Abstract
A murine monoclonal antibody (OC125) has been developed that reacts with each of six epithelial ovarian carcinoma cell lines and with cryopreserved tumor tissue from 12 of 20 ovarian cancer patients. By contrast, the antibody does not bind to a variety of nonmalignant tissues, including adult and fetal ovary. OC125 reacts with only 1 of 14 cell lines derived from nonovarian neoplasms and has failed to react with cryostat sections from 12 nonovarian carcinomas.
Full text
PDF

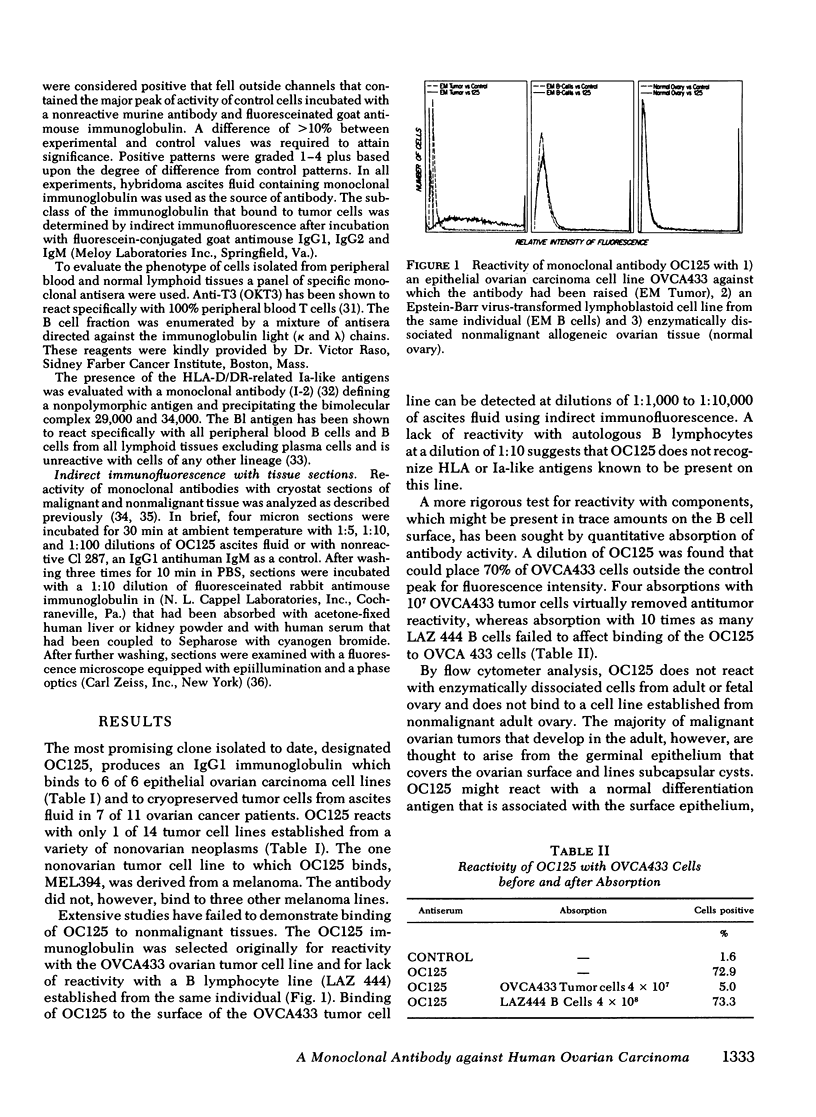
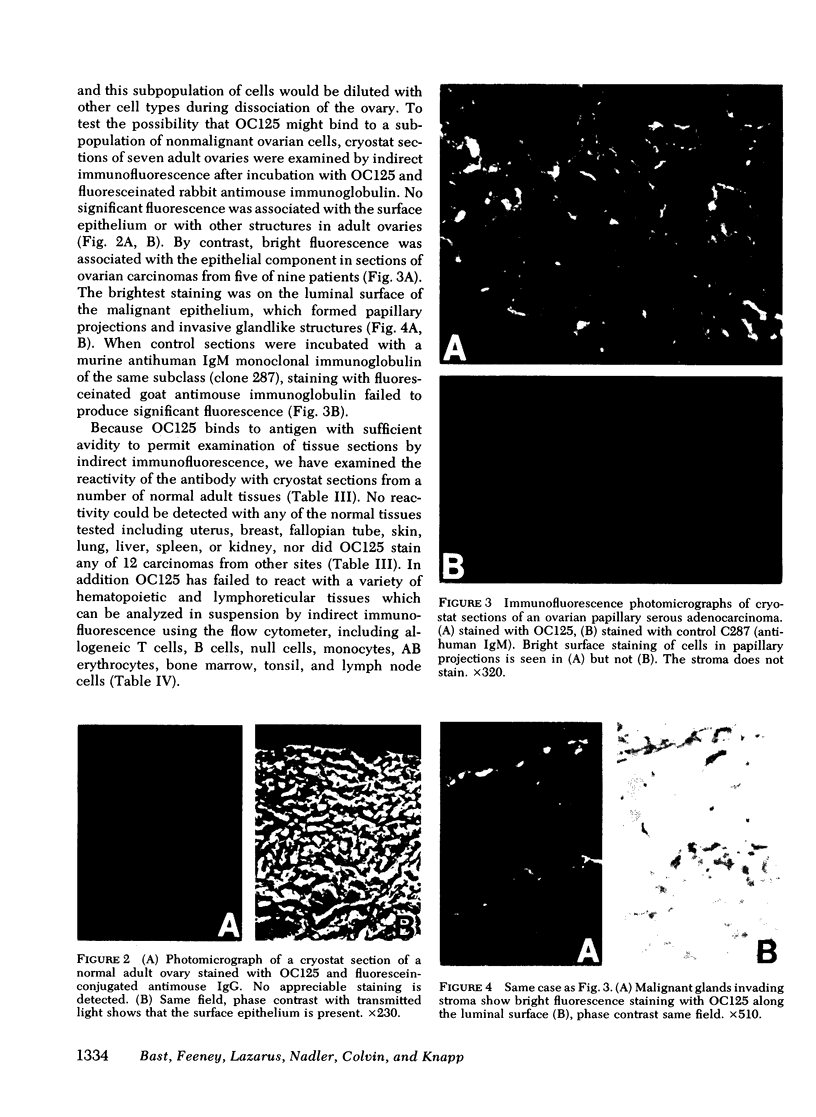



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Knapp R. C., Donahue V. C., Thurston J. G., Mitchell A. K., Feeney M., Schlossman S. F. Specificity of heteroantisera developed against purified populations of intact murine ovarian carcinoma cells. J Natl Cancer Inst. 1980 Feb;64(2):365–372. doi: 10.1093/jnci/64.2.365. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Knapp R. C., Mitchell A. K., Thurston J. G., Tucker R. W., Schlossman S. F. Immunotherapy of a murine ovarian carcinoma with Corynebacterium parvum and specific heteroantiserum. I. Activation of peritoneal cells to mediate antibody-dependent cytotoxicity. J Immunol. 1979 Nov;123(5):1945–1951. [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Colvin R. B., Gardner P. I., Roblin R. O., Verderber E. L., Lanigan J. M., Mosesson M. W. Cell surface fibrinogen-fibrin receptors on cultured human fibroblasts. Association with fibronectin (cold insoluble globulin, LETS protein) and loss in SV40 transformed cells. Lab Invest. 1979 Nov;41(5):464–473. [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. B., GRAHAM R. M. Autogenous vaccine in cancer patients. Surg Gynecol Obstet. 1962 Jan;114:1–4. [PubMed] [Google Scholar]
- Gall S. A., Walling J., Pearl J. Demonstration of tumor-associated antigens in human gynecologic malignancies. Am J Obstet Gynecol. 1973 Feb 1;115(3):387–393. doi: 10.1016/0002-9378(73)90595-4. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C. N., McHardy J. E., Curling O. M., English P. E., Levin L., Poulton T. A., Crowther M., Leighton M. Active specific immunotherapy for ovarian cancer. Lancet. 1976 Oct 23;2(7991):877–879. doi: 10.1016/s0140-6736(76)90539-0. [DOI] [PubMed] [Google Scholar]
- Imamura N., Takahashi T., Lloyd K. O., Lewis J. L., Jr, Old L. J. Analysis of human ovarian tumor antigens using heterologous antisera: detection of new antigenic systems. Int J Cancer. 1978 May 15;21(5):570–577. doi: 10.1002/ijc.2910210506. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Knauf S., Urbach G. I. Purification of human ovarian tumor-associated antigen and demonstration of circulating tumor antigen in patients with advanced ovarian malignancy. Am J Obstet Gynecol. 1977 Apr 1;127(7):705–710. doi: 10.1016/0002-9378(77)90242-3. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Herlyn D., Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Levi M. M., Keller S., