Abstract
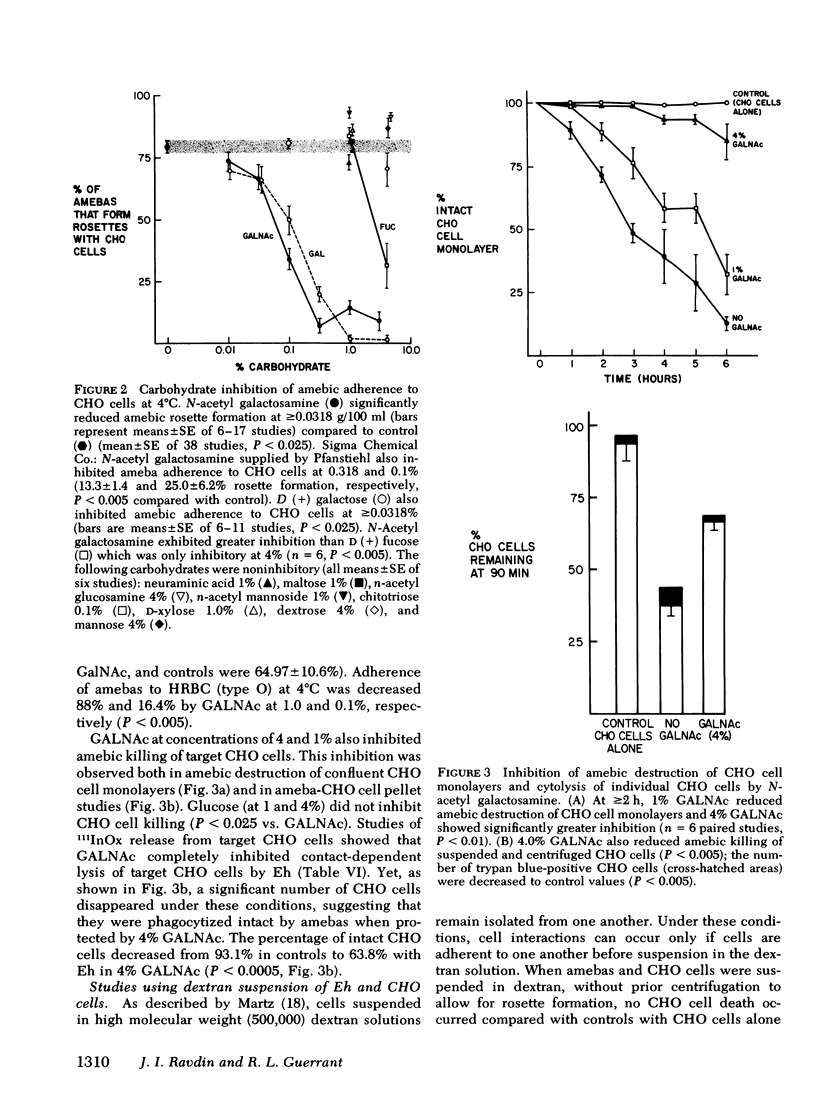
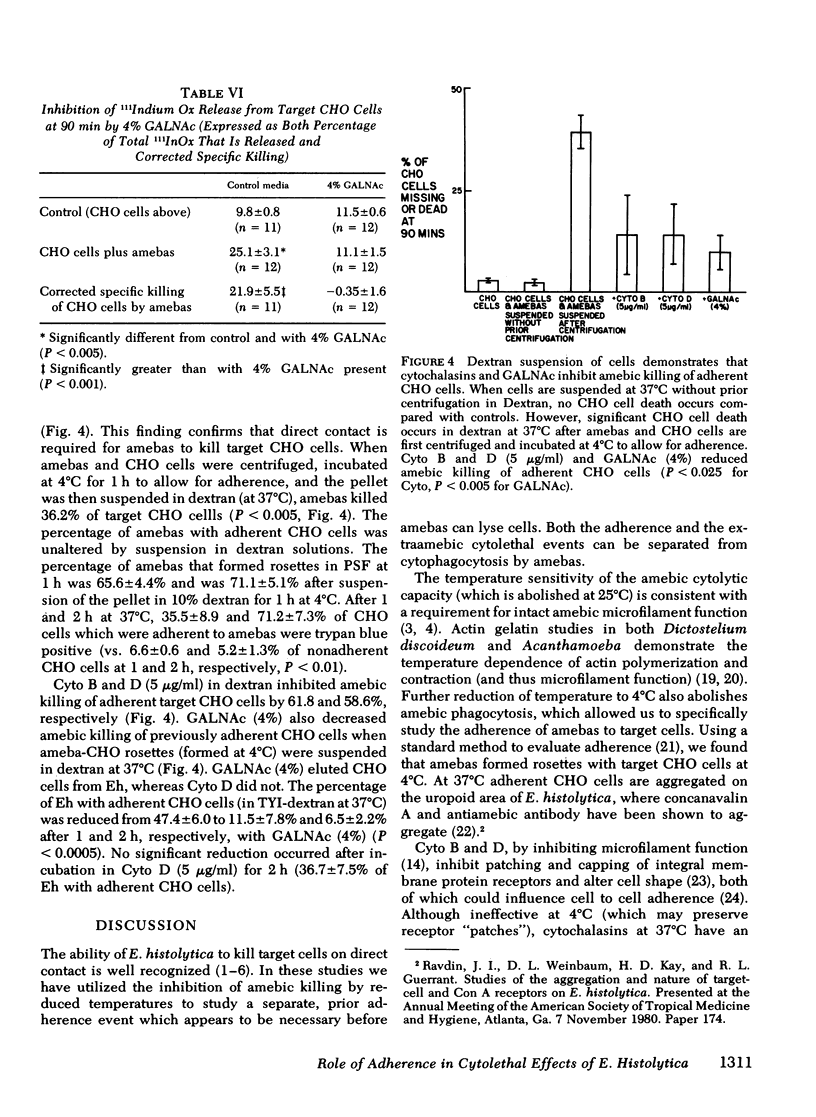
The enteric pathogen, Entamoeba histolytica, appears to cause disease by adhering to and then destroying mucosal barriers. Using an in vitro method of studying the interaction of E. histolytica with target cells (Chinese hamster ovary [CHO] and human erythrocytes [RBC]), we examined the mechanism of amebic adherence and its role in lysis of target cells. Killing and phagocytosis of target cells by amebas ceases at 4°C, allowing observation of adherence. Amebas adhere to CHO cells at 4°C, 78.9% formed rosettes (amebas with ≥3 adherent CHO cells each) at 2 h. At 37°C, cytochalasins B and D inhibit adherence of amebas to CHO cells (P < 0.0005). Amebas adhere to and kill CHO cells in media with <0.1 μM calcium and magnesium plus 10 mM EDTA, indicating that divalent cations are not required in the medium. Adherence of amebas to human RBC was not ABO blood group specific and showed greater adherence to human than bovine or sheep RBC (P < 0.005). Neither Fc nor complement receptors were found on amebas by standard rosette studies. The amebic adherence receptor is not trypsin (0.125%) sensitive nor inhibited by trypan blue (1 mM). N-acetyl-d-galactosamine (GALNAc) inhibited the adherence of amebas to CHO cells and human RBC (0.1 g/100 ml or 4.5 mM GALNAc, P < 0.005) by binding to a receptor on the amebic surface. GALNAc abolishes amebic cytolysis of target CHO cells (determined by 111Indium oxine release from CHO cells, P < 0.001) but not amebic phagocytosis of CHO cells. By suspending ameba-CHO cells rosettes in dextran, we found that GALNAc (1%) reversibly inhibits amebic adherence (P < 0.0005) and that cytochalasins decrease amebic killing of adherent CHO cells (P < 0.025).
These findings indicate that the adherence of E. histolytica to target cells requires microfilament function, is via a specific amebic receptor that has affinity for GALNAc, and is required to lyse cells. Inhibition of the adherence of E. histolytica may alter the pathogenicity of this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck F., Lloyd J. B., Griffiths A. Lysosomal enzyme inhibition by trypan blue: a theory of teratogenesis. Science. 1967 Sep 8;157(3793):1180–1182. doi: 10.1126/science.157.3793.1180. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Calderón J., de Lourdes Muñoz M., Acosta H. M. Surface redistribution and release of antibody-induced caps in entamoebae. J Exp Med. 1980 Jan 1;151(1):184–193. doi: 10.1084/jem.151.