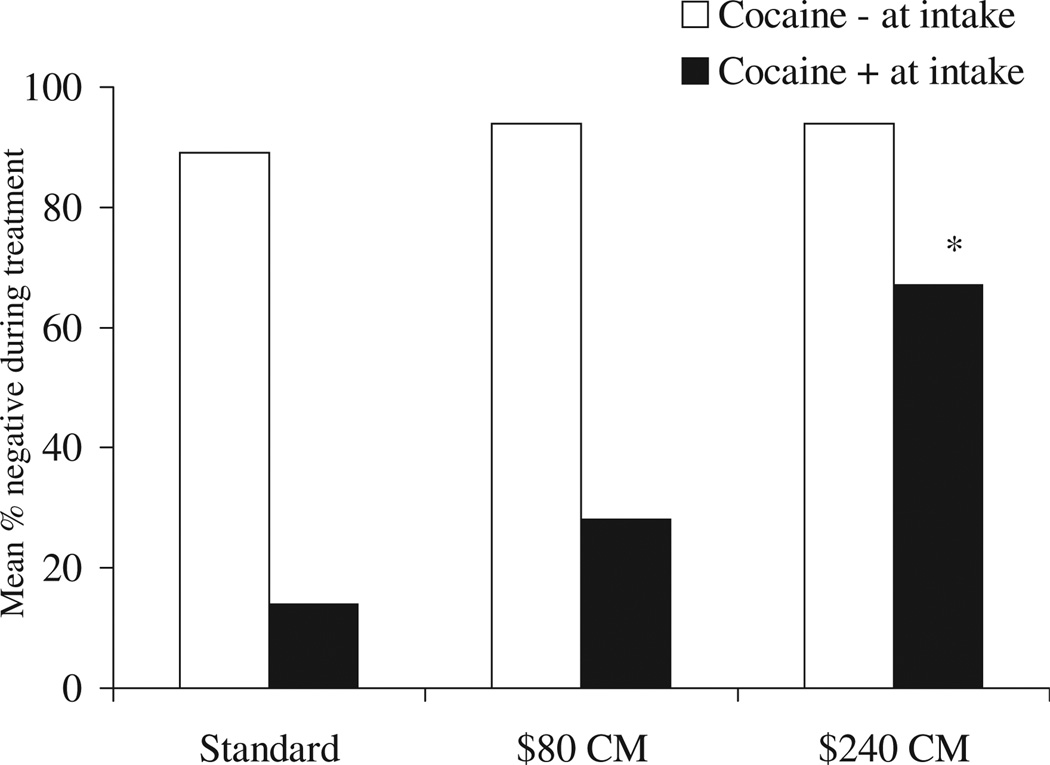
Retention rates and total number of samples submitted did not differ across groups, regardless of whether patients initiated treatment with a positive or negative urinalysis result (data not shown). However, as shown in Fig. 2, patients who initiated treatment with positive or negative urinalysis results responded differently to the CM interventions. Patients who initiated treatment with cocaine-negative samples were unlikely to provide positive samples during treatment regardless of their treatment assignment, and no differences across treatment conditions were noted for these patients, χ2(2) = 3.14, P > 0.20. In contrast, those who began treatment with a cocaine-positive sample evidenced different effects across conditions, χ2(2) = 14.13, P < 0.001. Patients with an initially positive result and assigned to the $240 CM condition (n = 14) had a lower percentage of drug-free specimens during treatment than patients with initially cocaine-positive samples who were assigned to the standard (n = 13), U = 23.5, P < 0.001, or $80 CM condition (n = 19), U= 56.5, P < 0.01. The $80 CM and standard conditions did not differ from one another, U= 92.5, P > 0.20.
Figure 2.
Percentage of during-treatment negative samples submitted by patients in the three treatment groups. Data are presented separately for patients who initiated treatment abstinent versus those who initiated treatment with a positive sample. * P < 0.01; group differs from both standard and $80 CM conditions
Cost of reinforcers
The mean number (and standard deviation) of draws earned for submitting negative specimens was 16 ± 21 and 24 ± 24 in the $80 and $240 contingent groups, respectively. In terms of draws for completion of goal-related activities, patients in the $80 condition earned an average of 20 ± 21 draws versus 26 ± 24 draws for patients in the $240 condition. In the $80 condition, patients won an average of 14 ± 16 mini prizes, 3 ± 4 medium prizes, and 0.2 ± 0.6 jumbo prizes. In the $240 condition, patients won an average of 21 ± 19 small, 3 ± 3 large, and 0.2 ± 0.4 jumbo prizes. The average cost of the prizes selected by patients in the $80 condition was $36 ± 66 and $68 ± 75 by the patients in the $240 condition. The overall cost of all prizes distributed was $4199.
