Abstract
Brain abscesses arise following parenchymal infection with pyogenic bacteria and are typified by inflammation and edema, which frequently results in a multitude of long-term health problems. The impact of adaptive immunity in shaping continued innate responses during late stage brain abscess formation is not known but is important, since robust innate immunity is required for effective bacterial clearance. To address this issue, brain abscesses were induced in TCR αβ knockout (KO) mice, since CD4+ and NKT cells represented the most numerous T cell infiltrates. TCR αβ KO mice exhibited impaired bacterial clearance during later stages of infection, which was associated with alterations in neutrophil and macrophage recruitment as well as perturbations in cytokine/chemokine expression. Adoptive transfer of either Th1 or Th17 cells into TCR αβ KO mice restored bacterial burdens and innate immune cell infiltrates to levels detected in WT animals. Interestingly, adoptively transferred Th17 cells demonstrated plasticity within the CNS compartment and induced distinct cytokine secretion profiles in abscess-associated microglia and macrophages compared to Th1 transfer. Collectively, these studies identify an amplification loop for Th1 and Th17 cells in shaping established innate responses during CNS infection to maximize bacterial clearance and differentially regulate microglial and macrophage secretory profiles.
Keywords: Th1, Th17, central nervous system, S. aureus, macrophage, microglia, neutrophil
INTRODUCTION
Brain abscess formation is consequent of chronic bacterial infections of the middle ear, sinuses, or teeth, or from bacterial dissemination from systemic sites (1). Once pyogenic bacteria invade the brain parenchyma, a localized area of cerebritis ensues, which develops into a purulent lesion encapsulated by a well-vascularized fibrotic wall. Long-term morbidity issues often arise in patients recovering from brain abscesses as a result of the extensive parenchymal damage typically associated with infection, which can manifest as seizures, cognitive deficits, and/or hemiparesis (2-4). Diagnosing and treating brain abscesses can be complicated by delays in appropriate treatment due to initial non-specific clinical presentation and may conclude in the rupture of untreated lesions within the ventricular space engendering an 80% mortality rate (1, 3). Currently, brain abscess therapy encompasses long-term systemic antibiotics (6-8 week duration) and surgery or guided needle aspiration to allow for drainage and a reduction in intracranial pressure (3). It is estimated that 1 in every 10,000 hospital admissions of an infectious disease nature in the United States results from a brain abscess, with S. aureus representing one of the most frequent causes (1, 5). Although only a few CNS infections to date have been attributed to methicillin-resistant S. aureus (MRSA), the emergence of community acquired MRSA (CA-MRSA) strains causing brain abscesses is increasing with infections occurring in otherwise healthy individuals (6, 7). Therefore, alternative approaches, such as immune modulation, could enhance treatment options once more is known about the anti-bacterial immune responses that ensue during brain abscess development and mechanisms responsible for maximal pathogen clearance. Indeed, immune-based approaches represent an attractive therapeutic alternative to antibiotics by minimizing direct mutational pressures on bacteria and decreasing the likelihood of developing resistant strains.
Previous studies from our laboratory and others have established the genesis of a rapid innate immune response during CNS abscess formation (8-11). For example, microglial and astrocyte activation is immediately evident within hours after infection followed by neutrophil accumulation within 12-24 h, which continues throughout abscess development (11-13). Macrophages accumulate along the abscess margins and are readily detected at day 3 post-infection and prior work from our laboratory has established that rapid pathogen recognition within the CNS compartment is essential for establishing a protective anti-microbial response (14).
Despite this information, the mechanisms responsible for maintaining a robust innate immune response to ensure pathogen clearance during late stage CNS infection remain relatively undefined. One possibility is that components of adaptive immunity, in particular various T cell populations, provide critical signals to perpetuate ongoing innate anti-bacterial responses. This possibility is supported by recent studies where Th17 cells impact innate immune responses via indirect effects on neutrophil recruitment (15-17) as well as NKT cells that span innate and adaptive immunity (18, 19). Although some information is available regarding the kinetics of T cell entry into brain abscesses (10), these studies were either performed with a laboratory-adapted S. aureus strain (20) or limited time points were examined (21). More importantly, the functional impact of T cell populations on brain abscess progression, the main subtypes involved, and their cross-talk with ongoing innate anti-bacterial responses have not yet been investigated and all represent novel aspects of the current study.
The objective of the current report was to assess the functional importance of major abscess-associated T cell subsets in modulating ongoing innate immune responses during infection. We demonstrate here that TCR αβ+ cells regulate bacterial burdens, neutrophil and macrophage influx, and shape the cytokine/chemokine milieu during brain abscess development. In mice that lacked αβ T cells, γδ T cell infiltrates were elevated, which may represent a compensatory response to facilitate bacterial clearance. Adoptive transfer of either purified Th1 or Th17 cells into TCR αβ knockout (KO) mice was capable of restoring bacterial burdens and alterations in neutrophil and macrophage influx/activation to levels observed in wild type (WT) animals, emphasizing the link between adaptive and innate immunity during CNS bacterial infection. Collectively, these results suggest that manipulating Th1 and Th17 cells could expedite S. aureus clearance from the CNS parenchyma and limit the extent of tissue damage.
MATERIALS AND METHODS
Mouse strains
TCR αβ KO (C57BL/6 background; CD45.2), CD1d KO, and B6/SJL mice congenic for the CD45 allele (CD45.1) on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). For the majority of studies, age- and sex-matched C57BL/6 mice were obtained from Charles River (Frederick, MD) as WT controls. To exclude potential variation arising from sub-strain differences between C57BL/6 and C57BL/6J mice, several adoptive transfer experiments were also performed with age-matched C57BL/6J animals purchased from The Jackson Laboratory. Both approaches produced nearly identical results, allowing the conclusion that sub-strain differences in the source of C57LB/6 mice did not impact the results obtained.
Bacteria strain and generation of experimental brain abscesses
S. aureus strain USA300, a CA-MRSA clinical isolate recovered from a patient with a fatal brain abscess (22) was encapsulated in agarose beads prior to injection as previously described (11). Mice were infected with 2 μl of live USA300 (1-2 × 104 cfu) by stereotaxic injection into the striatum and monitored daily for clinical signs of disease including hunched posture, ruffled fur, lethargy, and weight loss. The animal use protocol has been approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and is in accord with the National Institutes of Health (NIH) guidelines for the use of rodents.
Recovery of brain abscess-associated cells and FACS analysis
FACS analysis was utilized to characterize the relative percentages of abscess-associated T cell subsets and their infiltration kinetics, in addition to the effects of manipulating the T cell compartment on innate immune cell influx into brain abscesses. Briefly, mice were perfused to eliminate leukocytes from the vasculature, whereupon the entire infected hemisphere was collected to recover abscess-associated cells. Brain tissues were minced in HBSS supplemented with 10% FBS (HyClone, Logan, UT) and filtered through a 70 μm nylon mesh cell strainer, whereupon an aliquot of tissue homogenate from each animal was removed to quantitate bacterial burdens. Next, the resulting slurry was digested for 30 min at 37° C in HBSS supplemented with 2 mg/ml collagenase type I and 28 U/ml DNase I (both from Sigma-Aldrich, St. Louis, MO) to obtain a single-cell suspension. Following enzyme neutralization, cells were layered onto a discontinuous Percoll gradient (1.03 to 1.088 g/ml) and centrifuged at 2,400 rpm for 20 min at room-temperature in a swinging bucket rotor. After centrifugation, the myelin debris was carefully aspirated and the cell interface collected. Following extensive washes and incubation in Fc Block™ (BD Biosciences, San Diego, CA), cells were stained with directly conjugated antibodies for multi-color FACS including F4/80-FITC (Clone BM8), CD45-APC (Clone RA3-6B2), Ly-6G-PE (Clone 1A8), γδ TCR-PECy5 (Clone eBioGL3), NKp46-AlexaFluor® 647 (Clone 29A1.4), CD3ε-APC (Clone 17A2), TCRβ (Clone H57-597) MHC Class II-PE (Clone M5/114.15.2) as well as a CD1d-PE tetramer (NIH Tetramer Core Facility, Emory University). All antibodies were purchased from BD Biosciences with the exception of γδ TCR, NKp46, CD3ε, TCRβ, and MHC Class II (eBiosciences). For experiments comparing the impact of TCR αβ+ cells on proinflammatory mediator production from resident microglia (F4/80+, CD45lo-intermediate) versus infiltrating macrophages (F4/80+, CD45high), cell types were discriminated based on differential CD45 expression (11, 23-25). Cells were analyzed using a BD FACSAria (BD Biosciences) with compensation set based on the staining of each individual fluorochrome alone and correction for autofluorescence with unstained cells. Controls included cells stained with isotype control antibodies to assess the degree of non-specific staining. Results are presented as either the percentage or absolute numbers of each cell population recovered from brain abscesses of TCR αβ KO or WT mice normalized for differences in total cell recovery between the two strains.
Intracellular cytokine staining
Abscess-associated T cells were sorted from TCR αβ KO and WT mice by FACS as described above. FACS-purified CD3+ CD4+ and NKT cells were stimulated immediately ex vivo with a cocktail of PMA + ionomycin (Leukocyte Activation Cocktail) in the presence of GolgiPlug™ for 3 h, whereupon cells were incubated with Fc Block™ (all from BD Biosciences) to minimize non-specific antibody binding. Subsequently, T cells were fixed and permeabilized with a CytoFix/CytoPerm kit and stained for intracellular IL-17 or IFN-γ (all from BD Biosciences). Cells were analyzed using a BD FACSAria with compensation set based on the staining of each individual fluorochrome alone (CD4-FITC, IL-17-PE, and IFNγ-PE-Cy7) and correction for autofluorescence with unstained cells. Controls included cells stained with directly-conjugated isotype control antibodies to assess the degree of non-specific staining. Results are presented as the percentages or absolute numbers of each cell population recovered from brain abscesses of TCR αβ KO or WT mice normalized for differences in total cell recovery between the two strains.
T cell isolation and adoptive transfer
For experiments designed to evaluate the general impact of CD4+ versus CD8+ and NKT cells in brain abscesses of TCR αβ KO mice, NKT-depleted CD4+ T cells (CD4+, NK1.1−) were purified from the spleens of uninfected B6/SJL mice by FACS to facilitate the identification of adoptively transferred (CD45.1) from endogenous immune cells in TCR αβ KO animals (CD45.2). In studies to determine the importance of Th1 versus Th17 cells in brain abscess immunity, naïve CD4+ T cells (CD4+CD62L+CD44lo) were purified from spleens and lymph nodes of uninfected B6/SJL mice using a naïve CD4+ T cell column (R&D Systems, Minneapolis, MN). Naïve CD4+ T cells were seeded at 2 × 105 per well in 96-well plates with appropriate cytokine/antibody cocktails in RPMI medium supplemented with 10% FBS for five days with one-half medium replacement every other day (26, 27). The formulary for Th1 skewing medium included 8 μg/ml recombinant IL-12 and 5 ng/ml anti-IL-4, whereas Th17 skewing media contained 3 ng/ml TGF-β, 10 ng/ml IL-6, 5 ng/ml IL-1β, 10 ng/ml IL-23, 2 μg/ml anti-IL-4 (Clone 11B11), 2 μg/ml anti-IL-12, and 2 μg/ml anti-IFN-γ (Clone XMG1.2) (26, 27). In addition, both Th1 and Th17 media contained 50 U/ml of IL-2 and 5 μl/ml of CD3/CD28 DynaBeads (Invitrogen, San Diego, CA). Subsequently, TCR αβ KO mice (CD45.2) were injected with 106 B6/SJL Th1 or Th17 cells (CD45.1) in 100 μl of PBS into the retro-orbital sinus one day prior to brain abscess induction, whereas control animals received an equivalent volume of sterile PBS. Based on ICC staining results, the numbers of Th1 or Th17 cells injected into recipients was adjusted so that 106 cells of each cytokine specificity were injected (which increased the total number of adoptively transferred cells by approximately 10-15%).
Milli-plex multi-analyte bead arrays
To evaluate proinflammatory mediator expression profiles in brain abscess homogenates and microglia and macrophages recovered from brain abscesses of WT and TCR αβ KO mice, a custom-designed mouse cytokine/chemokine microbead array was utilized according to the manufacturer’s instructions (Milliplex; Millipore, Billerica, MA). This microbead array allows for the simultaneous detection of 19 individual inflammatory molecules in a single 75 μl brain homogenate including IL-1α, IL-1β, TNF-α, IFN-γ, IL-6, IL-9, IL-10, IL-12p70, IL-12p40, IL-15, IL-17, CXCL1/keratinocyte chemoattractant (KC), CXCL2/macrophage inflammatory protein-2 (MIP-2), CXCL9/monokine induced by IFN-γ (MIG), CXCL10/IFN-γ-induced protein 10 (IP-10), CCL2/monocyte chemoattractant protein-1 (MCP-1), CCL3/MIP-1α, CCL4/MIP-1β, and CCL5/regulated upon activation T cell expressed and secreted (RANTES). Results were analyzed using a Bio-Plex workstation (Bio-Rad) and adjusted based on the amount of total protein extracted from brain tissue homogenates or per 104 cells for normalization.
Statistical analysis
Significant differences between experimental groups were determined by one way analysis of variance (ANOVA) followed by the Holm-Sidak method for pair-wise multiple comparisons using SigmaStat (SPSS Science, Chicago, IL).
RESULTS
Brain abscesses are typified by CD4+ and NKT cell infiltrates
The current study utilized a S. aureus USA300 isolate with important clinical origins (i.e. recovered from an otherwise healthy patient who died from a brain abscess) (22), which is more reminiscent of strains causing natural CNS infections. To investigate the impact of adaptive immune cells on ongoing innate responses during brain abscess development, the infiltration kinetics of various T cell populations was first established. Although we recently examined the impact of TLR2 on T cell infiltration kinetics, these studies were performed with either a S. aureus laboratory adapted strain (20) or limited time points were examined (21). Here we report that CD4+ T cell infiltrates were first detectable on day 3 and progressively increased until day 14 after S. aureus exposure, with peak levels averaging 20-35% of the total leukocyte infiltrate (Fig. 1). In contrast, fewer CD8+ or γδ T cells were detected over the course of infection.
Figure 1. Brain abscesses are typified by CD4+ and NKT cell infiltrates.
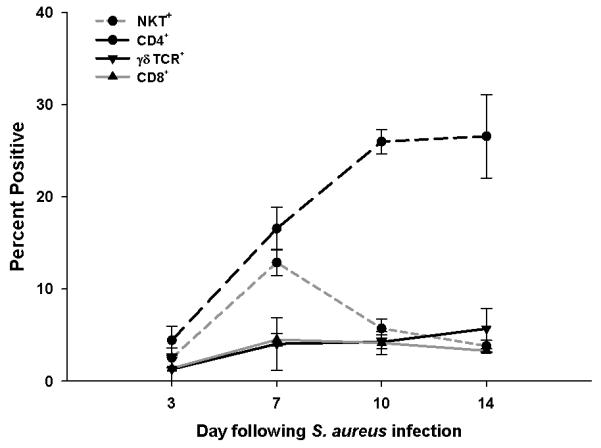
Abscess-associated cells were isolated from C57BL/6 mice at the indicated day post-infection and stained for FACS with NK1.1-FITC, CD4-PECy5, CD8-APC, and γδ TCR-PE. Results represent the percent positive cells of each population combined from a total of five independent experiments (mean ± SEM).
The influx of NK1.1+ (NKT) cells was more short-lived compared with the CD4+ population, with cells first detected at day 3 post-infection and peaked around day 7 (Fig. 1). Since NK1.1 is expressed on both NK and NKT cells, a NK cell-specific antibody (i.e. NKp46) was used to discriminate between these populations. This staining approach revealed that the majority of NK1.1+ cells in the infected brain were NKp46−, indicating that they were NKT cells (Supplemental Fig. 1A). Furthermore, CD1d tetramers were also used to confirm the presence of invariant NKT cells infiltrating brain abscesses (Supplemental Fig. 1B). An NKp46+NK1.1+ population was apparent in the spleens of the same mice, demonstrating that NK cells could be detected, although they did not represent a major brain abscess infiltrate (Supplemental Fig. 1A). Collectively, these findings demonstrate that cell populations known to express the αβ TCR represent the main adaptive infiltrates during brain abscess development.
IL-17 producing CD3+ CD4+ T cell infiltrates predominate during brain abscess evolution
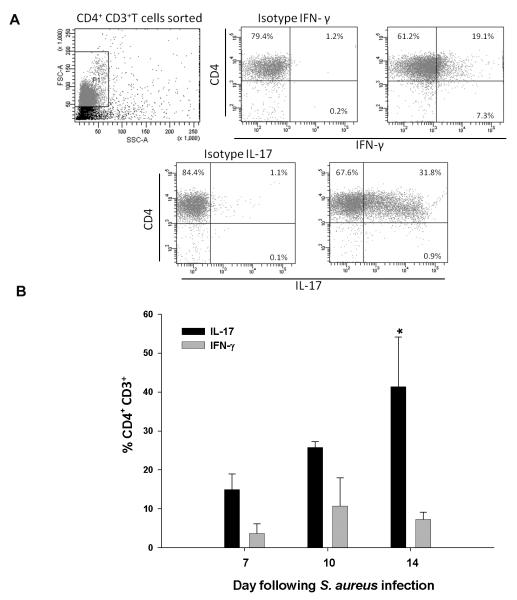
To characterize the cytokine expression profiles of infiltrating T cell populations over the course of infection, intracellular cytokine staining was performed. Abscess-associated CD3+CD4+ cells were found to produce both IFN-γ and IL-17 (Fig. 2); however, the percentages of CD3+CD4+ IL-17 expressing cells progressively increased over time, whereas CD4+ IFN-γ producing cells remained relatively constant (Fig. 2). Since both IL-17 and IFN-γ can elicit inflammatory mediator release from several innate immune cell populations, this suggested a link between adaptive and innate immune responses during late stage CNS parenchymal infection.
Figure 2. IL-17 producing CD4+ T cells progressively increase during brain abscess evolution.
Abscess-associated CD3+ CD4+ T cells were isolated from C57BL/6 mice at the indicated day post-infection by FACS, immediately stimulated ex vivo with PMA + ionomycin for 3 h, and stained for IL-17 or IFN-γ to demonstrate cytokine profiles. (A) Representative dot plots depicting cytokine staining at day 7 post-infection. Note that total CD4+ T cells were sorted and immediately processed for intracellular cytokine staining; therefore, CD4 staining was still present in the isotype controls for IL-17 and IFN-γ. (B) Changes in the percentages of IL-17 and IFN-γ producing CD4+ T cells over the course of infection. Significant differences between the percentages of IL-17- or IFN-γ-positive cells at day 14 are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
TCR αβ+ cells impact bacterial clearance during brain abscess development
Since the majority of brain abscess-associated T cell infiltrates are known to express the αβ TCR (i.e. CD4+, NKT, and CD8+ cells), we next examined the functional importance of these populations on a more global scale utilizing TCR αβ KO mice. TCR αβ KO mice were more sensitive to CNS S. aureus infection as revealed by reduced survival rates, which correlated with significantly elevated bacterial burdens compared to WT animals at days 7 and 14 post-infection (Fig. 3A and B, respectively). Importantly, αβ+ T cells did not impact S. aureus titers during early infection (i.e. day 3), which was expected since T cell influx was minimal at this time point. These results demonstrate that innate immune mechanisms are effective at controlling bacterial burdens during early infection; however, assistance from TCR αβ+ cells is required to maintain ongoing anti-bacterial responses at later time points following infection.
Figure 3. TCR αβ+ cells contribute to bacterial clearance.
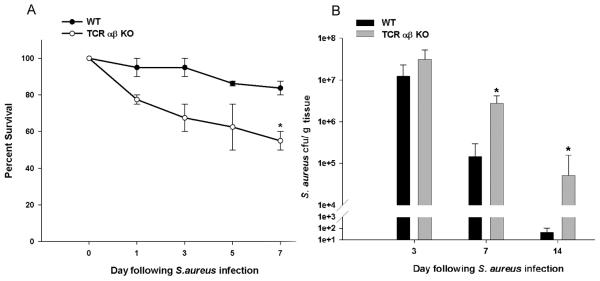
Brain abscesses were induced in TCR αβ KO and WT mice, whereupon percent survival (A) and bacterial burdens (B) were determined at the indicated time points post-infection. Significant differences between TCR αβ KO versus WT mice are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
TCR αβ+ cells impact chemokine expression and innate immune cell influx during later stages of brain abscess formation
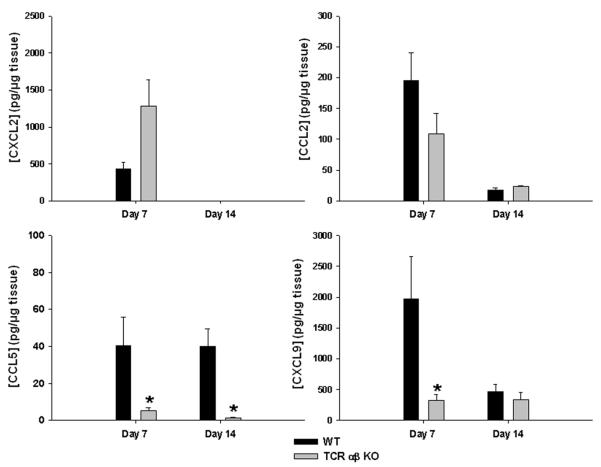
To investigate the impact of TCR αβ+ cells on the local cytokine and chemokine milieu, inflammatory mediator expression was quantitated in brain abscess homogenates using multi-plex microbead arrays. Interestingly, the neutrophil chemoattractant CXCL2 was elevated in lesions of TCR αβ KO mice, whereas CCL5 and CXCL9 were significantly decreased (Fig. 4).
Figure 4. TCR αβ+ cells impact chemokine production during brain abscess development.
Brain abscesses were induced in TCR αβ KO and WT mice (n= 4-5/group), whereupon abscess homogenates were collected at the indicated time points post-infection and CXCL2, CCL2, CCL5, and CXCL9 expression examined using a multi-analyte bead array with results normalized to total protein to correct for differences in tissue sampling size. Significant differences between TCR αβ KO versus WT mice are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
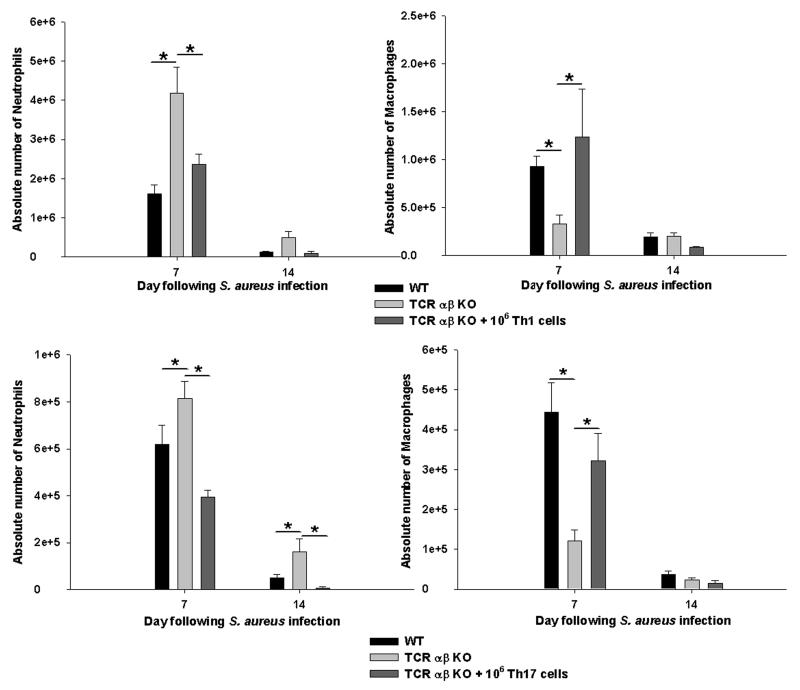
Since TCR αβ KO mice demonstrated impaired CNS bacterial clearance and alterations in chemokine expression, we next determined whether this could be explained by differences in the numbers and/or activation status of infiltrating innate immune cell populations. To examine this possibility, FACS analysis was used to quantitate neutrophils (Ly-6G+, F4/80−, CD45hi), macrophages (F4/80+, CD45hi), microglia (F4/80+, CD45lo-intermediate), and γδ T cells (γδ TCR+) associated with brain abscesses of TCR αβ KO and WT mice. Neutrophil influx was significantly higher in TCR αβ KO mice at day 7 post-infection (Fig. 5), which correlated with enhanced bacterial burdens and expression of the neutrophil chemokine CXCL2 compared to WT animals (Figs. 3 and 4, respectively). Previous studies from our laboratory have established the importance of neutrophils in the experimental brain abscess model (28) and that the degree of neutrophil infiltrates mirrors infection severity. Therefore, it was not unexpected that neutrophil influx was increased TCR αβ KO animals.
Figure 5. TCR αβ+ cells regulate neutrophil and macrophage infiltrates during late stage brain abscess development.
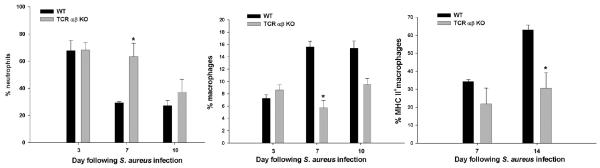
Abscess-associated cells were collected from TCR αβ KO and WT mice (n= 4-5/group), whereupon the percentages of neutrophils (F4/80−, CD45hi, Ly6G+), macrophages (F4/80−, CD45hi, Ly6G−), and MHC Class II+ macrophages were quantified by FACS. Significant differences between TCR αβ KO versus WT mice are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
In contrast, fewer macrophages infiltrated abscesses of TCR αβ KO mice at days 7 and 10 post-infection (Fig. 5), which correlated with decreases in CCL5 and CXCL9 expression (Fig. 4). In addition, those macrophages that infiltrated abscesses of TCR αβ KO mice were less activated, as revealed by diminished MHC Class II and inflammatory cytokine expression (Fig. 5 and data not shown). No significant changes in the relative percentages or absolute numbers of microglia were observed between abscesses of TCR αβ KO and WT mice (data not shown). Both neutrophil and macrophage infiltrates were equivalent between TCR αβ KO and WT mice at day 3 post-infection (Fig. 5), which was expected since T cell influx was minimal at this time point.
Although initial analysis of abscess-associated T cell populations did not reveal a significant γδ T cell infiltrate, we elected to evaluate this subset in TCR αβ KO mice since it represented the sole remaining T cell type. Interestingly, γδ T cells were significantly elevated in TCR αβ KO animals at days 10 and 14 post-infection (Fig. 6). This may represent a compensatory response to combat elevated bacterial burdens, since γδ T cells utilize their TCR as a pattern recognition receptor to identify microbial peptide antigens and elicit IL-17 and IFN-γ release (29). Indeed, the time frame where elevated γδ T cell influx was observed coincided with the decline in bacterial burdens in TCR αβ KO mice, although titers remained significantly elevated compared to WT animals (Fig. 3). Collectively, these results indicate that TCR αβ+ cells play an important role in regulating innate immune cell influx during CNS infection.
Figure 6. Loss of TCR αβ cells leads to exaggerated γδ T cell influx in brain abscesses.
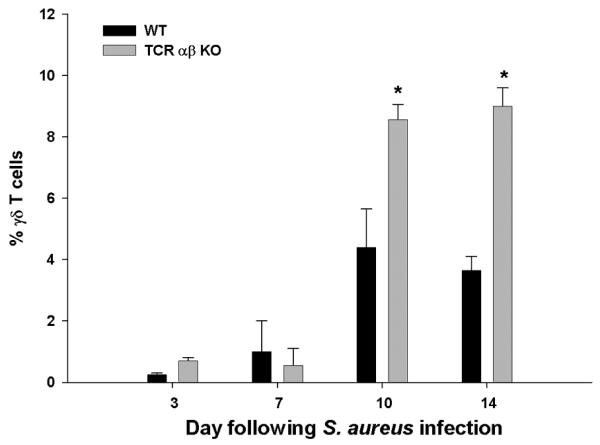
Abscess-associated cells were isolated from TCR αβ KO and WT mice (n= 4-5/group), whereupon the percentages of γδ T cells were identified by FACS. Significant differences between TCR αβ KO versus WT mice are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
Adoptive transfer of Th1 or Th17 cells facilitates bacterial clearance and restores innate immune responses in TCR αβ KO mice
To identify which αβ TCR population was most pivotal for maintaining innate immunity during brain abscess development, we performed initial adoptive transfer studies with total CD4+ T cells into TCR αβ KO mice since they represented the main abscess-associated T cell infiltrate. For these experiments, CD4+ T cells isolated from B6/SJL congenic mice (CD45.1) were depleted of NKT cells and adoptively transferred into TCR αβ KO animals (CD45.2) to facilitate their identification. Infiltration of adoptively transferred CD4+ T cells into brain abscesses of TCR αβ KO mice was demonstrated by the presence of CD45.1+ cells in the parenchyma (Supplemental Fig. 2). Importantly, CD4+ T cell adoptive transfer was capable of reducing S. aureus burdens at both days 7 and 14 post-infection compared to TCR αβ KO mice that did not receive T cells, with titers in the former approaching those observed in WT animals (Fig. 7). Similar restorative responses were also observed with regard to the ability of CD4+ T cell transfer to decrease neutrophil and enhance macrophage influx to levels observed in WT mice (Fig. 7). Together, these data indicate that CD4+ T cells are a major driving force to maintain ongoing innate immune responses during CNS infection, whereas CD8+ and NKT cells play a relatively minor role in comparison.
Figure 7. Bacterial clearance and innate immune cell influx is restored following adoptive transfer of CD4+ T cells into TCR αβ KO mice.
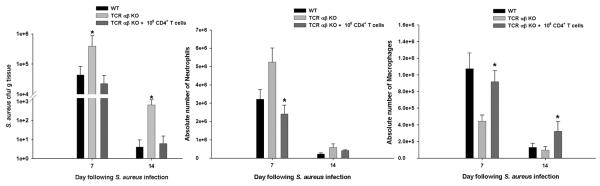
Brain abscesses were induced in WT, TCR αβ KO, and TCR αβ KO mice that received an adoptive transfer of 106 purified CD3+CD4+ T cells (NKT-depleted) 24 h prior to S. aureus infection (n= 4-5/group). Animals were euthanized at the indicated time points post-infection, whereupon bacterial burdens were quantitated and normalized to tissue wet weight (g), and neutrophil and macrophage infiltrates determined by FACS. Significant differences between PBS-injected TCR αβ KO mice versus TCR αβ KO animals receiving adoptively transferred T cells are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
To establish the contribution of Th1 versus Th17 cells in regulating ongoing innate immune responses during CNS abscess development, naïve CD4+ T cells were exposed to cytokine cocktails that skew towards a Th1 or Th17 phenotype (26, 27). Successful establishment of Th1 or Th17 polarization was confirmed by intracellular cytokine staining for IFN-γ and IL-17 prior to adoptive transfer, although some IFN-γ/IL-17 double-positive cells were also observed (Supplemental Fig. 3). Infiltration of adoptively transferred Th1 or Th17 cells into brain abscesses of TCR αβ KO mice was demonstrated by tracking CD45.1+ expression and the stability of each Th subtype upon recruitment into brain abscesses was evaluated by ICC staining. The majority of adoptively transferred Th1 and Th17 cells homed to the infected brain, whereas fewer cells were distributed in the draining deep and superficial cervical lymph nodes (data not shown). Interestingly, adoptive transfer of either Th1 or Th17 cells was capable of reducing S. aureus burdens at both days 7 and 14 post-infection compared to TCR αβ KO mice that did not receive T cells (Fig. 8). In addition, Th1 and Th17 adoptive transfer restored neutrophil numbers as well as macrophage infiltrates and MHC Class II expression, often to a greater extent than that observed in WT mice (Fig. 9 and data not shown). Together these findings suggest that both Th1 and Th17 cells play an important role in eliciting maximal innate immune responses to facilitate bacterial clearance during later stages of brain abscess development.
Figure 8. Bacterial clearance is enhanced in TCR αβ KO mice following Th1 or Th17 adoptive transfer.
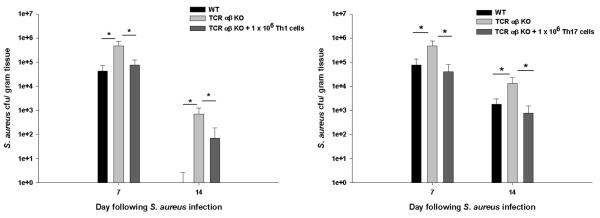
Brain abscesses were induced in WT, TCR αβ KO, and TCR αβ KO mice that received an adoptive transfer of either 106 in vitro skewed Th1 or Th17 cells 24 h prior to S. aureus infection (n= 4-5/group). Animals were euthanized at the indicated time points post-infection, whereupon bacterial burdens were quantitated and normalized to tissue wet weight (g). Significant differences between PBS-injected TCR αβ KO mice versus TCR αβ KO animals receiving adoptively transferred Th1 or Th17 cells are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
Figure 9. Th1 and Th17 cells regulate neutrophil and macrophage infiltrates during late stage brain abscess development.
Abscess-associated cells were collected from WT, TCR αβ KO, and TCR αβ KO mice that received an adoptive transfer of either 106 in vitro skewed Th1 or Th17 cells 24 h prior to S. aureus infection (n= 4-5/group). The absolute numbers of neutrophils (Ly-6G+, F4/80−, CD45hi) and macrophages (F4/80+, CD45hi) were quantitated by FACS. Significant differences between TCR αβ KO versus WT or TCR αβ KO mice receiving Th1 or Th17 cells are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
Th17 cells demonstrate plasticity following CNS infection
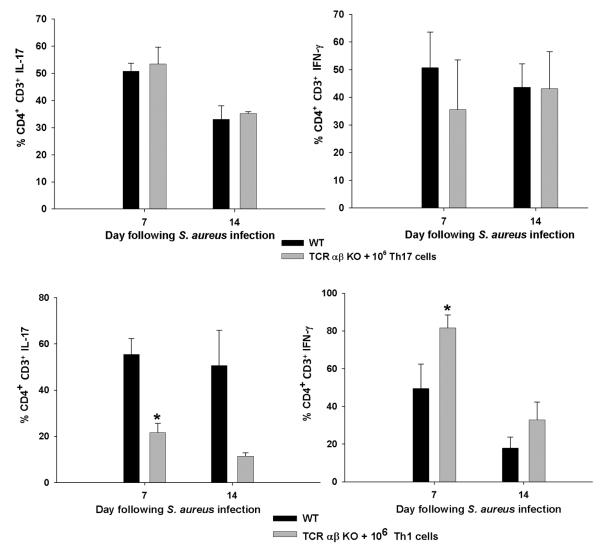
Interestingly, Th17 adoptive transfer was highly effective at promoting bacterial clearance in TCR αβ KO mice, particularly at day 14 post-infection (Fig. 8). This may be attributed to the more plastic nature of Th17 cells and their ability to acquire IFN-γ production when exposed to high levels of IL-12 and IFN-γ (30, 31). Indeed, this possibility was supported in our studies where a significant proportion of adoptively transferred Th17 cells, recovered from brain abscesses at days 7 and 14 post-infection, exhibited IFN-γ production (Fig. 10 and Supplemental Fig. 3). In contrast, the percentages of infiltrating Th1 cells remained similar to those originally transferred (Fig. 10 and Supplemental Fig. 4), which supports their relatively stable phenotype as described in the literature (32). Although a population of IFN-γ/IL-17 double-positive cells was observed during both Th1 and Th17 polarizing conditions in vitro, the frequency of double-positive CD4 T cells recovered from the infected brain following Th17 transfer was not significantly different (Supplemental Fig. 3). This finding suggests that the apparent plasticity of Th17 cells during CNS infection is not due to increased numbers of highly IFN-γ/IL-17 double-positive cells. The low levels of IL-17+ cells detected in brain abscesses of TCR αβ KO mice following Th1 transfer (Fig. 10) is likely due to the fact that a small percentage of adoptively transferred cells also produced IL-17 following in vitro skewing (Supplemental Fig. 3).
Figure 10. Th17 cells display plasticity in vivo by restoring both IL-17 and IFN-γ populations in brain abscesses of TCR αβ KO mice.
CD3+CD4+ T cells were recovered by FACS from brain abscesses of WT or TCR αβ KO mice receiving Th1 or Th17 adoptive transfer, whereupon T cells were stimulated immediately ex vivo with PMA + ionomycin for 3 h and stained for IL-17 and IFN-γ. Significant differences between the percentages of CD4+ IL-17 or IFN-γ positive cells in WT versus TCR αβ KO mice are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
Th1 and Th17 cells differentially influence inflammatory mediator secretion profiles in microglia and macrophages
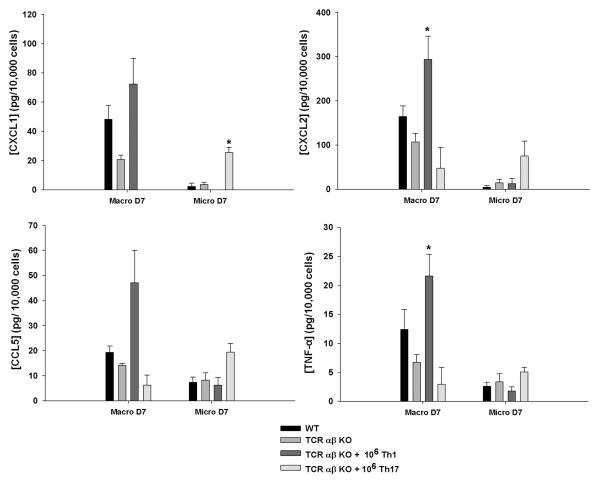
Although adoptive transfer of either Th1 or Th17 cells was capable of restoring defects in macrophage recruitment into brain abscesses of TCR αβ KO mice, it was not known whether these Th subsets would lead to differential secretory profiles of infiltrating macrophages or resident microglia in vivo. To address this question, microglia and macrophages were isolated from brain abscesses of WT, TCR αβ KO, and TCR αβ KO mice receiving adoptively transferred Th1 or Th17 cells by FACS, whereupon cells were incubated in vitro for a 24 h period without bacterial re-stimulation, in an attempt to capture cellular activation states that were established in vivo. Interestingly, abscess-associated macrophages appeared to be most affected by TCR αβ loss, as reflected by reductions in CXCL1, CXCL2, and TNF-α expression, whereas microglial secretory activity was more refractory to TCR αβ cell loss (Fig. 11).
Figure 11. Th1 and Th17 cells elicit distinct inflammatory mediator secretion profiles in abscess-associated macrophages and microglia.
Macrophages (F4/80+, CD45hi) and microglia (F4/80+, CD45lo-intermediate) were isolated by FACS from brain abscesses of WT, TCR αβ KO, or TCR αβ KO mice after adoptive transfer of Th1 or Th17 cells at day 7 post-infection. Abscess-associated macrophages and microglia were incubated for 24 h in vitro without bacterial re-stimulation, in an attempt to capture cellular activation states that were established in vivo, whereupon inflammatory mediator expression was evaluated by microbead array analysis. Results represent the amount of inflammatory mediator expression normalized per 104 cells. Significant differences in mediator production by macrophages or microglia recovered from PBS-injected TCR αβ KO mice versus TCR αβ KO animals receiving Th1 or Th17 cell transfer are denoted by asterisks (*, p < 0.05). Results represent the mean ± SEM combined from three independent experiments.
Interestingly, Th1 adoptive transfer significantly augmented macrophage activation as revealed by CXCL1, CXCL2, CCL5, and TNF-α release equal or exceeding WT levels, whereas Th17 cells had little effect (Fig. 11). In contrast, Th17 cells increased microglial cytokine/chemokine release but Th1 cells had minimal impact (Fig. 11). Significant proinflammatory mediator release was only detected in macrophages and microglia recovered from brain abscesses at day 7 but not day 14 following infection (Fig. 11 and data not shown, respectively), which may be a consequence of waning cell activation associated with declining bacterial burdens or alternatively, mediator levels falling below the limit of detection. Collectively, these results indicate that Th1 and Th17 cells target distinct antigen presenting cell populations in the context of established CNS infection.
DISCUSSION
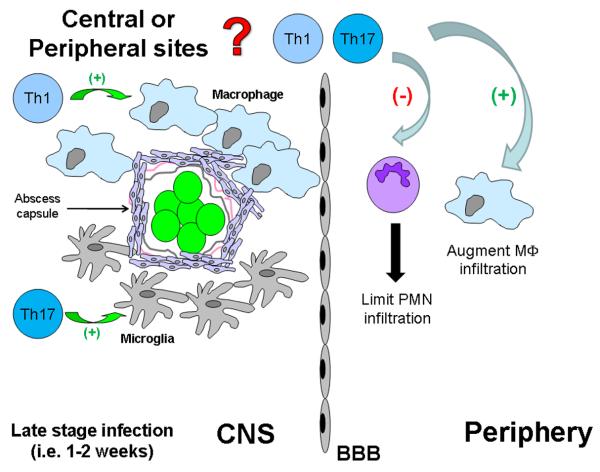
Although it is well established that innate immunity is required for optimal establishment of adaptive immune responses, comparatively fewer studies have examined the reciprocal relationship. The latter has received greater attention recently with the identification of Th17 cells regulating innate immune mechanisms (33-35). However, to the best of our knowledge there is no available information describing the ability of adaptive immunity to shape ongoing innate immune responses during CNS bacterial infection, which warrants investigation since a robust innate immune response is essential for efficient bacterial clearance (10, 11, 14). The current study revealed cross-talk between the adaptive and innate arms during CNS infection by demonstrating that Th1 and Th17 cells play an important role in expediting bacterial clearance and impacting neutrophil and macrophage recruitment and activation status (Fig. 12). In establishing that T cells positively regulate ongoing innate immune responses during brain abscess development, it may be possible to manipulate their activity to expedite bacterial clearance. This rapid response may equate to a reduction in tissue necrosis and decline in long-term neurological deficits that often accompany patients who recover from brain abscesses (2-4).
Figure 12. Th1 and Th17 cells impact established innate immune responses during later stages of brain abscess development.
CD3+CD4+ Th1 and Th17 cells regulate neutrophil and macrophage infiltrates during later stages of CNS parenchymal infection to expedite bacterial clearance. In addition, Th1 cells target infiltrating macrophages to regulate inflammatory mediator release, whereas Th17 cells appear to preferentially affect microglia. It remains to be determined whether these events are antigen-specific or instead result from the intense chemokine gradient generated during infection and non-specific T cell activation by staphylococcal superantigens.
Interestingly, our data demonstrated Th17 plasticity within the infected brain, which to our knowledge represents the first report of this process during CNS bacterial infection. Specifically, following Th17 transfer, the frequency of Th1 cells associated with brain abscesses was significantly increased compared to the smaller percentage of IFN-γ producing cells that were originally injected along with Th17 cells (since a 100% pure population of Th17 cells could not be attained with currently available cytokine cocktails). In contrast, the percentages of Th1 cells recovered from brain abscesses nearly equaled those that were originally transferred (Supplemental Fig. 4). These findings are in agreement with recent studies in models of experimental autoimmune encephalomyelitis where Th17 cells were found to acquire IFN-γ production, while Th1 cells were shown to be a more stable phenotype (30).
Currently, it is not possible to evaluate the impact of S. aureus-specific Th1 or Th17 cells in TCR αβ KO mice because immunodominant S. aureus antigens remain to be defined. Therefore, our approach was to expand naïve CD4+ cells from mice without prior S. aureus infection and skew cells towards a Th1 or Th17 phenotype in vitro prior to adoptive transfer into TCR αβ KO mice. Because of the strong chemokine gradients generated during infection, we cannot assess the impact of S. aureus-reactive versus non-specific T cell infiltrates that enter the infected CNS. In addition, it is important to note that S. aureus produces numerous superantigens that lead to the clonal activation of T cell subsets bearing specific Vβ TCRs. Indeed, a recent study from our laboratory has shown the preferential accumulation of Vβ8.2+ T cells in brain abscesses that are reactive with staphylococcal enterotoxin B (21, 36). Importantly, we also found that T cells are highly activated within brain abscesses and upon isolation continue to proliferate at least once in vitro without further re-stimulation (data not shown), suggesting the presence of potent T cell proliferation signals in vivo. This possibility is also strengthened by the fact that we only transferred a total of 106 Th1 or Th17 cells into TCR αβ KO mice, yet significant effects on innate immune mechanisms and bacterial burdens were observed, implying their in vivo expansion. However, it is likely that T cells were also driven to expand via homeostatic proliferation to fill the void in the T cell compartment in TCR αβ KO mice. Studies are currently in progress to evaluate whether adoptive transfer of CD4+ T cells from ovalbumin TCR transgenic mice impact innate responses during brain abscess development in TCR αβ KO mice to address the requirement for antigen specificity.
It is important to note similarities and differences between our model system and other recent studies examining CD4+ T cells and bacterial infections, since it emphasizes the importance of the site of infection and the immune cells that can most readily access various tissues. For example, Mc Loughlin et al. reported that abscesses did not form during S. aureus skin and soft tissue infections in TCR αβ KO mice, which were associated with efficient neutrophil recruitment and reduction in bacterial burdens (37, 38). In contrast, we showed that TCR αβ KO mice displayed impaired S. aureus clearance within the CNS, typified by enhanced neutrophil accumulation, likely in an attempt to contain the infection. It is important to note that the findings from Mc Loughlin et al. may be attributed to compensatory activity by epidermal γδ T cells, which can also produce IFN-γ and IL-17; however, this possibility was not examined by the authors. Indeed, we observed in the current study that γδ T cell influx was significantly elevated at later stages of brain abscess development in TCR αβ KO mice. The functional impact of these cells in controlling infection in the absence of other T cell populations remains uncertain. However, it is clear from our studies that CD4+ Th1 and Th17 cells are critical in shaping the intensity and duration of ongoing innate responses during late stage CNS infection. Although NKT cells can express CD4, their possible involvement was minimized during the sorting process by only collecting CD4+ NK1.1− cells. However, NKT cells have been reported to express several combinations of surface markers often with transient expression patterns and, therefore, a subset of NKT cells may still have been included in our adoptive transfer studies (18, 39). Because of this, a definitive role for NKT cells in regulating inflammation during brain abscess development is currently being explored in our laboratory using CD1d KO mice that lack all NKT subsets (40, 41).
Another intriguing finding was that Th1 and Th17 cells induced differential inflammatory secretion profiles in abscess-associated macrophages versus microglia. For example, adoptive transfer of Th1 cells led to enhanced chemokine and TNF-α production in macrophages, whereas microglia were not affected. In contrast, Th17 transfer led to increased mediator release from microglia but had minimal effects on macrophages. These findings indicate the existence of novel cross-talk between each Th subset and mononuclear phagocyte target; however, the specific modes of action responsible for these differences remain to be defined. Another interesting observation was that infiltrating macrophages were more sensitive to the loss of TCR αβ+ cells since inflammatory mediator release was dramatically reduced in macrophages recovered from abscesses of TCR αβ KO mice. In contrast, the extent of microglial activation, as measured by proinflammatory mediator release, was similar in microglia recovered from brain abscesses of WT and TCR αβ KO animals. Macrophage activation may be especially critical since the chemokines CXCL9 and CCL5 have also been shown to exhibit direct microbicidal activity (42-45) and are significantly attenuated in TCR αβ KO mice. Therefore, the reduced expression of these kinocidins may represent one mechanism responsible for the increased bacterial burdens observed in TCR αβ KO animals. Additionally, the marked decrease in MHC Class II expression in macrophages recovered from brain abscesses of TCR αβ KO mice is likely due to diminished IFN-γ levels, a major cytokine product of Th1 cells, which is known to upregulate MHC Class II expression. To our knowledge, this is the first report demonstrating differential effects of Th1 and Th17 cells on microglia versus macrophages during CNS infection, discerning the fact that specialized responses are triggered during inflammation. It will be interesting to determine whether these differences are localized to specific microdomains within the abscess environment; however, this question lies beyond the scope of the current report.
In summary, this study demonstrates the important role that adaptive immunity plays in shaping established innate immune responses during CNS infection. Specifically, we found that Th1 and Th17 cells facilitate bacterial clearance and neutrophil and macrophage infiltration/activation during the later stages of brain abscess formation. Importantly, Th17 cells infiltrating brain abscesses displayed plasticity and acquired the ability to produce IFN-γ. In addition, another novel aspect of our work was the finding that Th1 and Th17 cells provide distinct signals that culminate in unique secretory profiles of resident microglia and infiltrating macrophages. Collectively, this information could be used to heighten anti-microbial activity to expedite bacterial clearance from the CNS during infections concomitant with conventional antibiotic therapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Charles Kuszynski, Megan Michalak, and Victoria Smith in the UNMC Cell Analysis Facility for assistance with FACS analysis, Debbie Vidlak for performing Milli-Plex assays, and Dr. Costi Sifri for the USA300 isolate. We acknowledge the NIH Tetramer Core Facility at Emory University for providing the mCD1d-PE tetramer and Dr. Mark Hanke and Kari Nelson for editorial review of the manuscript.
Footnotes
This work was supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS) R01 NS040730 to T.K. and F31 NS070455 to M.M.H.
REFERENCES
- 1.Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- 2.Greenberg BM. Central nervous system infections in the intensive care unit. Semin Neurol. 2008;28:682–689. doi: 10.1055/s-0028-1105976. [DOI] [PubMed] [Google Scholar]
- 3.Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979–985. doi: 10.1016/j.jocn.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 4.Davis LE, Baldwin NG. Brain Abscess. Curr Treat Options Neurol. 1999;1:157–166. doi: 10.1007/s11940-999-0015-7. [DOI] [PubMed] [Google Scholar]
- 5.Jones ME, Draghi DC, Karlowsky JA, Sahm DF, Bradley JS. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann Clin Microbiol Antimicrob. 2004;3:3. doi: 10.1186/1476-0711-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naesens R, Ronsyn M, Druwe P, Denis O, Ieven M, Jeurissen A. Central nervous system invasion by community-acquired meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2009;58:1247–1251. doi: 10.1099/jmm.0.011130-0. [DOI] [PubMed] [Google Scholar]
- 7.Khan MA, Greig JR, Jayamohan J. Community-acquired methicillin-resistant Staphylococcus aureus brain abscess in an immunocompetent individual. Scand J Infect Dis. 2000;32:423–424. doi: 10.1080/003655400750045042. [DOI] [PubMed] [Google Scholar]
- 8.Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schluter D, Deckert M. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008;172:132–145. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157:647–658. doi: 10.1016/S0002-9440(10)64575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. doi: 10.1016/j.jneuroim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Garg S, Nichols JR, Esen N, Liu S, Phulwani NK, Syed MM, Wood WH, Zhang Y, Becker KG, Aldrich A, Kielian T. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro. 2009:1. doi: 10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scurlock AM, Frazer LC, Andrews CW, Jr., O’Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 18.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 19.Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr Biol. 2005;15:R429–431. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Nichols JR, Aldrich AL, Mariani MM, Vidlak D, Esen N, Kielian T. TLR2 deficiency leads to increased Th17 infiltrates in experimental brain abscesses. J Immunol. 2009;182:7119–7130. doi: 10.4049/jimmunol.0802656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidlak D, Mariani MM, Aldrich A, Liu S, Kielian T. Roles of Toll-like receptor 2 (TLR2) and superantigens on adaptive immune responses during CNS staphylococcal infection. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin Infect Dis. 2007;45:e113–117. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- 23.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 25.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 26.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- 29.Born WK, Yin Z, Hahn YS, Sun D, O’Brien RL. Analysis of gamma delta T cell functions in the mouse. J Immunol. 2010;184:4055–4061. doi: 10.4049/jimmunol.0903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurschus FC, Croxford AL, Heinen AP, Wortge S, Ielo D, Waisman A. Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3336–3346. doi: 10.1002/eji.201040755. [DOI] [PubMed] [Google Scholar]
- 31.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78:32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol. 2011 doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A. 2006;103:10408–10413. doi: 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weidenmaier C, McLoughlin RM, Lee JC. The zwitterionic cell wall teichoic acid of Staphylococcus aureus provokes skin abscesses in mice by a novel CD4+ T-cell-dependent mechanism. PLoS One. 2010;5:e13227. doi: 10.1371/journal.pone.0013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vosshenrich CA, Samson-Villeger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–158. doi: 10.1016/j.coi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, Garcia J, Wang C, Patten K, Stills HF, Alt FW, Snapper SB, Balk SP. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeaman MR. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–968. doi: 10.1086/516120. quiz 969-970. [DOI] [PubMed] [Google Scholar]
- 43.Yeaman MR, Yount NY. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5:727–740. doi: 10.1038/nrmicro1744. [DOI] [PubMed] [Google Scholar]
- 44.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 45.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.