Summary
Proliferation and differentiation are tightly coordinated to produce an appropriate number of differentiated cells, and often exhibit an antagonistic relationship. Developing T cells, that arise in the thymus from a minute number of bone marrow-derived progenitors, undergo a major expansion upon pre-TCR expression. The burst of proliferation coincides with differentiation towards the αβ T cell lineage – but the two processes were previously thought to be independent from one another, although both driven by signaling from pre-TCR and Notch receptors. Here we report that proliferation at this step was not only absolutely required for differentiation, but also that its ectopic activation was sufficient to substantially rescue differentiation in the absence of Notch signaling. Consistently, pharmacological inhibition of cell cycle machinery also blocked differentiation in vivo. Thus proliferation step is strictly required prior to differentiation of immature thymocytes.
Introduction
Cell numbers in multicellular organisms have to be precisely controlled – thus proliferation, differentiation and cell death are tightly coordinated both during ontogenesis and in the course of tissue self-renewal. Dysregulation of these processes can lead to developmental abnormalities or cancer. Adult tissues that undergo constant self-renewal, such as blood and epithelia, often contain slowly dividing stem cells that give rise to uncommitted, in many cases frequently dividing, progenitors that have lost their self-renewal capacity (in some tissues termed transient amplifying cells), which in turn give rise to non-proliferating terminally differentiated cells. Such hierarchical organization – with the stem cell undergoing a limited number of divisions and more mature progenitors being responsible for the production of the bulk of the tissue – is thought to be required to protect stem cell DNA from mutations that accumulate in the course of replication. Extinction of proliferation in the course of differentiation, on the other hand, allows control over the number of terminally differentiated cells.
The latter conjecture is often interpreted as an inherently antagonistic relationship between proliferation and differentiation. This antagonism however, goes beyond mere correlation, as inhibition of the cell cycle machinery in many cases can directly trigger differentiation, whereas its ectopic activation can suppress it (Zhu and Skoultchi, 2001). For example, forced expression of cyclin D1 (Ccnd1) impedes myocyte differentiation, whereas ectopic expression of cyclin-dependent kinase (Cdk) inhibitors promotes expression of some differentiation markers (Skapek et al., 1995) (Manceau et al., 2008). Similarly, repression of Ccnd1 expression by Jumnaji was shown to be required for cardiomyocyte differentiation, while ectopic expression of Ccnd1 interfered with this process (Nakajima et al., 2011). In many such cases, cell cycle proteins were suggested to interfere directly with the activity of factors involved in differentiation.
In contrast, in some cases proliferation seems to be required for developmental progression. For instance, during differentiation of helper T cells, acquisition of the ability to secrete IFNγ, IL-4 and IL-10, but not IL-2 was shown to be dependent on proliferation by some studies (Bird et al., 1998; Richter et al., 1999) but not others (Ben-Sasson et al., 2001; Laouar and Crispe, 2000). Such dependence of differentiation on proliferation was suggested to reflect cell cycle-coupled epigenetic changes required for developmental progression (Bird et al., 1998; Mullen et al., 2001; Murphy and Reiner, 2002). Finally, the two processes can occur in parallel but independently from each other (Brown et al., 2003). Thus the relationship between differentiation and proliferation strongly depends on the cellular context – and needs to be examined in each particular case.
In the case of T cell development, a small number of progenitors, that originate from hematopoietic stem cells, undergo a dramatic expansion in the thymus. Early T lineage progenitors (ETP) and more mature double negative (DN) 2 cells are proliferating. DN2 cells then progress to the DN3a stage. Though these cells are often thought to be quiescent in terms of proliferation, it has been suggested that they may also be slowly cycling (Petrie et al., 2000). At this stage, Tcrb, Tcrg, and Tcrd rearrangement takes place. Expression of the pre-TCR upon productive rearrangement of the Tcrb gene results in a burst of proliferation and in progression to the CD4+CD8+ double positive (DP) stage – a hallmark of αβ T cell lineage commitment. This process is called β-selection. The majority of DP cells are non-dividing, as are CD4 and CD8 single positive thymocytes. Thus one can view all thymocyte populations prior to the DP stage as transient amplifying cells, with the largest amplification triggered by β-selection.
Signals from a number of receptors, including the pre-TCR, the IL-7R, Notch (Ciofani et al., 2004; Maillard et al., 2006) and CXCR4 regulate proliferation and developmental progression upon β-selection. A recent study utilizing a reductionist feeder-free cell culture system identified pre-TCR and Notch signaling as the only two pathways strictly required for both proliferation and differentiation – with others playing modulatory roles (Tussiwand et al., 2011).
The burst of proliferation that takes place upon β-selection serves to amplify cells with productive Tcrb rearrangements, and thus maximizes TCR diversity – as the same TCRβ can later pair with different TCRα chains. It was previously reported that proliferation per se was not required for developmental progression, as Myc-deficient DN3 thymocytes were able to generate DP cells in the apparent absence of cell division (Dose et al., 2006). Moreover, DP cells were found in the thymi of Cyclin D3 (Ccnd3) deficient mice, where thymocyte proliferation is severely impaired (Sicinska et al., 2003). These results suggested that proliferation and differentiation after β-selection coincide, but are independent of each other. However, careful analysis of thymocytes from Ccnd3 and Myc deficient mice reported here revealed that, like for WT thymocytes, only those cells that divide several times gave rise to DP cells. Moreover, reversible inhibition of proliferation by several compounds with biochemically distinct modes of action, which block cell cycle at different stages, also resulted in a complete block of DP cell progression. Treatment of mice with a Cdk4 and Cdk6 inhibitor led to a complete block in the DN3 to DP transition, suggesting that the same rules apply in vivo. Ectopic activation of the cell cycle machinery, although unable to compensate for the lack of pre-TCR signaling, substantially rescued differentiation of precursors in the absence of Notch ligands. Rescue of proliferation and differentiation in the absence of Notch signaling was also achieved by ectopic expression of a single Notch target – the transcription factor Myc – a known positive regulator of proliferation and metabolism. Another transcription factor - E47 (encoded by Tcf3) - was required to make developmental progression dependent on proliferation as Tcf3−/− cells were able to progress to the DP stage without cell division. Thus, at the β-selection checkpoint, proliferation was required for differentiation of αβ T cells, and its ectopic activation was sufficient to rescue differentiation in the absence of one of the two key molecular pathways required at this stage – Notch signaling.