DISCUSSION
Results from this study suggest that the prize reinforcement CM procedure is efficacious in reducing cocaine use. The beneficial effects appear to be magnitude-dependent, with the low magnitude condition tested in this study not being particularly efficacious. Patients who initiate treatment with a positive urinalysis result may have better outcomes when they receive CM than when they receive standard treatment without CM. In contrast, CM may not be necessary for patients who initiate treatment with a cocaine-negative urinalysis result. These results will be discussed, along with their implications for the use of CM as an add-on to standard therapy at community-based substance abuse treatment programs.
This study found that the $240 CM condition engendered more beneficial effects than standard treatment on the substance use outcome measures. Whether duration of continuous abstinence or percentages of negative samples submitted were used, CM treatment out-performed standard treatment. These results are consistent with a variety of other studies demonstrating beneficial effects of CM in improving outcomes of cocaine-abusing patients in drug-free treatment modalities (Higgins et al. 1994, 2000a) and in methadone maintenance programs (Silverman et al. 1996; Griffith et al. 2000; Petry & Martin 2002).
A magnitude effect was noted on some of the outcome measures, with the larger magnitude CM condition generally being more efficacious than the lower magnitude condition. The $240 CM condition was more efficacious than the $80 CM condition with percentage of negative samples submitted. Compared to the $80 CM condition, the $240 CM condition also showed a trend toward engendering longer durations of continuous abstinence. Some other studies have demonstrated magnitude effects when money or vouchers are used as the reinforcer for cigarette (Stitzer & Bigelow 1983, 1984; Stitzer et al. 1986) or cocaine abstinence (Silverman et al. 1999). The $80 CM condition, in contrast, did not improve outcomes significantly relative to standard treatment.
Beneficial effects of CM did not extend to other indices of psychosocial problems as measured by ASI composite scores. While time effects were noted on many of the ASI composite scores, no group or group × time interactions emerged. Similar results are noted in other CM studies, which generally find that beneficial effects of CM are limited to the behavior that is targeted (Higgins et al. 1994, 2000a; Petry 2000). Compliance with goal-related activities was reinforced in this study, and patients earned slightly over half their draws for activity completion. Perhaps because goals and activities were individualized, group effects of CM on reducing psychosocial problems were not evident. Some patients focused on enhancing family relationships, while others improved health or legal status. Given that such a range of activities was reinforced, significant effects of CM on ASI composite scores would be difficult to detect.
In CM studies that reinforce only abstinence, 30–50% of patients never access the reinforcer (Stitzer, Iguchi & Felch 1992; Silverman et al. 1996; Iguchi et al. 1997). Reinforcing activity compliance was included to increase the probability that patients would earn reinforcement. All but two patients in the CM conditions earned at least one draw during treatment, and all but four (95%) won at least one prize. While most all patients assigned to a CM condition earned at least some reinforcement, dismantling designs are needed to address whether the beneficial effects are related to the reinforcing abstinence, compliance with activities or the two behaviors concurrently.
Most studies of CM conducted in non-methadone maintained samples find beneficial effects on retention in treatment (see Petry 2000 for review). No differences in retention were noted across groups in the present study, and only a minority of patients remained in treatment for the full 12 weeks. The lack of group differences may be related to the lower overall magnitude of reinforcement used in this study compared with voucher studies. It may also be related to the implementation of this study in community-based clinics, as opposed to research-based treatment centers in which patient case-loads are substantially lower (Higgins et al. 1993, 1994, 2000a). Regardless of the reason, the similar retention rates noted across groups in this study suggest that any group-related differences in abstinence are not a reflection of greater retention in treatment or the number of urine samples submitted.
Preston et al. (1998) noted that methadone-maintained cocaine-using patients who initiated CM treatment with cocaine-negative urine samples were more likely to benefit from the experimental treatment than those who initiated CM treatment with positive samples. The present study found a somewhat similar effect in a drug-free treatment setting. Patients who initiated treatment with a cocaine-negative urine sample provided a higher percentage of cocaine-negative urine samples throughout treatment than patients who initiated treatment with a cocaine-positive urine sample.
