Abstract
Attention deficit/hyperactivity disorder (ADHD) is attributed to dysfunction of the prefrontal cortex. Methylphenidate, an inhibitor of dopamine and norepinephrine transporters (DAT and NET, respectively), is a standard treatment for ADHD. The Spontaneously Hypertensive Rat (SHR) is a well-established animal model of ADHD. Our previous results showed that methylphenidate treatment in adolescent SHR enhanced cocaine self-administration during adulthood, and alterations in DAT function in prefrontal cortex play a role in this response. Importantly, prefrontal cortex subregions, orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC), have been shown to have distinct roles in ADHD and cocaine self-administration. In the current study, SHR, Wistar-Kyoto (WKY) and Wistar (WIS) rats received a therapeutically relevant dose of methylphenidate (1.5 mg/kg, p.o.) or vehicle during adolescence and then OFC and mPFC DAT function and cellular expression were assessed during adulthood. In both OFC and mPFC, no strain differences in Vmax or Km for dopamine uptake into synaptosomes were found between vehicle-treated SHR, WKY and WIS. Methylphenidate increased DAT Vmax in SHR mPFC and decreased DAT Vmax in WKY OFC. Also, methylphenidate decreased DAT Km in WIS OFC. Further, methylphenidate did not alter DAT cellular localization, indicating that methylphenidate treatment during adolescence regulated DAT function in SHR mPFC in a traffickingindependent manner. Thus, the increase in mPFC DAT function was an SHR-specific long term consequence of methylphenidate treatment during adolescence, which may be responsible for the treatment-induced alterations in behavior including the observed increases in cocaine self-administration.
Keywords: Addiction, Attention Deficit/Hyperactivity Disorder, Cocaine, Dopamine transporter function, Methylphenidate, Spontaneously Hypertensive Rat
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is a highly debilitating, heterogeneous disorder typically diagnosed in childhood, and characterized by age-inappropriate levels of inattention, impulsivity and hyperactivity. The neurobiological etiology of this disease is not understood completely [1]. ADHD is ascribed partially to dopaminergic deficits in prefrontal cortex [2–4]. Further, ADHD is associated with increased dopamine transporter (DAT) expression in striatum and with specific polymorphisms in the DAT gene [5, 6].
The Spontaneously Hypertensive Rat (SHR) is the most widely accepted rodent model of ADHD and displays all of the behavioral diagnostic characteristics of ADHD [7, 8]. SHR display diminished dopamine (DA) release from prefrontal cortical and striatal slices in vitro [9], but greater DA release from nucleus accumbens in vivo [10]. Also, DAT function and expression levels in frontal cortex and striatum were greater in SHR than in control rats [11, 12]. Taken together, these previous findings strengthen the predictive value of the SHR for evaluating the consequences of long-term pharmacotherapeutic treatment of ADHD on DA neurochemistry and related behaviors.
Methylphenidate (Ritalin®) is a gold standard treatment for ADHD, providing successful relief from ADHD symptoms. In SHR, methylphenidate improves attention and decreases hyperactivity [8, 13]. In terms of underlying neurochemical mechanisms of action, methylphenidate acts as an inhibitor of striatal DAT function and prefrontal cortical DAT and norepinephrine transporter (NET) function, increasing extracellular DA concentrations and DA receptor occupancy [14–17]. Furthermore, methylphenidate decreases basal firing rates of striatal neurons [16], which has been suggested to strengthen corticostriatal signals, thus contributing to its pharmacological effects. Based on these findings, DAT is critically involved in the dopaminergic dysfunction associated with ADHD and serves as an important molecular target for the treatment of ADHD.
Adults with ADHD have been reported to have a higher risk of developing substance use disorders compared to individuals without ADHD [18]. In comparison to the general population, those with ADHD have a 35% higher incidence of cocaine abuse [19]. However, the impact of prior treatment with methylphenidate on cocaine abuse liability in this population is controversial. While some studies suggest methylphenidate treatment is protective against cocaine abuse [20–22], others indicate that methylphenidate exposure during adolescence may increase cocaine abuse liability [23–25]. Also, the mechanisms underlying the high comorbidity between ADHD and cocaine abuse are not well understood. Since cocaine competitively inhibits DAT, which leads to a compensatory increase in DAT cell surface expression and function [26–28], one explanation for the comorbitity of ADHD and cocaine abuse may be a greater DAT expression and function in these individuals.
Another explanation for the comorbidity of ADHD and cocaine abuse may be the preexisting impairments in cortically-controlled executive function, including increased impulsivity and risk-taking behavior in this population [29, 30]. Compared to control subjects, boys with ADHD showed decreased orbitofrontal cortex (OFC) activation using fMRI during a delayed discounting task that measures impulsivity [31]. Also, decreased dorsolateral prefrontal cortex (DLPFC) activation was associated with working memory deficits in ADHD individuals compared to controls [32]. Unmedicated ADHD individuals also showed increased deactivation of fMRI signals in the medial prefrontal cortex (mPFC) assessed during the Stroop test with emotional interference, compared to medicated ADHD and control individuals [33]. With respect to understanding molecular mechanisms in cortical areas involved in ADHD, it is important to note that the DLPFC in primates is considered to be functionally analogous to the subregions of mPFC in rodents, including the Fr2 and the anterior cingulate cortex [34]. Thus, functional impairments in these cortical regions (OFC, DLPFC and mPFC) are strongly associated with behavioral deficits in ADHD; however, few studies have evaluated molecular mechanisms in the OFC and mPFC of SHR.
Of importance, cocaine abuse is associated with impulsive behavior and with lasting neurochemical changes in these same cortical regions [35]. Individuals abusing cocaine display reduced response inhibition in the Stroop test and increased glucose metabolism in OFC, compared with demographically-matched controls [36]. In cocaine users, reduced inhibitory control in the Go-No Go tasks was associated with decreased mPFC activation using fMRI [37]. In contrast, increased activation of DLPFC was found in cocaine-dependent individuals during the Stroop test and in response to cocainerelated cues [38, 39]. With respect to animal models, outbred rats self-administering cocaine showed decreased cocaine-seeking and taking behavior following inactivation of OFC and mPFC [40–43], thereby supporting the critical involvement of OFC and mPFC function in cocaine abuse. Thus, both impulsivity and functional impairments of the OFC, DLPFC and mPFC are associated with ADHD and cocaine abuse, and these commonalities may in part underlie their comorbidity.