Results
A tight link between proliferation and differentiation at the DN3 to DP transition
We noticed that when CFSE-labeled pre-β-selection DN3a thymocytes were plated on OP9-DL1 monolayers, only cells that had divided four times or more progressed to the DP stage efficiently (Figure 1A). Both proliferation and differentiation at this stage of T cell development are triggered by productive rearrangement of Tcrb that results in the expression of the pre-TCR. The simplest explanation for this correlation was that the time required for a β-selected cell to progress to the DP stage was sufficient for this number of cell divisions. Indeed, several studies suggest that progression to the DP stage can take place in the absence of proliferation. When Myc was deleted in DN3 cells, CD4 and CD8 were upregulated on thymocytes in OP9-DL1 co-cultures in the absence of proliferation in culture (Dose et al., 2006). However, total DN3 cells that include both pre- and post-β-selection cells were used in this study. When we revisited this question using pre-β-selection DN3a cells from these mice, we noticed that only a minor fraction of the cells progressed to the DP stage, and that this progression was always associated with cell division (Figure 1B). In contrast, DN3b cells, some of which underwent proliferation in vivo, were able to progress to the DP stage without further cell division in culture (Figure 1B). Ex vivo DN3b cells had more of the non-deleted Myc allele than did their DN3a precursors (Figure S1) – suggesting that cells which did not delete Myc had a better chance to progress further in development. These DN3b cells, however, seemed to delete Myc quickly thereafter as very low levels of the non-deleted allele could be detected in their immediate progeny - the transitional CD25int DN3b-DN4 population (Figure S1). It is unclear whether all the cells that progressed beyond the DN3a stage deleted Myc late (or had a higher starting level of the Myc protein for other reasons) or whether some differentiation could take place in the true absence of Myc. In either case, it is safe to conclude that, similar as in WT cells, differentiation of Lck-Cre Mycfl/fl DN3a precursors is associated with cell division.
Figure 1. αβ T cell differentiation is associated with proliferation in WT and genetically manipulated mice.
A. WT DN3a cells were sorted, labeled with CFSE and placed in OP9-DL1 co-culture. CFSE dilution, CD4 and CD8 expression was analyzed 4 days later. CFSE dilution vs CD4 and CD8 expression (summed electronically) (left) and expression of CD4 and CD8 individually on divided and undivided cells (center and right) is shown. B. DN3a and DN3b cells from WT and Mycfl/fl Lck-Cre were treated and analyzed as in A. C. CellTrace Violet-labeled Ccnd3−/− and Ccnd3+/- DN3a cells were placed in OP9-DL1 co-cultures for 4 (top panels) or 8 (bottom panels) days. Dilution of CellTrace Violet, and expression of CD4 and CD8 was assessed. Numbers above CellTrace Violet dilution plots represent the number of divisions. Note the dulling of CellTrace Violet from day 4 to day 8. Representative results of two independent experiments are shown. See also Figure S1.
It was also shown that cyclin D3 (Ccnd3) was required for efficient proliferation at the β-selection checkpoint, even though Ccnd3−/− thymi contained some DP thymocytes (Sicinska et al., 2003). Accordingly, Ccnd3−/− DN3a cells divided slower in culture (Figure 1C). If WT cells were starting to upregulate CD4 and CD8 coreceptors after four divisions simply because differentiation takes the same amount of time as several cell cycles, then slowly dividing cyclin D3-deficient cells should progress to DP stage synchronously with WT cells – but after going through fewer divisions. This was not the case: decelerated proliferation of Ccnd3−/− precursors was associated with delayed progression to the DP stage (Figure 1C). Again, only cells that divided several times activated CD4 and CD8 expression. These observations hinted at the intriguing possibility that proliferation was required for the progression to DP stage.
Progression through the cell cycle is required for αβ T cell differentiation
To test whether proliferation upon β-selection was required for differentiation of αβ lineage cells we aimed to assay the differentiation status of DN3 cells under conditions where cell cycle progression was inhibited. To this end we utilized PD0332991 – a highly specific inhibitor of cyclin-dependent kinases 4 and 6. To increase survival of the cells in the presence of this and other compounds, Vav-Bcl2 transgenic thymocytes were used in the experiments utilizing inhibitors. Similar results but with lower cell yields were obtained with WT cells (Figure S2A and not shown). Addition of PD0332991 to DN3a-OP9-DL1 co-cultures blocked proliferation of the thymocytes (Figure 2A) without any obvious toxic effects on the Bcl2-transgenic precursors or feeder cells. The block of proliferation coincided with a block in DP progression (Figure 2B). When the cells were resorted from these cultures and plated on fresh OP9-DL1 feeders in the absence of inhibitor, they regained the ability to divide – and only the dividing cells progressed to the DP stage (Figure 2A, B).
Figure 2. Proliferation is required for αβ T cell differentiation.
CellTrace violet-labeled Vav-Bcl2 transgenic DN3a cells were cultured on OP9-DL1 monolayers in the presence or absence of 1μM PD0332991 cdk inhibitor for two days; undivided undifferentiated cells were resorted and cultured for additional three days with or without the inhibitor. Expression of CD4 and CD8 (A, B) and CellTrace violet dilution (A) was analyzed at both time points. C, D. CellTrace violet-labeled Vav-Bcl2 transgenic DN3a cells were cultured in the presence or absence of the indicated inhibitors (for concentrations see Supplemental Experimental Procedures). On day 4, CD4 and CD8 upregulation (C) and CellTrace violet dilution (D) were analyzed. See also Figure S2.
Although this result supported the hypothesis that the wave of proliferation upon β-selection was required for αβ lineage differentiation, alternative explanations were possible. It was suggested recently that Cdk6 activity itself may be directly required for αβ T cell differentiation (Hu et al., 2009; Hu et al., 2011). It was also possible that PD0332991 had off-target effects unrelated to cell cycle that led to a block in T cell development. Thus we tested a panel of cell cycle inhibitors that block cells at G1, S or G2-M stages. All inhibitors that blocked proliferation also interfered with developmental progression (Figure 2C, D). At no concentration of any inhibitor could the block in differentiation be uncoupled from the block in differentiation (Figure S2B, results for PD0332991, purvalanol A and for the thymidine block are shown). Importantly, multiple inhibitors blocked cells at the S and G2-M stages of the cell cycle and thus should not interfere with Cdk6 function (shown below for thymidine block), arguing against the possibility that the block in differentiation was a direct consequence of the missing Cdk6 activity. Although all of the inhibitors blocked thymocytes at the expected cell cycle stage, few affected density (aphidicolin) or morphology (L-mimosine, colcemid, nocodazole) of OP9 cells. Further experiments were mostly performed with PD0332991, the inhibitor described above, and the thymidine block - 1mM thymidine treatment that causes feedback inhibition of nucleotide synthesis and blocks cells at the S stage – neither had any adverse effect on OP9DL1 survival or morphology. As expected PD0332991, but not thymidine, interfered with Cdk activity as judged by severely reduced phosphorylation of their key target – Rb – on Ser773 (a cyclin D-Cdk4 and 6 specific site) (Figure S2C). The thymidine block could be rescued both in terms of proliferation and differentiation by supplementing the medium with nucleosides (Figure S2D). To exclude the possibility that the inhibitors kill DP cells, we cultured sorted Vav-Bcl2 Tg DP cells in their presence or absence, and found similar cell numbers (Fig. S2E).