An interesting interaction effect emerged in this study. The CM treatment was most beneficial for the patients who were least likely to do well in standard treatment— those who initiated treatment with positive urine specimens. Patients initiating treatment with a positive urine sample provided a 4–5 times higher percentage of negative samples during treatment if they received the $240 CM treatment than if they received standard treatment. Intermediary effects were noted in the $80 CM condition, which fared about twice as well as the standard condition in terms of percentage of negative samples. In contrast, those who initiated treatment with a negative urine specimen did relatively well in treatment regardless of whether or not they received CM, with about 89–94% of the urine specimens submitted being drug-free. The lack of a beneficial effect of CM among patients who entered treatment abstinent may be related to a ceiling effect, as few of these patients submitted positive urine specimens during treatment.
If replicated in other settings and with larger samples, these data may suggest that, in a time of cost constraints, CM treatments should be aimed primarily at cocaine-using patients who initiate drug-free treatment with positive samples. The average cost of prizes won by patients in the high magnitude CM condition was $86, and significant reductions in cocaine use were noted with this level of reinforcement. Although other costs including urine testing and personnel time to purchase prizes are associated with CM, the direct costs appear low, especially in relation to voucher CM procedures. (See Petry & Martin 2002 for a discussion of the advantages and disadvantages of the prize CM system relative to the voucher CM system.) By including aspects of the voucher system such as allowance for patient preferences, access to high magnitude rewards and bonuses for continuous abstinence, the prize CM system was successful in engendering beneficial effects among patients who otherwise do poorly in treatment.
Given these relatively low costs, implementation of prize reinforcement CM may be possible in community clinics. Although an average cost of $86 per patient may still be considered prohibitive in some settings, clinics can solicit prize donations from retailers (Amass 1997). If the interaction effect between baseline drug use and efficacy of CM is replicated in prospective studies then CM, and especially higher magnitude CM, could be applied only to the patients most likely to benefit from them. Finally, societal costs associated with cocaine abuse may far exceed the costs of prizes, or even hundreds to thousands of dollars in vouchers, provided by CM treatments. Larger studies, conducted at sites across the country, are ongoing to evaluate the cost–effectiveness of CM in terms of impact on criminal activity, unemployment and medical sequelae related to drug abuse. Finally, future work must also address training therapists to administer the techniques and the efficacy of the procedures when administered by community-based therapists.
Long-term evaluations of CM on drug use as well as other psychosocial outcome measures are also necessary. Higgins, Badger & Budney (2000b) found that the longest duration of continuous abstinence during treatment is a significant predictor of 6-month outcomes. In a study of prize reinforcement CM in methadone patients, beneficial effects of CM were maintained for 2 months following the removal of the reinforcement (Petry & Martin 2002). Although beneficial effects of this CM system were noted in the short term, the majority of patients did not remain in treatment for the full 12 weeks of this study. Strategies to enhance treatment participation and improve outcomes over longer periods of time need to be investigated. These may include booster CM sessions or a longer duration of CM treatment with reductions in probability of reinforcement once substantial periods of abstinence have been achieved (e.g. Kirby et al. 1998). CM procedures may also be useful to enhance participation in other forms of treatment that have more enduring effects, such as cognitive–behavioral therapy (Carroll et al. 1994).
In summary, this study provides further evidence of the efficacy of this prize reinforcement CM procedure for reducing drug use in community-based, drug-free substance abuse treatment programs. Additional research is needed to evaluate the cost–effectiveness of this approach and to delineate important parameters, such as the probabilities of reinforcement, optimal durations of treatment and targets of reinforcement, under which these procedures engender beneficial effects, and the patients for whom the procedures are most beneficial and cost effective.
ACKNOWLEDGEMENTS
We thank the patients and staff at BlueRidge Center and Alcohol and Drug Recovery Centers, Inc. for their participation in and support of this project. This research was supported by NIH grants P50-DA09241, R01-DA13444, R29-DA12056, P50-AA03510, K05-DA00089 (BJR), K02-DA00248 (KMC) and General Clinical Research Center Grant M01-RR06192.