Valuable insights into the mechanisms underlying comorbidity of ADHD and cocaine abuse may be obtained using an animal model of ADHD that exhibits high cocaine self-administration behavior. However, appropriate interpretation of results obtained from ADHD rat models with strong face validity, such as SHRs, requires cautious selection of suitable reference control groups [57]. Studies that employ the SHR-progenitor strain, Wistar-Kyoto (WKY) as the only reference control [7] have been criticized. For example, hyperactivity in SHR appears to have been overestimated when the only control employed was WKY, because WKY are hypoactive relative to outbred control rats [58, 59]. Neurochemical studies also reveal differences between WKY and outbred control rat strains. WKY show altered dopaminergic function compared to outbred Wistar (WIS) rats (e.g., decreased striatal DA D1 and D2 receptors and increased striatal D3 receptors; [60, 61]; decreased accumbal DAT expression [62]; and decreased mPFC DA content [63]). Thus, inclusion of both the more commonly used WKY control and the WIS control as comparators to SHR provides a more complete understanding of the behavioral and neurochemical mechanisms underlying ADHD pathology and of the effects of long term ADHD pharmacotherapeutics.
Using this optimized experimental design, our previous results showed that SHR treated with a therapeutically relevant oral dose of methylphenidate during adolescence exhibit increased cocaine self-administration in adulthood compared to vehicleadministered SHR and methylphenidate-administered WKY and WIS controls [44]. The increase in cocaine self-administration was associated with decreased DAT function in whole prefrontal cortex, which includes both OFC and mPFC. OFC and mPFC are distinct based on cytoarchitecture and behavioral function, but are also highly interconnected [45, 46]. Thus, the current study determined DAT function and expression in OFC and mPFC in adult SHR following a therapeutically relevant oral dose of methylphenidate administered during adolescence.
2 Materials and methods
2.1 Materials
(±)-Methylphenidate hydrochloride, desipramine hydrochloride, paroxetine hydrochloride, nomifensine maleate, pargyline, ascorbic acid, ethylenediaminetetraacetic acid (EDTA), sucrose, β-mercaptoethanol, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 3-hydroxytyramine (DA), sodium chloride and magnesium sulfate were purchased from Sigma-Aldrich (St. Louis, MO). The current experiments used racemic methylphenidate, which also is administered to ADHD patients under the trade name Ritalin®. α-d-Glucose, L-ascorbic acid, and monobasic potassium phosphate were purchased from Aldrich Chemical Co. (Milwaukee, WI), AnalaR-BHD Ltd. (Poole, UK) and Mallinckrodt (St. Louis, MO), respectively. [3H]DA (dihydroxyphenylethylamine,3,4-[7-3H]; specific activity, 30.3 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences Inc. (Boston, MA). All other chemicals in the uptake assay buffers were purchased from Fisher Scientific Co. (Pittsburgh, PA).
For the cell surface localization assays, antibodies recognizing rat DAT (C-20; goat polyclonal antibody), demethylated protein phosphatase 2A–C (PP2A-C; 4B7; mouse monoclonal antibody), anti-mouse IgG conjugated with horseradish peroxidase (IgG-HRP; sc2954 chicken polyclonal antibody) and anti-goat IgG-HRP (sc2020; donkey polyclonal antibody) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against alpha-1 Na+/K+ ATPase type-1 (mouse monoclonal antibody) were obtained from Abcam (Cambridge, MA). Antibodies recognizing β-actin (A 5441, mouse monoclonal antibody) were purchased from Sigma-Aldrich (St. Louis, MO). Sulfosuccinimidobiotin (sulfo-NHS-biotin), d-biotin and immunoPure immobilized monomeric avidin gel were purchased from Pierce Chemical (Rockford, IL). Complete protease inhibitor cocktail tablets were obtained from Roche diagnostics (Indianapolis, IN). Immunobilon-P PVDF membranes were procured from Millipore. HyGLO Quickspray Chemiluminescent HRP Antibody Detection Reagent and HyblotCL autoradiography films were purchased from Denville Scientific Inc. (Metuchen, NJ). All other chemicals in the buffers for cell surface localization assays were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA).