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Condeelis J. S., Taylor D. L. The contractile basis of amoeboid movement. V. The control of gelation, solation, and contraction in extracts from Dictyostelium discoideum. J Cell Biol. 1977 Sep;74(3):901–927. doi: 10.1083/jcb.74.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg J. L. The biochemistry of intercellular recognition. Adv Comp Physiol Biochem. 1978;7:105–226. doi: 10.1016/b978-0-12-011507-5.50008-2. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Eaton R. D., Meerovitch E., Costerton J. W. The functional morphology of pathogenicity in Entamoeba histolytica. Ann Trop Med Parasitol. 1970 Sep;64(3):299–304. doi: 10.1080/00034983.1970.11686695. [DOI] [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Attachment and short-term maintenance of motility and viability of Entamoeba histolytica in a defined medium. J Protozool. 1980 May;27(2):220–225. doi: 10.1111/j.1550-7408.1980.tb04685.x. [DOI] [PubMed] [Google Scholar]
- Guckian J. C., Christensen W. D., Fine D. P. Trypan blue inhibits complement-mediated phagocytosis by human polymorphonuclear leukocytes. J Immunol. 1978 May;120(5):1580–1586. [PubMed] [Google Scholar]
- Guerrant R. L., Brush J., Ravdin J. I., Sullivan J. A., Mandell G. L. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981 Jan;143(1):83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Heterocytolysis by macrophages activated by bacillus Calmette-Guérin: lysosome exocytosis into tumor cells. Science. 1974 Apr 26;184(4135):468–471. doi: 10.1126/science.184.4135.468. [DOI] [PubMed] [Google Scholar]
- JARUMILINTA R., KRADOLFER F. THE TOXIC EFFECT OF ENTAMOEBA HISTOLYTICA ON LEUCOCYTES. Ann Trop Med Parasitol. 1964 Sep;58:375–381. doi: 10.1080/00034983.1964.11686259. [DOI] [PubMed] [Google Scholar]
- Kay H. D., Fagnani R., Bonnard G. D. Cytotoxicity against the K562 erythroleukemia cell line by human natural killer (NK) cells which do not bear free Fc receptors for IgG. Int J Cancer. 1979 Aug;24(2):141–150. doi: 10.1002/ijc.2910240204. [DOI] [PubMed] [Google Scholar]
- Knight R., Bird R. G., McCaul T. F. Fine structural changes at Entamoeba histolytica rabbit kidney cell (RK 13) interface. Ann Trop Med Parasitol. 1975 Jun;69(2):197–202. doi: 10.1080/00034983.1975.11687001. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Mirelman D. Lectin activity in Entamoeba histolytica trophozoites. Infect Immun. 1980 Jul;29(1):221–225. doi: 10.1128/iai.29.1.221-225.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz E. Mechanism of specific tumor-cell lysis by alloimmune T lymphocytes: resolution and characterization of discrete steps in the cellular interaction. Contemp Top Immunobiol. 1977;7:301–361. doi: 10.1007/978-1-4684-3054-7_9. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Keister D. B., Natovitz P. C. Entamoeba histolytica "toxin": fetuin neutralizable and lectin-like. Am J Trop Med Hyg. 1980 Jan;29(1):26–30. doi: 10.4269/ajtmh.1980.29.26. [DOI] [PubMed] [Google Scholar]
- McCaul T. F., Bird R. G. Surface features of Entamoeba histolytica and rabbit kidney (RK13) cell surface changes after trophozoite contact--observations by scanning electron microscopy. Int J Parasitol. 1977 Oct;7(5):383–388. doi: 10.1016/0020-7519(77)90063-7. [DOI] [PubMed] [Google Scholar]
- McCaul T. F., Poston R. N., Bird R. G. Entamoeba histolytica and Entamoeba invadens: chromium release from labeled human liver cells in culture. Exp Parasitol. 1977 Dec;43(2):342–352. doi: 10.1016/0014-4894(77)90039-x. [DOI] [PubMed] [Google Scholar]
- Mora Galindo J., Martinez-Palomo A., Chavez B. Interacción entre Entamoeba histolytica y el epitelio cecal del cobayo. Arch Invest Med (Mex) 1978;9 (Suppl 1):261–274. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Plaut M., Bubbers J. E., Henney C. S. Studies of the mechanism of lymphocyte-mediated cytolysis. VII. Two stages in the T cell-mediated lytic cycle with distinct cation requirements. J Immunol. 1976 Jan;116(1):150–155. [PubMed] [Google Scholar]
- Pollard T. D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol. 1976 Mar;68(3):579–601. doi: 10.1083/jcb.68.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Reitherman R. W., Barondes S. H. Distinct lectin activities from six species of cellular slime molds. Exp Cell Res. 1975 Oct 1;95(1):159–166. doi: 10.1016/0014-4827(75)90621-7. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Piessens W. F. Tumor cell killing by macrophages activated in vitro with lymphocyte mediators. III. Inhibition by cytochalasins, colchicine, and vinblastine. Cell Immunol. 1978 Jul;38(2):276–285. doi: 10.1016/0008-8749(78)90058-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Phillips B. P. Electron microscope studies of experimental Entamoeba histolytica infection in the guinea pig. I. Penetration of the intestinal epithelium by trophozoites. Am J Trop Med Hyg. 1975 Jan;24(1):34–48. doi: 10.4269/ajtmh.1975.24.34. [DOI] [PubMed] [Google Scholar]
- Trissl D., Martínez-Palomo A., de la Torre M., de la Hoz R., Pérez de Suárez E. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978 Nov 1;148(5):1137–1143. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Frost P., Cummings G. D. Isotope-release cytotoxicity assay with the use of indium-111: advantage over chromium-51 in long-term assays. J Natl Cancer Inst. 1978 Jul;61(1):183–188. doi: 10.1093/jnci/61.1.183. [DOI] [PubMed] [Google Scholar]