One explanation for the observed developmental block would be the inability of the cells to rearrange their Tcrb in the presence of the inhibitors. To address this we first carefully assessed the influence of the compounds on the frequency of IC TCRβ+ cells. The staining was highly specific as judged by lack thereof on Rag2-deficient cells. As after four days of culture IC TCRβ+ cells in the absence the inhibitors had divided, we calculated their ‘adjusted frequency’ (as if they did not divide) on the basis of CellTrace Violet dilution as described in Figure S2F. This approach allowed a direct comparison of TCRβ expression between cells cultured with or without inhibitors. Several compounds, including PD0332991 used throughout this study, did not have any adverse effect on TCRβ expression, while others caused some decrease in frequency of IC TCRβ+ cells (but in all such cases the frequency was several fold higher than in the post-sort population) (Figure S2F). We then sorted and cultured HY TCRβ-transgenic DN3a cells; their differentiation was likewise blocked by the inhibitors (Figure S2G). We conclude therefore that the developmental block observed in the absence of proliferation cannot be attributed to the inability to rearrange Tcrb.
Blocking of the cell cycle did not merely interfere with CD4 and CD8 coreceptor induction – as upregulation of RORγt, a key regulator of DP thymocyte survival (Sun et al., 2000), was also severely inhibited in the absence of proliferation (Figure 3A). Likewise, CD25, normally downregulated upon β-selection, remained equally expressed on IC TCRβ positive and negative cells when proliferation was inhibited (Figure 3B). Interestingly, however, the Tcra locus, which normally becomes transcriptionally active and is rearranged only at the DP stage, was still rearranged in the presence of some but not all of the inhibitors - as judged by presence of surface TCRβ+ cells some of which stained with a cocktail of Vα antibodies (Figure S3). The fact that some inhibitors allowed for surface TCR expression and others did not may reflect the destabilization of Rag2 in the S-phase, thought to be mediated by direct phosphorylation from Cdks (Lee and Desiderio, 1999; Li et al., 1996). Thus, although proliferation was required for execution of developmental program broader than CD4 and CD8 upregulation, some important events in αβ T cell differentiation – such as Tcra rearrangement – were proliferation-independent. Nevertheless, as no conventional αβ T cell can develop in the mere absence of just the co-receptors, it is fair to conclude that proliferation is required for αβ T cell differentiation.
Figure 3. Proliferation dependence of the αβ T cell program is not limited to CD4 and CD8 coreceptor upregulation and holds true in vivo.
A, B CellTrace violet-labeled Vav-Bcl2 transgenic DN3a cells were cultured on OP9-DL1 monolayers in the presence or absence of 1μM PD0332991 or 1 mM thymidine for 3 days, stained for surface expression of CD25 and intracellular expression of TCRβ and RORγt. RORγt (A) and CD25 (B) expression is plotted against CellTrace violet dilution (top) or is compared in TCRβ+ and TCRβ− cells (bottom). C. WT mice were treated with PD0332991 or vehicle for 5 days as described in Supplemental Experimental Procedures. Expression of CD4 and CD8 on Lin− (Gr1− CD11b−CD11c−CD19−Ter119−NK1.1−) thymocytes (left), surface expression of TCRβ on Lin−CD4+CD8+ cells (center) and CD44 vs CD25 expression on DN Lin−TCRβ− thymocytes (right) are shown. See also Figure S3.
We next sought to find in vivo evidence for a proliferation requirement in αβ T cell differentiation. As all the available genetic tools (such as Cyclin D3 knock-out) block cell cycle progression of β-selected thymocytes only partially, we again took a pharmacological approach and treated wt mice with PD0332991 by gastric gavage. Overall thymic cellularity of treated mice was reduced about 30-fold, but DN3 cells were still present on day 5 after the beginning of treatment. Their progression to the DP stage was, however, completely blocked as judged by a reduction of the DP compartment to less than 1% of the remaining thymocytes and, most strikingly, a complete absence of newly generated TCRβ−/low DP cells (Figure 3C). Although this in vivo experiment by itself could not discriminate between cell-autonomous and non-cell-autonomous effects of the compound, together with the bulk of cell culture data presented above it provided support for the notion that proliferation was indispensible for DP progression both in vitro and in vivo.
The ability to differentiate is acquired gradually over several cell divisions
As developing thymocytes showed a strong correlation between the number of cell divisions and their differentiation status – with only those that divided four times or more, progressing to the DP stage efficiently (Figure 1A) – we next tested how many cell divisions were in fact required. To this end we plated CellTrace violet labeled DN3a thymocytes on OP9-DL1 monolayers, cultured them for three days, resorted cells that went through various numbers of cell divisions, and placed them into secondary cultures in the presence or absence of 1mM thymidine. In the absence of the inhibitor, all sorted populations were able to generate DP cells. Importantly, the efficiency of the DP progression was very similar for all populations that divided at least once in primary culture (Figure 4). However, the presence of the inhibitor revealed that the ability to differentiate was acquired gradually over several cell divisions, plateauing at around 4–5 divisions (Figure 4). Thus not only progression through cell cycle was required for αβ T cell differentiation, but several rounds of cell division were necessary to make this process efficient.
Figure 4. Several rounds of cell division are required for efficient progression to the DP stage.
Vav-Bcl2 transgenic DN3a cells were sorted, labeled with CellTrace violet and placed in OP9-DL1 co-culture. On day 3 individual CellTrace violet peaks corresponding to different number of cell divisions were sorted and cells were placed into secondary OP9-DL1 co-culture with or without 1 mM thymidine to block further divisions. Expression of CD4 and CD8 was assessed after two days of secondary culture. Representative FACS plots (A) and frequency of DP cells in the cultures (B) is shown. As the cell cycle block was not always complete, letting some cells undergo one more division, additional gating on cells that did not divide in secondary cultures was applied for cultures in the presence of thymidine. Representative results of three independent experiments are shown.
Ectopic activation of proliferation is insufficient to drive DP progression in the absence of pre-TCR expression
Both pre-TCR and Notch signaling is strictly required for DP progression – and both pathways are mitogenic at this stage. We therefore asked whether ectopic activation of proliferation may rescue development in the absence of one of them. To this end we first utilized retroviral overexpression of various components of cell cycle machinery in Rag2-deficient DN3 cells with a Vav-Bcl2 transgene (to compensate for the pro-survival role of the pre-TCR), generated in OP9-DL1 co-culture from hematopoietic stem cells. Previously, it was shown that ectopic Bcl2 expression driven by Lck (Linette et al., 1994) but not Eμ (Maraskovsky et al., 1997) regulatory elements in Rag−/− mice could cause the appearance of DP cells. Indeed, Vav-Bcl2 tg Rag2−/− mice had 30–40% of DP cells in the thymus (Figure S4A). However, in OP9-DL1 co-cultures, DP progression of Vav-Bcl2 tg Rag2−/− DN3 cells was extremely slow, with small numbers of DP cells detectable as late as day 9, correlating with the very slow proliferation of these cells (Figure S4B, C). This provided us with a time window sufficient for overexpression strategies.
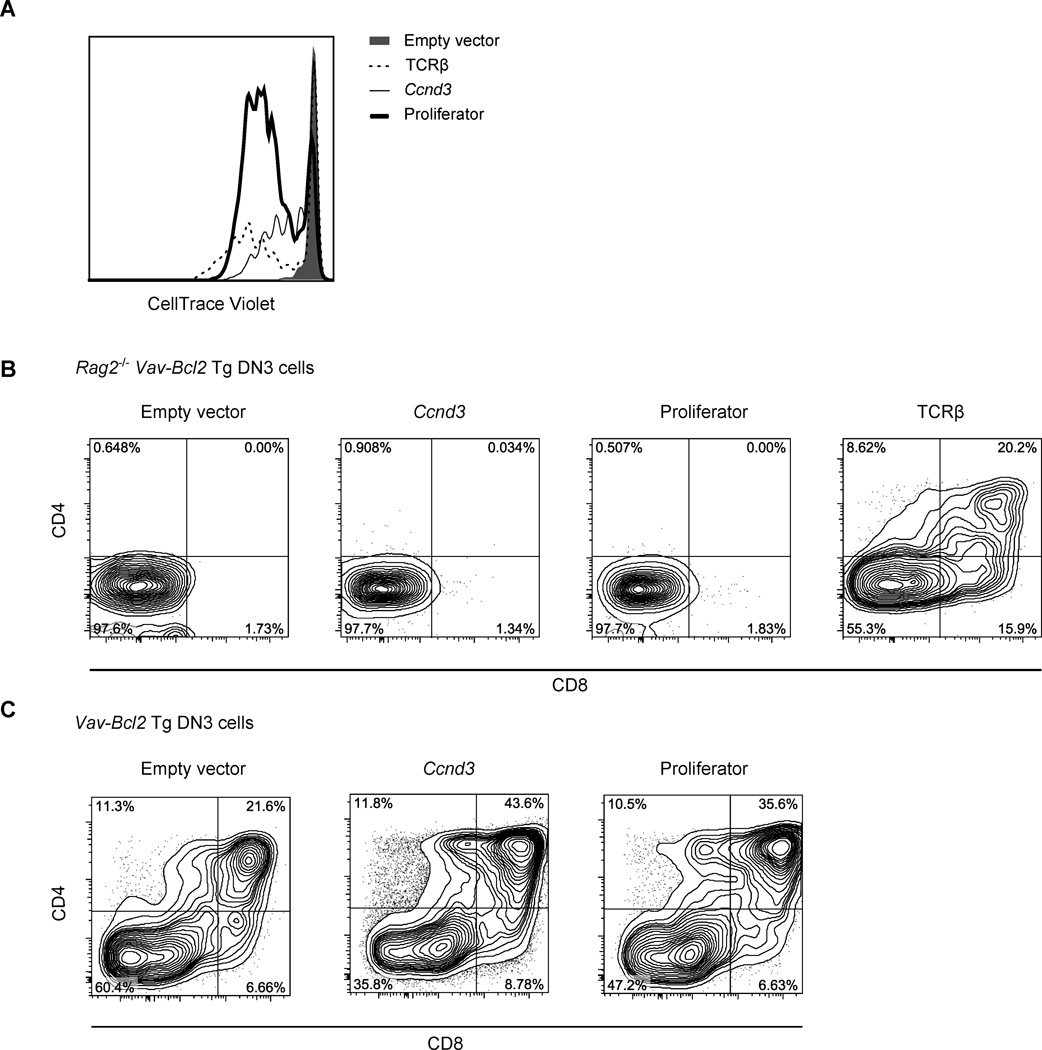
Entry into the cell cycle and transition through the G1 phase are governed by the coordinated action of the D- and E-type cyclins, which activate their catalytic partners, cyclin-dependent kinases Cdk4, Cdk6 and Cdk2. Indeed, abundance of cyclin D3 protein was shown to increase dramatically upon β-selection (Sicinska et al., 2003), and expression of cyclins E1 (Ccne1) and E2 (Ccne2) was likewise induced in DN3b cells (Immgen.org). We observed that forced expression of individual cyclins drove some proliferation of Vav-Bcl2 tg Rag2−/− DN3 cells; however the proliferation rate was lower than that driven by TCRβ expression (Figure 5B, cyclin D3 overexpression is shown). Thus we designed a cassette (termed ‘proliferator’) containing cyclins D3 and E1 together with their partner Cdks (Cdk6 and Cdk2 respectively) all joined by P2A peptides (Figure S5A) that cause ribosomal skipping – thus four polypeptide chains are generated from one mRNA. Expression of this cassette resulted in a burst of proliferation comparable to that induced by TCRβ expression when both delivered retrovirally into Vav-Bcl2 tg Rag2−/− DN3 cells (Figure 5A). However, unlike TCRβ expression, it was insufficient to drive developmental progression of the cells (Figure 5B). Importantly, neither ‘proliferator’, nor cyclin D3 alone interfered with differentiation of Rag2-sufficient cells (Figure 5C). Thus, although proliferation was required for DP progression, its ectopic activation was insufficient to drive differentiation of αβ T cells in the absence of pre-TCR signaling.
Figure 5. Ectopic activation of proliferation is insufficient to compensate for the lack of pre-TCR signaling.
A, B. DN3 cells generated in OP9-DL1 co-cultures from Vav-Bcl2 transgenic Rag2−/− HSCs were infected with retroviruses encoding IRES-GFP, Ccnd3-IRES-GFP, proliferator-IRES-GFP, or TCRβ-IRES-GFP, sorted, labeled with CellTrace Violet and plated again on OP9-DL1 monolayers. Six days later CellTrace violet dilution (A, gated on GFP+ cells) as well as CD4 and CD8 upregulation (B) was assessed. Representative results of four independent experiments are shown. C. Differentiation of Vav-Bcl2 Tg, Rag-sufficient cells treated as in B is shown. See also Figure S4.
Ectopic activation of proliferation rescues differentiation in the absence of Notch signaling
Notch signaling is another pathway crucial for the progression of cells to the DP stage. We thus tested whether expression of the ‘proliferator’ cassette could rescue proliferation and differentiation in the absence of Notch signaling. To this end, we infected DN3a cells generated in OP9-DL1 co-culture from Vav-Bcl2 transgenic hematopoietic stem cells with the ‘proliferator’-encoding retrovirus or empty vector (transgenic expression of Bcl2 was required to compensate for the well-established pro-survival role of Notch signaling at this stage (Ciofani et al., 2004; Kreslavsky et al., 2010)) as well as to circumvent the low tolerance of wt DN3a cells to proliferator expression (Figure S5B). As our previous experiments demonstrated that proliferator was able to drive division of pre-TCR negative cells (Figure 5), gating on intracellular TCRβ positive cells - to focus on the cells that actually had the potential to differentiate - was applied in these experiments. Both empty vector and proliferator infected cells were able to divide and differentiate on OP9-DL1 feeders (Figure 6A). As expected, in the absence of Notch signaling, empty vector-infected cells were almost completely stalled both in terms of proliferation and differentiation. However, expression of proliferator forced cells to divide even in the absence of Notch signaling – although the rescue of proliferation, unlike in the case of pre-TCR signaling, varied between experiments. However, in all cases where cells divided three or more times, proliferation was accompanied by a substantial rescue of differentiation (Figure 6A).
Figure 6. Ectopic activation of proliferation rescues DP progression in the absence of Notch signaling.
A. DN3 cells generated in OP9-DL1 co-cultures from Vav-Bcl2 transgenic HSCs were infected with retroviruses encoding IRES-GFP or proliferator-IRES-GFP, sorted, labeled with CellTrace Violet and plated again on OP9-DL1 or control OP9 monolayers. Six days later cells were harvested, stained for surface CD45, CD4 and CD8 expression, fixed, permeabilized and stained for intracellular TCRβ. CellTrace violet dilution (right) as well as CD4 and CD8 upregulation (left) were analyzed in CD45+IC TCRβ+GFP+ cells. Representative results of five independent experiments in which proliferator-infected cells divided three times or more are shown. B, C. Cells were generated, infected with Myc-IRES-GFP or empty vector, cultured and analyzed as in A. Myc-infected cells were cultured in presence or absence of 1μM PD0332991. CellTrace violet dilution (B, right), CD4 and CD8 upregulation (B, left) were analyzed in CD45+IC TCRβ+GFPhi cells. C shows GFP vs CellTrace violet and GFP vs CD8 plots for Myc-infected CD45+IC TCRβ+ cells. Representative results of three independent experiments. See also Figure S5.
Notch is thought to regulate proliferation of β-selected thymocytes at least in part via direct induction of Myc (Weng et al., 2006). Thus we tested if ectopic Myc expression could drive proliferation in the absence of Notch signaling and whether that in turn might rescue their differentiation. To this end Vav-Bcl2-transgenic DN3a cells were generated from HSC and infected with Myc-IRES-GFP retrovirus or empty vector and placed on OP9-DL1 or control OP9 feeders (WT cells did not tolerate high level of Myc expression (Figure S5C)). GFP+ Myc-infected DN3a cells proliferated and efficiently progressed to the DP stage in the absence of Notch signaling (Figure 6B) and both proliferation and differentiation were restricted to the GFPhi population (Figure 6C). Notch-independent proliferation and differentiation of Myc-IRES-GFP-expressing cells was readily blocked by PD0332991 suggesting that induction of proliferation was an important part of Myc-mediated rescue (Figure 6B). We next tested how the level of Myc that enables DP progression in the absence of Notch ligands relates to the level physiologically induced by Notch signaling. Specificity of the Myc antibody was first confirmed by staining thymocytes infected with a retrovirus encoding mouse Myc-IRES-GFP (Figure S5D). As it was previously reported with a Myc-GFP reporter, Myc was downregulated in DP cells (Figure S5F, left). Strikingly, the level of Myc protein dropped to a virtually undetectable in the cells cultured without Notch signaling, suggesting that Myc expression was strictly Notch-dependent at this stage of T cell development (Figure S5F, center). Ectopic expression of mouse Myc that was able to rescue progression to the DP stage (Figure S5E) restored Myc expression in the absence of Notch signaling to a physiological level, although expectedly DP cells failed to downregulate ectopically expressed Myc (Figure S5F, right) while the presence of Notch signaling resulted in overexpression (not shown). Thus, activation of the cell cycle machinery – directly or via ectopic expression of a single Notch target – Myc – can compensate, at least in part, for the lack of Notch signaling at the β-selection checkpoint.
Transcription factor E47 imposes the dependence of differentiation on proliferation
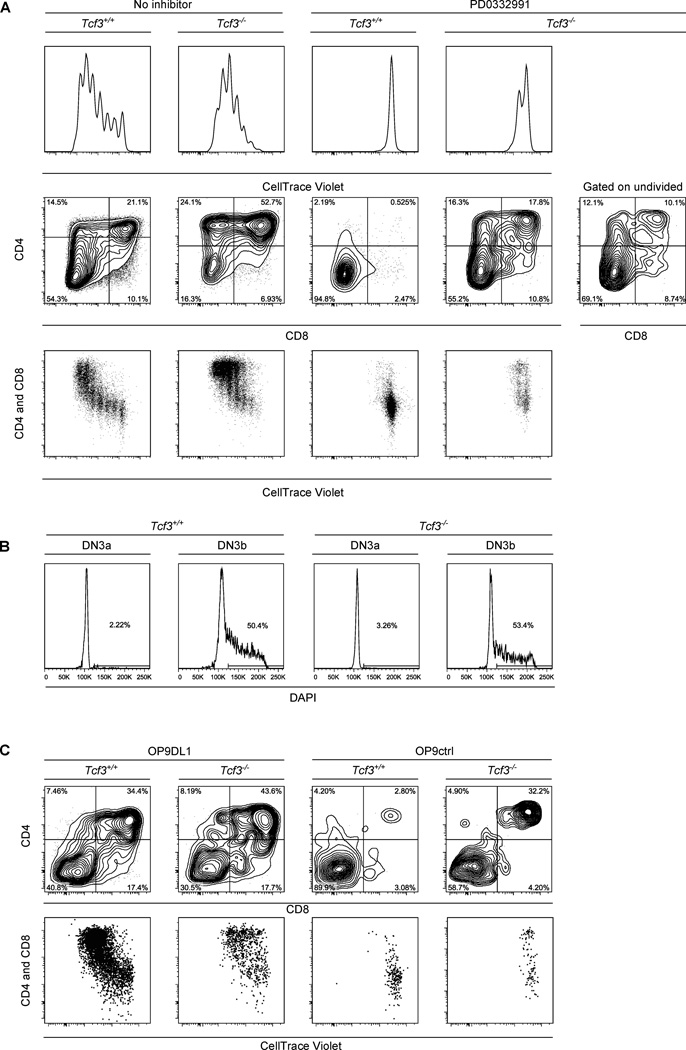
E47, one of the two E-protein family transcription factors encoded by Tcf3 gene, was suggested to play the role of a gatekeeper of differentiation at the β-selection checkpoint, as its activity drops upon β-selection and in its absence thymocytes progress to the DP stage even without TCR rearrangement (Engel et al., 2001). We compared differentiation of DN3a cells from wt and E47-deficient (referred here and below as Tcf3−/−, note that only a E47-specific exon is disrupted in this mouse (Bain et al., 1997)) mice in the presence or absence of the cell cycle inhibitor. Strikingly, Tcf3−/− DN3a thymocytes, unlike their WT counterparts, were able to progress to the DP stage without a single cell division (Figure 7A) (note that only a fraction of undivided cells would express the pre-TCR). Even without cell cycle inhibitor, E47 deficient precursors upregulated coreceptors after fewer cell divisions than their WT counterparts in parallel cultures (Figure 7A, C). These results are unlikely to be explained by excessive proliferation of Tcf3−/− cells in vivo as WT and null mutant DN3a cells did not show major differences in their cell cycle status (Figure 7B). Importantly, sorted E47-deficient cells did not exhibit increased frequency of IC TCRβ+ cells (not shown) and thus represent bona fide DN3a cells. Precursors, deficient in another E protein – HEB (encoded by Tcf12) – that has both overlapping and distinct functions with E47 in T cell development, failed to differentiate when proliferation was blocked (Figure S6A). In fact, DN3a cells from Tcf12fl/flIL7RCre mice, used in these experiments, exhibited proliferation-dependent accumulation of CD8+CD4− ISP cells consistent with the in vivo phenotype of Tcf12 knock-outs (Barndt et al., 1999) and slower proliferation. Thus expression of E47, but not HEB, made further development of DN3a cells dependent on proliferation.
Figure 7. Tcf3−/− DN3a cells can progress to the DP stage without proliferation.
A. WT or Tcf3−/− DN3a cells were labeled with CellTrace violet and placed in OP9-DL1 cocultures in the presence or absence of 1μM PD0332991. CellTrace violet dilution as well as CD4 and CD8 expression was analyzed on day 3. B. Cell cycle status of ex vivo WT or Tcf3−/− DN3a and DN3b thymocytes was analyzed by DAPI staining. C. WT or Tcf3−/− DN3a cells (from bm chimeras, as described in the Supplemental Experimental Procedures) were labeled with CellTrace violet, placed on OP9-DL1 or control OP9 monolayers and analyzed as in A. See also Figure S6.
E47 is believed to be inactivated upon β-selection by rapid induction of Id3, which heterodimerizes with E-proteins and prevents their binding to DNA, and by a decline in E47 protein abundance. However, neither was affected by proliferation as judged by normal induction of an Id3-GFP reporter in the presence of cell cycle inhibitors and lack of obvious downregulation of the product of Tcf3GFP fusion allele where the GFP level correlated with the cell size rather than with the number of divisions (Figure 6C, D).
As Tcf3−/− thymocytes were able to progress to the DP stage without proliferation, and the results above suggest that a mitogenic role of Notch signaling in DN3 to DP transition maybe in part upstream of its role in differentiation, we next assessed development of Tcf3−/− precursors in the absence of Notch signaling. Like WT precursors, these cells failed to proliferate without Notch signaling, but, unlike WT cells, still were able to generate DP cells at variable, but substantial frequencies (30±17% for ko vs 2.4±1.5% for WT, n=5) in the absence of Notch ligands (Figure 7C). Thus E47 deficient cells were not only able to progress to DP stage without proliferation but also could make this developmental transition in the absence of Notch signaling.
Discussion
Proliferation and differentiation are often antagonistic. Here we report that, to the contrary, pre-TCR-induced Notch-dependent proliferation of β-selected thymocytes was indispensable for their differentiation, and that several cell divisions were required for efficient progression to the DP stage. Moreover, development in the absence of Notch, but not in the absence of the pre-TCR, was substantially rescued by ectopic activation of the cell cycle machinery in DN3 thymocytes.
Why proliferation triggered by β-selection is required for the differentiation of αβ T cells remains a mystery. One possibility is that a component of the cell cycle machinery activated at a certain stage of the cycle plays a direct role in the induction of differentiation. Indeed, it was suggested that Cdk6 was directly involved in thymocyte differentiation, perhaps through modulation of Notch signaling (although evidence for the latter is incomplete) (Hu et al., 2011). We demonstrated, however, that blocking the cell cycle as late as S and G2-M phases (which allowed for normal activation of Cdk6 in G1 as demonstrated here by normal phosphorylation of Rb in the case of thymidine block) interfered with differentiation. Moreover, progression through several cell cycles was required for efficient differentiation. Nevertheless, it remains possible that periodic activity of the cell cycle machinery (or some of its components), rather than cell division per se, is directly required for progression to DP.
In the system where proliferation was shown to be required for helper T cell differentiation it was suggested to act via cell cycle-coupled epigenetic changes which in turn were necessary for derepression of lineage-specific genes (Bird et al., 1998; Mullen et al., 2001; Murphy and Reiner, 2002). In one particular example, treatment of naive T cells differentiating towards Th1 or Th2 lineages with 5-azacytidine, a methyltransferase inhibitor that interferes with DNA methylation, enabled expression of cytokine genes after a lower number of divisions (Bird et al., 1998). However, treatment of DN3a cells with 5-azacytidine not only failed to enhance differentiation, but in fact interfered both with their proliferation and developmental progression (not shown). Nevertheless, the idea that proliferation-dependent epigenetic changes are required for the progression to the DP stage remains plausible.
Although the full mechanism that couples differentiation to proliferation has yet to be described, the transcription factor E47 encoded by Tcf3 gene was identified here as one of its gears, as without it the two processes could be readily uncoupled. However, neither of the two ways of E47 inactivation that were shown to act in thymocytes (pre-TCR-induced expression of Id3 and a decline in E47 protein abundance) were dependent on proliferation. It is thus possible that different means of E protein inactivation described in other systems (reviewed in (Braunstein and Anderson, 2012)) also play a role at the β-selection and are coupled to proliferation. It is also conceivable that the history of E47 expression (possibly through epigenetic changes), rather than its actual expression at β-selection checkpoint, is required to make the DN3 to DP transition dependent on proliferation. Consistent with the later hypothesis, our attempts to delete floxed allele of Tcf3 in culture with recombinant tat-Cre treatment or ER-Cre, unlike a germline deficiency, was insufficient to uncouple differentiation from proliferation (not shown) – although this may be due to an insufficient deletion efficiency or residual E47 protein. The fact that HEB deficiency failed to uncouple differentiation from proliferation may indicate that this function is qualitatively unique for E47 or that quantitative differences in overall E protein activity are changed differently by the removal of HEB and E47. Of interest, Tcf3−/− DN3a cells were also able to progress to DP stage in the absence of Notch signaling. Notch signaling can downmodulate E protein activity (Nie et al., 2003) and does so in cooperation with pre-TCR at β-selection checkpoint (Lauritsen et al., 2009). Consistent with this model, decreased E protein abundance in Tcf3−/− cells may render αβ differentiation partially Notch-independent.
Genetic manipulation of several cell cycle-related genes was previously reported to cause alterations in T cell development. Knock-out of Ccnd3 (Sicinska et al., 2003), Cdk6 (Hu et al., 2009), a kinase-dead knock-in of Cdk6 (Hu et al., 2011) as well as ectopic expression of the cell cycle inhibitor p19Arf (encoded by Cdkn2a) under the Lck promoter (Miyazaki et al., 2008) all led to a prominent block in the DP progression – in line with observations reported here. Often such results were interpreted as evidence for a role of the cell cycle protein of interest in the differentiation of thymocytes. Our results support this view, but suggest that their role in T cell differentiation may at least in part be downstream of their role in thymocyte proliferation. Surprisingly, Notch signaling likewise seems to exemplify here the model where the role of a molecular pathway in cell differentiation is at least in part downstream of its mitogenic function.
Notch signaling is required for the DN3 to DP progression (Ciofani et al., 2004; Maillard et al., 2006), however the exact function of Notch at this transition remained elusive. Early in T cell development, Notch is required for the induction of key transcription factors that specify T cell fate – Bcl11b, Hes1 and TCF-1 (encoded by Tcf7) (reviewed in (Rothenberg, 2012)). However, by the DN3 stage Notch signaling is not required for the maintenance of Bcl11b and Tcf7 expression (unpublished observations). Hes1, which is still regulated by Notch in DN3 thymocytes, is already dispensable at this stage (Wendorff et al., 2010). It was previously shown that Notch signaling plays a crucial role via regulation of cell survival and metabolism at the β-selection checkpoint (Ciofani and Zuniga-Pflucker, 2005). However, ectopic Bcl2 expression, although compensating for the pro-survival effects of Notch, could not rescue differentiation (Kreslavsky et al., 2010). Our observation that proliferation was required for the differentiation, together with the well-established mitogenic role of Notch signaling at this stage, prompted us to test whether ectopic activation of the proliferation machinery would rescue T cell differentiation in the absence of Notch signaling. This was indeed the case. The achieved rescue of proliferation was partial, which may explain the substantial but incomplete rescue of differentiation. It is also likely that other functions of Notch, such as regulation of thymocyte metabolism (Ciofani and Zuniga-Pflucker, 2005), may contribute to the DP progression.
We next sought to understand the molecular mechanism downstream of Notch that licenses β-selected thymocytes to proliferate. It was shown that the transcription factor Myc - a common downsream effector of multiple mitogenic signals – is a direct Notch1 target in T cell acute lymphoblastic leukemia (T-ALL), and that its expression at the β-selection checkpoint is likewise Notch-dependent (Weng et al., 2006). DN3 cells exhibited severely reduced proliferation upon conditional deletion of Myc ((Dose et al., 2006) and Figure 1). However, it was recently reported that ectopic expression of Myc was able to rescue expansion but not differentiation of thymocytes at β-selection checkpoint in the absence of Notch signaling (Wong et al., 2012) – potentially contradicting our observations with the proliferator construct. To resolve this discrepancy we reassessed the effects of Myc overexpression on Notch dependence of developing thymocytes. Strikingly, Vav-Bcl2 transgenic DN3a cells, expressing high amounts of Myc-IRES-GFP in the absence of Notch signaling were rescued both in terms of proliferation and differentiation. Non-bcl2 transgenic cells, however, could not be rescued, possibly because they expressed lower levels of Myc-IRES-GFP, a likely explanation for the seeming discrepancy with Wong et al. Different sources of the precursors (fetal liver vs. adult HSC), absence of IL-7, that is inhibitory for DP progression (Tussiwand et al., 2011) in our cultures or different level of Myc expression may also contribute to the difference. An obvious threshold in Myc expression (Figure 6C) that was required for the rescue is a plausible explanation of our own previous failure to render T cell differentiation and proliferation Notch-independent by this approach on ex vivo thymocytes that are poorly infectable (Kreslavsky et al., 2010). Importantly, level of Myc ectopic expression required to rescue progression to the DP stage was within physiological range.
Here we demonstrate that two pathways – pre-TCR and Notch signaling – both of them required for proliferation and conducive for DP progression – regulated these processes in different ways. Whereas the mitogenic role of the pre-TCR was required but not sufficient to support DP progression – indicating an additional direct effect of this signaling pathway on differentiation – the mitogenic role of Notch appeared to be to a large extent upstream of its function in supporting the differentiation of DN3 thymocytes to the DP stage. A situation where the same signaling pathway regulates both developmental progression and proliferation of a cell is common both in normal cell differentiation and in tumorigenesis. It may be thus of interest to test whether the relationship between proliferation and differentiation in some of these systems resembles Notch or pre-TCR signaling at the β-selection checkpoint. The ‘proliferator‘ cassette described here may be a useful tool to address this question in other developmental systems. Further study of the molecular mechanisms underlying the link between differentiation and proliferation may illuminate the initiating mechanisms of tumorigenesis – in particular, that of T-ALL - a common human cancer that originates specifically at β-selection.
Experimental Procedures
Antibodies, Plasmids, Chemicals
Antibodies and plasmids were gifts, purchased or generated, as specified in the Supplemental Experimental Procedures. Cell cycle inhibitors were purchased.
Retroviral Infections
Virus was made by co-transfecting 293T cells with plasmid and pEco packaging vector. Day 2 supernatants were filtered and used to spin-infect DN3 cells generated from LSK.
OP9 Cocultures
For LSK cocultures, precursors were plated on confluent OP9 monolayers and grown with Il-7 and Flt3, fresh media and cytokines was provided every 4 days. DN3 cocultures were performed without Il-7.
Flow Cytometry and Cell Sorting
Flow cytometry was performed on FACS Aria. Sorted cells were of ≥98%. Depletion of DP cells was done with magnetic beads. Cell surface, intracellular, CFSE, and CellTrace Violet staining is described further in detail in the Supplemental Experimental Procedures. For cell cycle staining, cells were fixed, permeabilized, treated with RNAse A and stained with DAPI.
Mice
Wild type and engineered mice were kept at animal facilities of the DFCI, Harvard Medical School or of the Department of Molecular Biology, UCSD according to institutional, state and federal guidelines, or purchased from the Jackson laboratory or Taconic.
Supplementary Material
Highlights.
β-selection-induced proliferation is required for αβ T cell differentiation
Proliferation drives differentiation without Notch but not pre-TCR signaling
Notch target Myc rescues differentiation in the absence of Notch signaling
Transcription factor E47 imposes the dependence of differentiation on proliferation
Acknowledgments
We would like to thank J.C. Zuniga-Pflucker, J.M. Adams, M. Dose, F. Gounari, H. Cantor, and A. Capobianco for providing reagents and mice, and G. Turchinovich, D. Gray, M. Herold, H.-J. Kim, C. Daniel and S. Schlenner for helpful discussions. The authors are grateful to V. Schmidt and G. Singh for technical assistance. These studies were supported by National Institutes of Health Grants R01 A145846 and R01 A151378.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bain G, Maandag ECR, Riele HPJt, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. Both E12 and E47 Allow Commitment to the B Cell Lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- Barndt R, Dai MF, Zhuang Y. A novel role for HEB downstream or parallel to the pre-TCR signaling pathway during alpha beta thymopoiesis. J Immunol. 1999;163:3331–3343. [PubMed] [Google Scholar]
- Ben-Sasson SZ, Gerstel R, Hu-Li J, Paul WE. Cell division is not a "clock" measuring acquisition of competence to produce IFN-gamma or IL-4. J Immunol. 2001;166:112–120. doi: 10.4049/jimmunol.166.1.112. [DOI] [PubMed] [Google Scholar]
- Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Anderson MK. HEB in the spotlight: Transcriptional regulation of T-cell specification, commitment, and developmental plasticity. Clinical & developmental immunology. 2012;2012:678705. doi: 10.1155/2012/678705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G, Hughes PJ, Michell RH. Cell differentiation and proliferation--simultaneous but independent? Experimental Cell Research. 2003;291:282–288. doi: 10.1016/s0014-4827(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Dose M, Khan I, Guo Z, Kovalovsky D, Krueger A, von Boehmer H, Khazaie K, Gounari F. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood. 2006;108:2669–2677. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Johns C, Bain G, Rivera RR, Murre C. Early Thymocyte Development Is Regulated by Modulation of E2a Protein Activity. The Journal of Experimental Medicine. 2001;194:733–746. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MG, Deshpande A, Enos M, Mao D, Hinds EA, Hu GF, Chang R, Guo Z, Dose M, Mao C, et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 2009;69:810–818. doi: 10.1158/0008-5472.CAN-08-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MG, Deshpande A, Schlichting N, Hinds EA, Mao C, Dose M, Hu G-f, Van Etten RA, Gounari F, Hinds PW. CDK6 kinase activity is required for thymocyte development. Blood. 2011;117:6120–6131. doi: 10.1182/blood-2010-08-300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Gleimer M, Garbe AI, von Boehmer H. alphabeta versus gammadelta fate choice: counting the T-cell lineages at the branch point. Immunol Rev. 2010;238:169–181. doi: 10.1111/j.1600-065X.2010.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Crispe IN. Functional Flexibility in T Cells: Independent Regulation of CD4+ T Cell Proliferation and Effector Function In Vivo. Immunity. 2000;13:291–301. doi: 10.1016/s1074-7613(00)00029-7. [DOI] [PubMed] [Google Scholar]
- Lauritsen JPH, Wong GW, Lee S-Y, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zúñiga-Pflücker JC, Wiest DL. Marked Induction of the Helix-Loop-Helix Protein Id3 Promotes the γδ T Cell Fate and Renders Their Functional Maturation Notch Independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Desiderio S. Cyclin A/CDK2 regulates V(D)J recombination by coordinating RAG-2 accumulation and DNA repair. Immunity. 1999;11:771–781. doi: 10.1016/s1074-7613(00)80151-x. [DOI] [PubMed] [Google Scholar]
- Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–589. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Maillard I, Tu L, Sambandam A, Yashiro-Ohtani Y, Millholland J, Keeshan K, Shestova O, Xu L, Bhandoola A, Pear WS. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203:2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau M, Gros J, Savage K, Thome V, McPherron A, Paterson B, Marcelle C. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes & development. 2008;22:668–681. doi: 10.1101/gad.454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Miyazaki K, Itoi M, Katoh Y, Guo Y, Kanno R, Katoh-Fukui Y, Honda H, Amagai T, van Lohuizen M, et al. Thymocyte proliferation induced by pre-T cell receptor signaling is maintained through polycomb gene product Bmi-1-mediated Cdkn2a repression. Immunity. 2008;28:231–245. doi: 10.1016/j.immuni.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Mullen AC, Hutchins AS, Villarino AV, Lee HW, High FA, Cereb N, Yang SY, Hua X, Reiner SL. Cell cycle controlling the silencing and functioning of mammalian activators. Current Biology. 2001;11:1695–1699. doi: 10.1016/s0960-9822(01)00533-4. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Genet. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Inagawa M, Uchida C, Okada K, Tane S, Kojima M, Kubota M, Noda M, Ogawa S, Shirato H, et al. Coordinated regulation of differentiation and proliferation of embryonic cardiomyocytes by a jumonji (Jarid2)-cyclin D1 pathway. Development (Cambridge, England) 2011;138:1771–1782. doi: 10.1242/dev.059295. [DOI] [PubMed] [Google Scholar]
- Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie HT, Tourigny M, Burtrum DB, Livak F. Precursor Thymocyte Proliferation and Differentiation Are Controlled by Signals Unrelated to the Pre-TCR. The Journal of Immunology. 2000;165:3094–3098. doi: 10.4049/jimmunol.165.6.3094. [DOI] [PubMed] [Google Scholar]
- Richter A, Löhning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. Exp. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg EV. Transcriptional drivers of the T-cell lineage program. Current Opinion in Immunology. 2012;24:132–138. doi: 10.1016/j.coi.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Tussiwand R, Engdahl C, Gehre N, Bosco N, Ceredig R, Rolink AG. The preTCR-dependent DN3 to DP transition requires Notch signaling, is improved by CXCL12 signaling and is inhibited by IL-7 signaling. European Journal of Immunology. 2011;41:3371–3380. doi: 10.1002/eji.201141824. [DOI] [PubMed] [Google Scholar]
- Wendorff AA, Koch U, Wunderlich FT, Wirth S, Dubey C, Bruning JC, Macdonald HR, Radtke F. Hes1 Is a Critical but Context-Dependent Mediator of Canonical Notch Signaling in Lymphocyte Development and Transformation. Immunity. 2010;33:671–684. doi: 10.1016/j.immuni.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes & development. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Knowles GC, Mak TW, Ferrando AA, Zúñiga-Pflücker JC. HES1 opposes a PTEN-dependent check on survival, differentiation and proliferation of TCRβ-selected mouse thymocytes. Blood. 2012 doi: 10.1182/blood-2011-12-395319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Skoultchi AI. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.