REFERENCES
- Aiken M. A program for balancing the allocation of subjects in a clinical trial. Computers and Biomedical Research. 1982;15:519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, Maany I. A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug and Alcohol Dependence. 1997;46:79–85. doi: 10.1016/s0376-8716(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Amass L. Financing voucher programs for pregnant substance abusers through community donations. In: Harris LS, editor. Problems of Drug Dependence, 1997, Proceedings of the 58th Annual Scientific Meeting NIDA Research Monograph. Washington, DC: Government Printing Office; 1997. p. 60. NIH publication no. 174. [Google Scholar]
- Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Rockville, MD: US Department of Health and Human Services; 1998. [Google Scholar]
- Carroll KM, Nich C, McLellan AT, McKay JR, Roun-saville BJ. ‘Research’ versus ‘real-world’ patients: representativeness of participants in clinical trials of treatments for cocaine dependence. Drug and Alcohol Dependence. 1999;54:171–177. doi: 10.1016/s0376-8716(98)00161-6. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Power MD, Bryant KJ, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers: psychopathology and dependence severity as predictors of outcome. Journal of Nervous and Mental Disease. 1993;18171 doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Crits-Cristoph P. Empirically supported individual and group psychological treatments for adult mental disorders. Journal of Consulting and Clinical Psychology. 1998;66:37–52. doi: 10.1037//0022-006x.66.1.37. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Flynn PM, Kristiansen PL, Porto JV, Hubbard RL. Costs and benefits of treatment for cocaine addiction in DATOS. Drug and Alcohol Dependence. 1999;57:167–174. doi: 10.1016/s0376-8716(99)00083-6. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE. ‘Mini-mental state.’ A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: application to the NIMH Treatment of Depression Collaborative Research Program. Archives of General Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug and Alcohol Dependence. 2000;58:55–56. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Hedeker DH. MIXREG: a Fortran Program for Mixed-Effects Linear Regression Models. Rockville, MD: NIMH Division of Services Research; 1993. [computer program] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer-term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000b;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger GJ. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden D, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one year of follow-up. Journal of Consulting and Clinical Psychology. 2000a;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Belding M, Morral A, Lamb R. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. Journal of Consulting and Clinical Psychology. 1997;65:421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Pottieger AE. Drug use and street crime in Miami: an (almost) twenty-year retrospective. Substance Use and Misuse. 1998;33:1839–1870. doi: 10.3109/10826089809059324. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences initiation of cocaine abstinence. Journal of Consulting and Clinical Psychology. 1998;66:761–767. doi: 10.1037//0022-006x.66.5.761. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. Evaluation of patients treated for pathological gambling in a combined alcohol, substance abuse and pathological gambling treatment unit using the Addiction Severity Index. British Journal of Addictions. 1991;86:1017–1028. doi: 10.1111/j.1360-0443.1991.tb01863.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Metzger DS, Grissom GR, Woody GE, Luborsky L, O’Brien CP. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: role of treatment services. Journal of Consulting and Clinical Psychology. 1994;62:1141–1158. doi: 10.1037//0022-006x.62.6.1141. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: reliability and validity in three centers. Journal of Nervous and Mental Diseases. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in general clinic settings. Drug and Alcohol Dependence. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM. Validity of the Addiction Severity Index in assessing gambling problems. Journal of Nervous and Mental Diseases. 2003;191:399–407. doi: 10.1097/01.NMD.0000071589.20829.DB. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: contingency management for the treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Finocche C. Contingency management for group treatment: a demonstration project in an HIV drop-in program. Journal of Substance Abuse Treatment. 2001;21:89–96. doi: 10.1016/s0740-5472(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Martin B. Reinforcing compliance with non-drug-related activities. Journal of Substance Abuse Treatment. 2001;20:33–44. doi: 10.1016/s0740-5472(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Preston LK, Silverman K, Higgins ST, Brooner RK, Mon-toya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Journal of Studies on Alcohol. 1998;59:631–639. doi: 10.15288/jsa.1998.59.631. [DOI] [PubMed] [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, Stitzer ML. The brief abstinence test: voucher-based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Petry NM, Carroll KM. Single versus multiple drug targets in substance abuse clinical trials research. Drug and Alcohol Dependence. 2003;70:117–125. doi: 10.1016/s0376-8716(03)00033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: effects of pay amount. Behavioral Therapy. 1983;14:647–656. [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: within-subject effects of pay amount. Journal of Applied Behavioral Analysis. 1984;17:477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer MS, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. Journal of Consulting and Clinical Psychology. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Rand CS, Bigelow GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 1999 National Household Survey on Drug Abuse. Rock-ville, MD: National Clearinghouse for Alcohol and Drug Information (NCADI); 2000. [Google Scholar]