2.2 Animals and Treatments
Male SHR and WKY (inbred control) rats at postnatal day 25 (P25) were obtained from Charles River Laboratories (Kingston, NY), and male WIS (outbred control) rats at P25 were obtained from Charles River Laboratories (Raleigh, NC). Rats were individually housed with free access to food and water in a colony room maintained on a 12-h light:dark cycle (lights on 07:00 h) in the Division of Laboratory Animal Resources (University of Kentucky, Lexington, KY). From P28 to P55, rats were administered methylphenidate (1.5 mg/kg, p.o. in oyster crackers, Monday - Friday) or vehicle (1 ml/kg, tap water in oyster crackers) to mimic the clinically relevant plasma concentrations, route of administration and weekly pattern of dosing [8, 47, 48, 80]. Specifically, a dose of 1.5 mg/kg delivered orally produces peak plasma levels of methylphenidate in rats comparable to plasma levels (8–40 µg/ml) obtained in the ADHD population [47, 48]. Oyster crackers containing methylphenidate or water were placed in the individual home cage and consumption was monitored to ensure reproducible dosing between days and between animals. Rats consumed the crackers within 3 min of presentation. P28 to P55 includes a time period from early adolescence to late adolescence [49, 50], the typical age during which methylphenidate is administered clinically. Rats were also maintained under mild food restriction (90% of their free-feeding body weight) to mimic the conditions of our previously published studies [44] to allow for comparison of current neurochemical findings with those previously reported. Further, all rats consumed the entire daily allotment of food, and body weight did not differ between the methylphenidate- and vehicle-treated groups (data not shown). After P55, methylphenidate treatment ended and ad libitum access to food was reinstated. The number of days varied between the last day of treatment (P55) and the day (P77-P84) that the neurochemical assay was conducted. Separate cohorts of rats were used for the DA uptake assays and the DAT cellular distribution assays. Rat handling procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and were performed in accordance with the 1996 version of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3 DA Uptake Assay
DAT function was assessed using kinetic analysis of [3H]DA uptake into OFC, mPFC and striatal synaptosomes using a previously published procedure [51] with minor modifications. For each experiment, OFC, mPFC and striatum from both hemispheres of one methylphenidate-treated and of one vehicle-treated rat of the same strain and age (P77-P84) were homogenized in separate glass homogenizers, each containing 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16–20 passes of a Teflon pestle. Synaptosomal suspensions were subjected to two centrifugation steps (2,000g, 10 min, 4 °C followed by 20,000g, 17 min, 4 °C) using an Avanti-J30I centrifuge (Beckman Coulter, Brea, CA). Resulting pellets were resuspended in 2.2 ml (OFC and mPFC synaptosomes) or 2.4 ml (striatal synaptosomes) ice-cold uptake buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). OFC and mPFC samples (100 µl aliquots of the 2.2 ml synaptosomal suspensions) and striatal samples (30 µl aliquot of the 2.4 ml synaptosomal suspension) were incubated for 5 min in a metabolic shaker (Dubnoff incubator; Precision Scientific, Winchester, VA) at 34 °C in a saturated 95% O2/5% CO2 atmosphere in the absence or presence of 10 µM nomifensine. Nomifensine, a DAT inhibitor, was used to obtain nonspecific [3H]DA uptake. OFC and mPFC suspensions also contained 5 nM each of paroxetine and desipramine to prevent [3H]DA uptake by serotonin transporters and NETs, respectively. Subsequently, 1 of 7 final concentrations (0.01–1.0 µM) of [3H]DA was added to the buffer, and incubation of the mPFC, OFC and striatal synaptosomal suspensions continued for 5, 5 and 10 min, respectively. [3H]DA uptake was terminated by addition of 3 ml of ice-cold assay buffer, immediately followed by filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 hr at 4 °C) using a cell harvester (Biochemical Research and Development Laboratories, Gaithersburg, MD). Values for total and nonspecific [3H]DA uptake were obtained from the amount of radioactivity retained on the filters as determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences, Downers Grove, IL). Protein concentrations were determined with bovine serum albumin standards using the Bradford method [52]. Specific [3H]DA uptake was obtained by subtracting nonspecific uptake from total uptake, and the values were used to determine kinetic parameters (Vmax and Km) by employing the commercially-available GraphPad Prism 5.0 program (GraphPad Software Inc., San Diego, CA).
2.4 DAT cellular distribution assay
Synaptosomal pellets of OFC, mPFC and striatum were resuspended in 1.25 ml (OFC and mPFC) or in 3 ml (striatum) of ice-cold sucrose solution. Synaptosomal protein concentrations were determined as previously described. Biotinylation and Western blotting assays were performed using a previously published method [53]. Briefly, synaptosomal suspensions contained about 1 mg protein (OFC and mPFC) or 500 µg protein (striatum). Suspensions were incubated with shaking for 1 hr at 4 °C in 500 µl of 1.5 mg/ml sulfo-NHS biotin in PBS/Ca/Mg buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.4), which labels all surface proteins with biotin. Free sulfo-NHS biotin was removed by centrifugation (8000g, 4 min, 4 °C) using a model 5417R centrifuge (Eppendorf, Hauppauge, NY) followed by washing with 1 ml ice-cold 100 mM glycine in PBS/Ca/Mg buffer, and these steps were repeated twice. Then, samples were centrifuged using the same conditions and washed with 1 ml ice-cold PBS/Ca/Mg buffer, and these steps were repeated twice. Subsequently, OFC, mPFC and striatal synaptosomes were lysed by sonication for 2–4 s followed by incubation, with continuous shaking for 20 min at 4 °C in Triton X-100 buffer (150, 150 and 300 µl, respectively; 10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µM pepstatin, 250 µM phenylmethysulfonyl fluoride, pH 7.4). Lysates underwent centrifugation (21,000g, 20 min, 4 °C). Supernatants constituted the total protein fraction. To obtain the cell surface and intracellular fractions, 2/3 of the supernatant was incubated with Avidin beads with shaking for 1 hr at room temperature, and samples centrifuged (17,000g, 4 min, 4 °C). Supernatants constituted the non-biotinylated fraction (intracellular fraction). Pellets contained the Avidin-conjugated biotinylated proteins (cell surface fraction). Pellets were washed three times with 1% Triton-X-100 and incubated with Lamelli buffer containing 5% v/v β-mecaptoethanol to free the cell surface proteins from the Avidin-biotin complex. Total, non-biotinylated and biotinylated fractions were frozen at −20 °C until Western blot analysis.
The total, non-biotinylated and biotinylated fractions were thawed and subjected to gel electrophoresis and Western blotting as described previously [54] using a gel running apparatus and Trans-Blot® SD Semi-Dry Transfer Cell, respectively (Bio-Rad Laboratories, Inc., Hercules, CA). Blots were incubated (overnight, 4 °C) with primary antibody for DAT, followed by secondary antibody (1 hr, room temperature). DAT protein was detected using enhanced chemiluminescence and HyblotCL autoradiography films (Denville Scientific Inc.). Blots were probed simultaneously for detection of Na+/K+ ATPase (a plasma-membrane enriched protein), PP2A (an intracellular protein) for determining efficiency of biotinylation of surface proteins, and β-actin (a cytoskeletal protein) to control for loading of proteins. Band densities, expressed as relative optical density (arbitrary units), were determined for DAT and β-actin in total, non-biotinylated and biotinylated fractions using ImageJ software (http://imagej.nih.gov/ij).
2.5 Statistical Analysis
Data analyses were conducted using SPSS Statistics Version 19 (SPSS Inc., IBM Company, Armonk, NY). Data are reported as mean ± S.E.M. and n represents the number of rats per group. To determine if there was a between group difference in the number of days between the cessation of treatment and the conduct of the neurochemical assay, post-treatment periods were analyzed by two-way ANOVAs, with strain and treatment as between-subject factors. Km values for DA were expressed as µM and were log transformed to adjust for the skewed distribution, and then analyzed by two-way ANOVA, with strain and treatment as between-subject factors. Vmax values were expressed as pmol/mg/min. Vmax values for the individuals in the methylphenidate-treated group were expressed as a percent of the mean Vmax value for the vehicle-treated control group and analyzed using one-way ANOVAs followed by Tukey’s post-hoc test to compare between methylphenidate-treated strains. Within each strain, Vmax values from methylphenidate-treated rats were compared to the vehicle-control (100%) using Student’s one-sample t-test for matched subjects [55]. For cellular distribution assays, total, non-biotinylated and biotinylated fractions were analyzed using mixed-model ANOVAs with strain and treatment as between-subject factors. Day of experiment was used as a covariate to account for day-to-day variations in experimental conditions [56]. Outliers were eliminated using the Grubbs test (GraphPad Software; http://www.graphpad.com/quickcalcs/Grubbs1.cfm). When appropriate, Tukey’s post-hoc analyses were used to determine between group differences.
3 Results
3.1 Maximum Velocity (Vmax) of DA Uptake by DAT
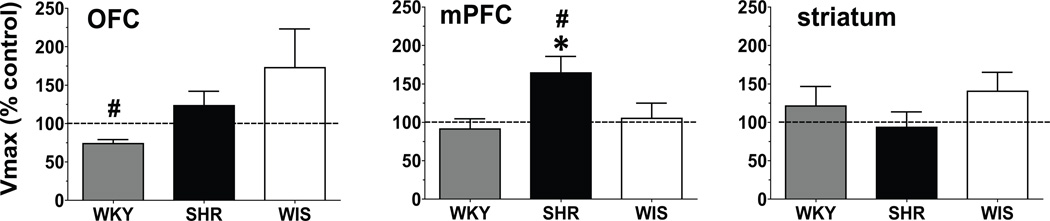
No between-group differences and no interaction of methylphenidate × strain were found for the number of days between the cessation of treatment and the DA uptake assays (interaction terms: OFC F[2, 38] = 0.51; mPFC (F[2, 45] = 0.08; striatum F[2, 30] = 0.10; ps > 0.05). In the vehicle-control groups, no strain differences in Vmax of [3H]DA uptake for either OFC (F[2, 17] = 0.34, p > 0.05), mPFC (F[2, 24] = 0.70, p > 0.05) or striatum (F[2, 16] = 0.30, p > 0.05; Table 1) were found. Figure 1 shows the strain comparisons after methylphenidate treatment. Methylphenidate treatment during adolescence increased (164 ± 21.6% of control) Vmax in the mPFC of adult SHR compared to SHR vehicle-control (t[6] = 2.98, p < 0.05; Figure 1). Also, Vmax in mPFC was greater in methylphenidate-treated SHR compared to methylphenidate-treated WKY and WIS (F[2, 18] = 4.36, p < 0.05; Figure 1b). Vmax was decreased (74.0 ± 5.12% of control) in OFC of the methylphenidate-treated WKY group compared with the WKY vehicle-control (t[7] = 5.09, p < 0.005), but was not different from the methylphenidate-treated WIS and SHR (F[2, 20] = 2.55, p > 0.05; Figure 1). Methylphenidate treatment during adolescence did not alter DAT function in adult striatum (F[2,14] = 0.92, p > 0.05; Figure 1).
Table 1.
Maximal velocity of [3H]DA uptake by DAT in OFC, mPFC and striatum in vehicle-treated adult SHR, WKY and WIS rats
| Brain regionsa | WKY | SHR | WIS |
|---|---|---|---|
| OFC | 4.3 ± 0.8 | 3.0 ± 0.8 | 2.8 ± 0.7 |
| mPFC | 4.0 ± 0.5 | 3.3 ± 0.6 | 3.0 ± 0.6 |
| striatum | 19 ± 2.5 | 17 ± 2.5 | 21 ± 3.4 |
Values are mean ± S.E.M. in pmol/mg/min. n = 6/group for striatal samples, and n = 8–10/group for OFC and mPFC samples
Fig. 1.
Vmax for DAT in OFC, mPFC and striatum from methylphenidate-treated WKY (grey bars), SHR (black bars) and WIS (open bars) rats expressed as pmol/mg/min as a percentage of vehicle control (dotted line). Values are mean ± S.E.M. # Different from the vehicle control, p < 0.05. * Different from WKY and WIS, p < 0.05. n = 8–10/group for OFC and mPFC; n = 6/group for striatum.
3.2 Affinity (Km) for DA at DAT
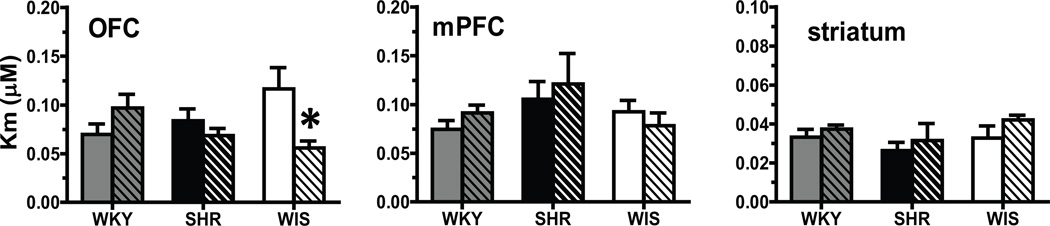
In mPFC and striatum, no methylphenidate × strain interaction for the Km for [3H]DA at DAT was found (mPFC, F[2, 45] = 1.08; striatum, F[2, 29] = 0.20; ps > 0.05; Figure 2). In OFC, a methylphenidate × strain interaction (F[2, 38] = 6.14, p < 0.005) was found, but main effects of strain (F[2, 38] = 0.18, p > 0.05) and treatment (F[1, 38] = 2.33, p > 0.05) were not obtained (Figure 2). Post-hoc evaluation of the interaction term revealed that methylphenidate treatment during adolescence decreased the Km value by 50% in WIS compared to the WIS vehicle-control group (p < 0.01).
Fig. 2.
Km for DAT in OFC, mPFC and striatum from methylphenidate-treated (striped) and vehicle-treated (plain) WKY (grey bars), SHR (black bars) and WIS (open bars) rats. Values are expressed in µM as mean ± S.E.M. * Different from vehicle, p < 0.05. n = 8–10/group for OFC and mPFC; n = 6/group for striatum.
3.3 DAT cell surface distribution
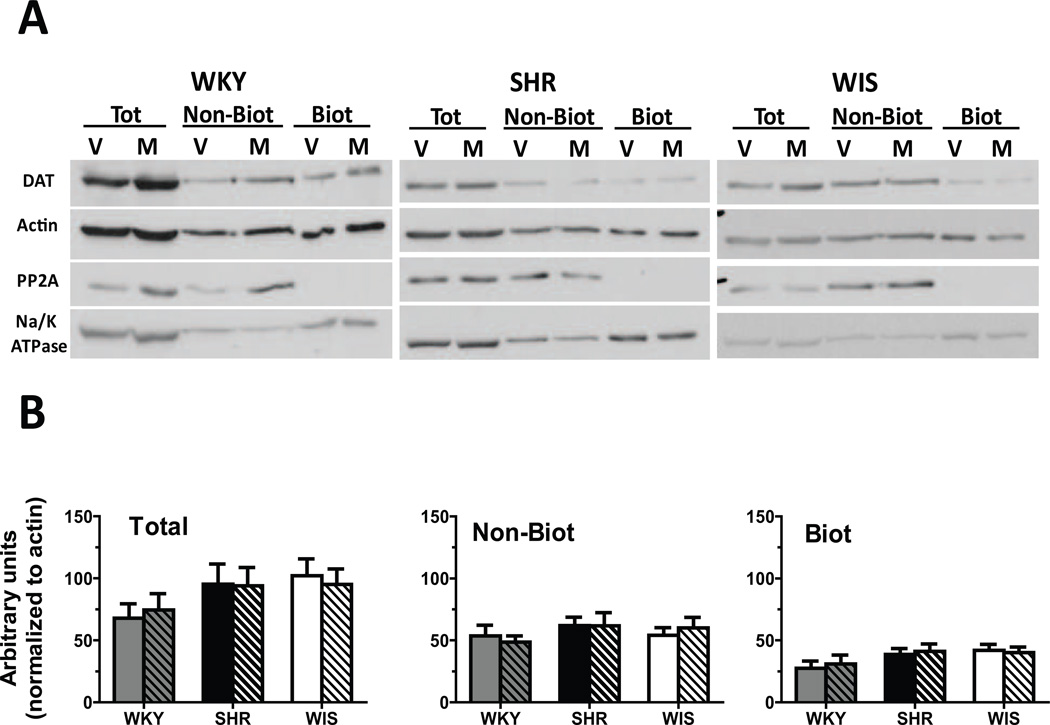
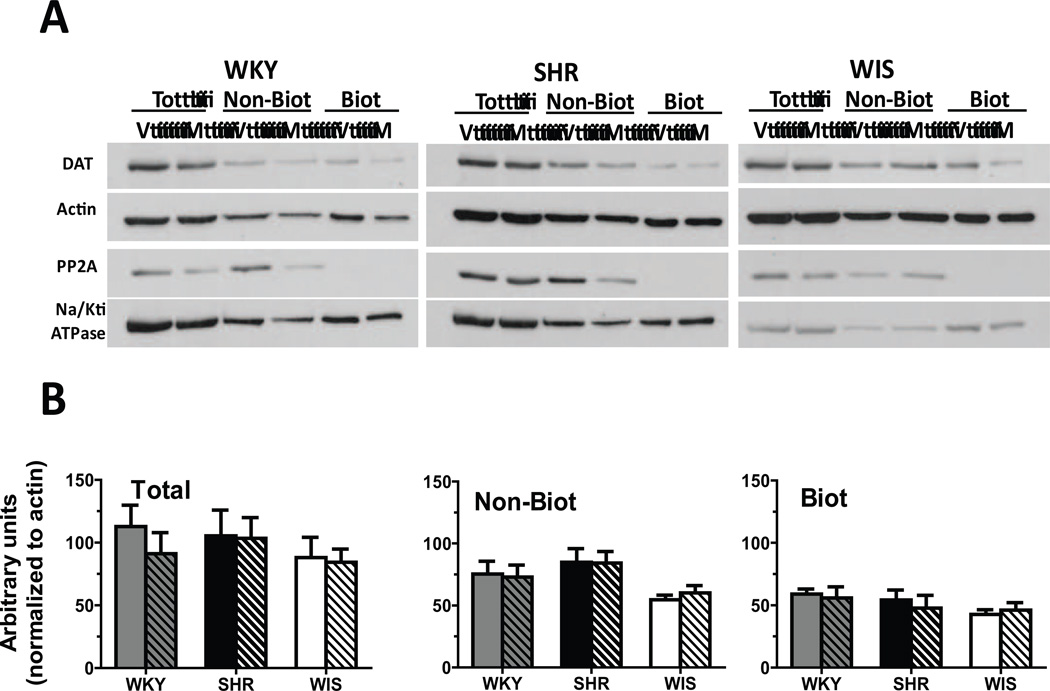
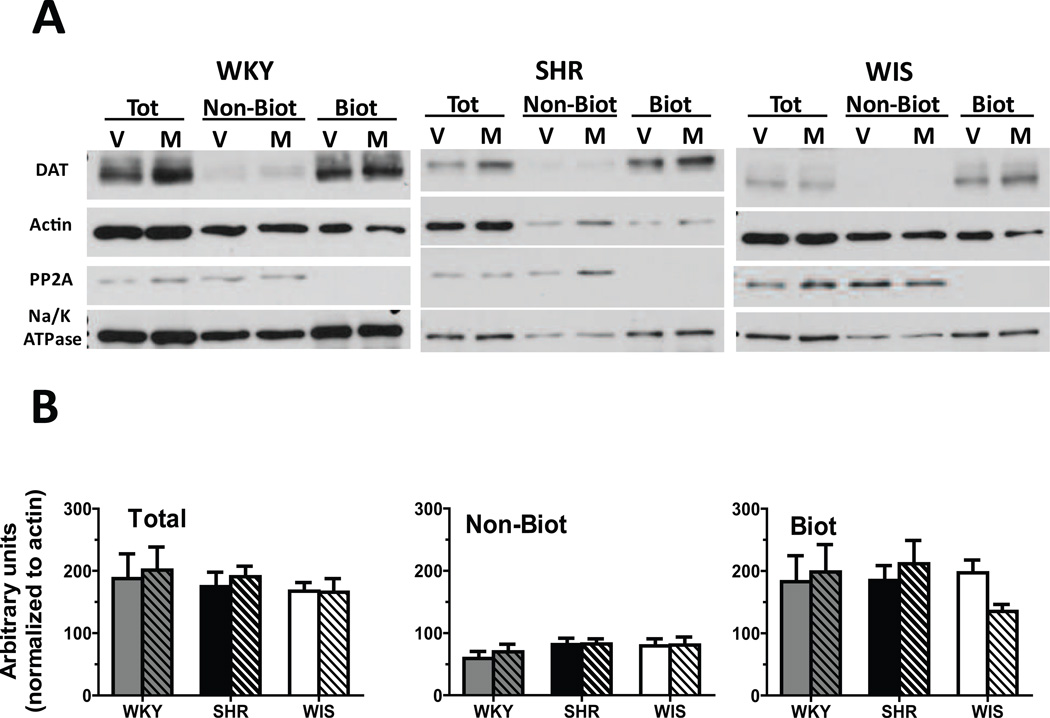
No between-group differences and no methylphenidate × strain interaction was found for the number of days between the cessation of treatment and the DAT cell surface distribution assays (F[2, 36] = 0.0, p > 0.05). DAT expression and distribution did not differ between treatment groups or between strains for either the total, non-biotinylated or biotinylated fractions in OFC (Figure 3), mPFC (Figure 4) or striatum (Figure 5). Results from the statistical analyses of the strain × treatment interaction, and the main effects of treatment and strain are provided in Table 2.
Fig. 3.
(A) Representative blots for cellular distribution of DAT in OFC synaptosomes from methylphenidate-treated (M) and vehicle-treated (V) WKY, SHR and WIS rats. Actin was used to monitor uniform protein-loading while Na+/K+ ATPase and PP2A served to ascertain the efficiency of biotinylation of surface proteins. (B) Distribution of DAT between total, non-biotinylated (Non-Biot; intracellular) and biotinylated (Biot; cell surface) fractions of OFC synaptosomes from methylphenidate-treated (striped) and vehicle-treated (plain) WKY (grey bars), SHR (black bars) and WIS (open bars) rats. Values are mean ± S.E.M in arbitrary units. n = 6–7/group.
Fig. 4.
(A) Representative blots for cellular distribution of DAT in mPFC synaptosomes from methylphenidate-treated (M) and vehicle-treated (V) WKY, SHR and WIS rats. Actin was used to monitor uniform protein-loading while Na+/K+ ATPase and PP2A served to ascertain the efficiency of biotinylation of surface proteins. (B) Distribution of DAT between total, non-biotinylated (Non-Biot; intracellular) and biotinylated (Biot; cell surface) fractions of mPFC synaptosomes from methylphenidate-treated (striped) and vehicle-treated (plain) WKY (grey bars), SHR (black bars) and WIS (open bars) rats. Values are mean ± S.E.M in arbitrary units. n = 6–7/group.
Fig. 5.
Representative blots for cellular distribution of DAT in striatal synaptosomes from methylphenidate-treated (M) and vehicle-treated (V) WKY, SHR and WIS rats. Actin was used to monitor uniform protein-loading while Na+/K+ ATPase and PP2A served to ascertain the efficiency of biotinylation of surface proteins. (B) Distribution of DAT between total, non-biotinylated (Non-Biot; intracellular) and biotinylated (Biot; cell surface) fractions of striatal synaptosomes from methylphenidate-treated (striped) and vehicle-treated (plain) WKY (grey bars), SHR (black bars) and WIS (open bars) rats. Values are mean ± S.E.M in arbitrary units. n = 5–6/group.
Table 2.
Statistics comparing DAT levels in total, non-biotinylated (Non-biot) and biotinylated (Biot) fractions of OFC, mPFC and striatum from methylphenidate-and vehicle-treated adult SHR, WKY and WIS rats
| F-statistics | Treatment main effect (Ft) |
Strain main effect (Fs) |
Treatment × Strain interaction (Fi) |
|
|---|---|---|---|---|
| OFC | Total | Ft[2, 17] = 0.65 | Fs[2, 17] = 0.15 | Fi[2, 17] = 0.13 |
| Non-biot | Ft[2, 17] = 0.00 | Fs[2, 17] = 0.54 | Fi[2, 17] = 1.13 | |
| Biot | Ft[2, 16] = 0.06 | Fs[2, 16] = 2.12 | Fi[2, 16] = 0.97 | |
| mPFC | Total | Ft[2, 18] = 1.74 | Fs[2, 18] = 0.46 | Fi[2, 18] = 0.82 |
| Non-biot | Ft[2, 18] = 0.06 | Fs[2, 18] = 3.11 | Fi[2, 18] = 0.62 | |
| Biot | Ft[2, 18] = 0.04 | Fs[2, 18] = 1.36 | Fi[2, 17] = 0.37 | |
| striatum | Total | Ft[2, 14] = 0.75 | Fs[2, 14] = 1.03 | Fi[2,14] = 0.66 |
| Non-biot | Ft[2, 14] = 2.50 | Fs[2, 14] = 0.75 | Fi[2,14] = 1.22 | |
| Biot | Ft[2, 13] = 0.09 | Fs[2, 13] = 0.41 | Fi[2,13] = 1.64 | |
The main effect of treatment (Ft), strain (Fs) and treatment × strain interaction (Fi) have been reported individually; ps > 0.05.
4 Discussion
In the current study, effects of methylphenidate treatment during adolescence on DAT function and expression in adulthood were determined in the SHR model of ADHD. WKY and WIS rats served as inbred and outbred control groups, respectively. Surprisingly, treatment of the WIS and WKY during adolescence with a therapeutically relevant dose of methylphenidate, and then cessation of methylphenidate treatment in adulthood, resulted in lasting changes in DAT function in OFC. Specifically, in OFC from WIS, an increase in affinity of DAT for DA was found, with no alteration in maximal uptake of DA. In contrast, in OFC from WKY, the methylphenidate treatment paradigm resulted in no change in affinity for DA, and a decreased maximal DA uptake, which was trafficking independent. These results suggest that a misdiagnosis of ADHD, and subsequent treatment with methylphenidate during adolescence, could result in lasting alterations in OFC DAT function. Thus, with respect to the current data, the outbred control (Wistar) has greater value than the inbred control (WKY) for comparisons to SHR, due to the greater stability of DAT function despite methylphenidate treatment in the outbred control. Moreover, in the SHR model of ADHD, the methylphenidate treatment regimen produced persistent increases in mPFC DAT function that were trafficking independent. Thus, the increase in mPFC DAT function was a long term consequence of methylphenidate treatment during adolescence, was specific to the SHR strain, and may be responsible for the treatment-induced alterations in behavior. Furthermore, the current results emphasize the importance of the ADHD animal model, and the appropriate control strains, for investigations of neurochemical mechanisms underlying long-term effects of ADHD pharmacotherapeutics.
The current study found that DAT function and cellular distribution in mPFC, OFC and striatum did not differ between SHR, WKY and WIS vehicle-control groups. With regard to striatum, the current results support previous findings showing no difference in total DAT expression and function between SHR and WKY [44, 64–66]. The current results further extend these previous findings by showing that cell surface DAT distribution similarly is not different between these inbred strains. In contrast, others have reported greater DAT function and greater total DAT expression in SHR compared to WKY [11, 12]. Inconsistencies in the results may be explained in that greater DAT function in adult SHR was observed following exposure of striatal synaptosomes to a single concentration (0.022 µM) of [3H]DA [12]; whereas, the current results were obtained from a comprehensive analysis of DAT kinetic parameters (Km and Vmax) using a wide [3H]DA concentration range (0.01–1.0 µM). No differences in the kinetic parameters for DAT were found between SHR, WKY and WIS. Furthermore, inconsistencies in the levels of striatal DAT expression may be explained by the use of [3H]GBR 12935 to assess expression, since this radioliand binds to both intracellular and cell surface DAT protein [11]. However, an explanation for the discrepancy in the analysis of striatal DAT protein by Western blot is not apparent currently. Close inspection of the clinical literature also reveals inconsistencies regarding striatal DAT levels in ADHD. While greater striatal DAT expression has been observed [5], others report that DAT levels are lower in left caudate in ADHD individuals, and not different from control in other subregions of striatum [67]. A recent meta analysis reported that striatal DAT levels in ADHD individuals may depend on previous stimulant treatment, with lower DAT density in medication naïve and higher DAT density in previously medicated ADHD individuals [68]. Thus, ADHD is a complicated condition that cannot be confined to an explanation regarding alterations in DAT function and/or expression in striatum.
Compared to WKY and WIS controls, SHR treated with methylphenidate during adolescence exhibited improvements in neurocognitive function in adolescence, as demonstrated by performance on maze- and operant-based visual discrimination learning tasks, and increased cocaine self-administration in adulthood [44]. Specifically, the increase in cocaine self-administration was characterized as a more rapid acquisition of cocaine self-administration, greater responding across a range of cocaine doses, and higher progressive ratio breakpoints. Further, methylphenidate dose and treatment during adolescence resulted in a decrease in DAT function in whole prefrontal cortex in SHR and an increase in DAT function in this brain region in WKY and WIS, compared to the respective vehicle controls [44]. In contrast, the identical methylphenidate treatment regimen in the current study resulted in an increased DAT function in SHR mPFC, whereas no changes were observed in OFC, compared to vehicle control. These subregions of prefrontal cortex have distinct cytoarchitecture, connectivity and differentially contribute to behavioral processes including decision making and impulsivity [45, 69]. As such, the apparent discrepancy with respect to DAT function between the previous and current results may be due to methylphenidate effects in specific cortical subregions. Evidence that mPFC and OFC are critically involved in cocaine self-administration comes from studies in which local inactivation with lidocaine decreased cocaine-seeking and taking behavior [40–43]. Following methylphenidate treatment during adolescence, the increase in mPFC DAT function would be expected to decrease extracellular DA concentration in mPFC. Consistent with these findings, mPFC DA depletion resulted in an increased motivation for and increased sensitivity to cocaine [70, 71]. Thus, mPFC may be at the intersection of methylphenidate effects on DAT function and the increased cocaine self-administration observed following adolescent methylphenidate treatment.
A limitation of the current study was that a single dose of methylphenidate was tested. Nevertheless, this dose of methylphenidate is therapeutically-relevant [47, 48] and has been shown to ameliorate behavioral deficits in the adult and adolescent SHR in working memory and behavioral flexibility tasks [8, 81]. The increased DAT function in SHR mPFC in the current study did not correspond with a concomitant increase in cell surface localization of DAT. Thus, the methylphenidate-induced long-term changes in DAT function in mPFC were not dependent on trafficking of DAT to the plasma membrane. Although the biotinylation method of evaluating protein trafficking differentiates intracellular and cell surface proteins [72, 73], this method does not differentiate between cholesterol-rich lipid raft and cholesterol-deficient non-raft regions of the cell surface membrane [74, 75]. DAT expressed in the lipid raft membrane compartment is more sensitive to protein kinase C mediated phosphorylation [75]. Increased phosphorylation of DAT results in reduced DA uptake, and conversely, decreased DAT phosphorylation results in increased DAT function [76–78]. Furthermore, phosphorylation at serine7 in DAT transitions the protein into a low affinity state [79]. Taken together, the methylphenidate-induced increase in DAT function in mPFC in SHR rats observed in the current study may be explained by localized DAT expression in the cholesterol-deficient non-raft regions of the cell surface membrane in mPFC, reducing DAT phosphorylation and increasing function.
5 Conclusions
In conclusion, the present study demonstrates that methylphenidate treatment during adolescence increases DAT function during adulthood in the mPFC of SHR, the ADHD model, and not in the control strains. The increased DAT function in mPFC may underlie the increased cocaine self-administration observed in methylphenidate-treated SHR.
Acknowledgements
We thank Agripina Deaciuc for technical assistance and Dr. Richard Charnigo for assisting with statistical analyses. This work was supported by a National Institute of Health grant R01 DA011716 and a Kentucky Opportunity Fellowship (S.S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Levy F. Dopamine vs noradrenaline: Inverted-U effects and ADHD theories. Aust N Z J Psychiatry. 2009;43:101–108. doi: 10.1080/00048670802607238. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiat. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 7.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, et al. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- 9.Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Brain Res. 1995;676:343–351. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- 10.Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav. 2008;90:184–197. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, et al. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfo P, Machado NJ, Kofalvi A, Takahashi RN, Cunha RA. Caffeine regulates frontocorticostriatal dopamine transporter density and improves attention cognitive deficits in an animal model of attention deficit hyperactivity disorder. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.04.011. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 14.Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: Most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984;104:277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- 15.Andersen PH. The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 16.Engert V, Pruessner JC. Dopaminergic and noradrenergic contributions to functionality in ADHD: The role of methylphenidate. Curr Neuropharmacol. 2008;6:322–328. doi: 10.2174/157015908787386069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiat. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. Am J Addict. 1998;7:156–163. [PubMed] [Google Scholar]
- 19.Levin FR, Evans SM, Kleber HD. Practical guidelines for the treatment of substance abusers with adult attention-deficit hyperactivity disorder. Psychiatr Serv. 1999;50:1001–1003. doi: 10.1176/ps.50.8.1001. [DOI] [PubMed] [Google Scholar]
- 20.Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19–23. [PubMed] [Google Scholar]
- 21.Winters KC, Lee S, Botzet A, Fahnhorst T, Realmuto GM, August GJ. A Prospective examination of the association of stimulant medication history and drug use outcomes among community samples of ADHD youths. J Child Adolesc Subst Abuse. 2011;20:314–329. doi: 10.1080/1067828X.2011.598834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A metaanalytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 23.Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attentiondeficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- 25.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 26.Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochemical and Biophysical Research Communications. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- 27.Mandt BH, Zahniser NR. Low and high cocaine locomotor responding male Sprague-Dawley rats differ in rapid cocaine-induced regulation of striatal dopamine transporter function. Neuropharmacology. 2010;58:605–612. doi: 10.1016/j.neuropharm.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113:15057–15066. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism, Clinical and Experimental Research. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groman SM, James AS, Jentsch JD. Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess GC, Depue BE, Ruzic L, Willcutt EG, Du YP, Banich MT. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol Psychiat. 2010;67:632–640. doi: 10.1016/j.biopsych.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner J, Maia TV, Fair D, Peterson BS, Sonuga-Barke EJ, Nagel BJ. The attenuation of dysfunctional emotional processing with stimulant medication: An fMRI study of adolescents with ADHD. Psychiatry Research. 2011;193:151–160. doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a refrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ, Review Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. The American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 39.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiat. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grakalic I, Panlilio LV, Quiroz C, Schindler CW. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience. 2010;165:313–324. doi: 10.1016/j.neuroscience.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. The European Journal of Neuroscience. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 42.Kantak KM, Yager LM, Brisotti MF. Impact of medial orbital cortex and medial subthalamic nucleus inactivation, individually and together, on the maintenance of cocaine self-administration behavior in rats. Behavioural Brain Research. 2013;238:1–9. doi: 10.1016/j.bbr.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaineseeking behavior. Pharmacology, Biochemistry, and Behavior. 2010;96:347–353. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–847. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert SJ, Burgess PW. Executive function. Curr Biol. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 47.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 51.Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharmacol. 2011;19:275–284. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 54.Salvatore MF, Apparsundaram S, Gerhardt GA. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging. 2003;24:1147–1154. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 55.Vadum AC, Rankin NO. Psychological research : methods for discovery and validation. Boston, Mass: McGraw-Hill; 1998. [Google Scholar]
- 56.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer series in statistics. New York: Springer; 2000. p. 1. online resource (xxii, 568 p.) [Google Scholar]
- 57.Sagvolden T, Johansen EB, Woien G, Walaas SI, Storm-Mathisen J, Bergersen LH, et al. The spontaneously hypertensive rat model of ADHD--the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD) J Neurosci Methods. 2007;162:42–48. doi: 10.1016/j.jneumeth.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar-Kyoto (WKY) and Wistar rats. Life Sci. 2008;83:74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sci. 2006;79:772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 62.Jiao X, Pare WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 63.De La Garza R, 2nd, Mahoney JJ., 3rd A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 64.Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, et al. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50:848–857. doi: 10.1016/j.neuint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Miller EM, Pomerleau F, Huettl P, Russell VA, Gerhardt GA, Glaser PE. The spontaneously hypertensive and Wistar Kyoto rat models of ADHD exhibit subregional differences in dopamine release and uptake in the striatum and nucleus accumbens. Neuropharmacology. 2012;63:1327–1334. doi: 10.1016/j.neuropharm.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Womersley JS, Hsieh JH, Kellaway LA, Gerhardt GA, Russell VA. Maternal separation affects dopamine transporter function in the spontaneously hypertensive rat: an in vivo electrochemical study. Behavioral and Brain Functions. 2011;7:49. doi: 10.1186/1744-9081-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. The American Journal of Psychiatry. 2012;169:264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- 69.Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Research Reviews. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Research. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- 71.McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacology, Biochemistry, and Behavior. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 72.Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase Cinduced internalization of the dopamine transporter is mediated by a clathrindependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 74.Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, et al. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- 75.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. Journal of Neurochemistry. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]
- 77.Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochemical and Biophysical Research Communications. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. The Journal of Pharmacology and Experimental Therapeutics. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- 79.Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, et al. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. The Journal of Biological Chemistry. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.American Academy of Pediatrics Committee on Children With Disabilities and Committee on Drugs. Medication for children with attentional disorders. Pediatrics. 1996;98(2 Pt 1):301–304. [PubMed] [Google Scholar]
- 81.Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: Methylphenidate vs. atomoxetine treatments. Behavior and Brain Research. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]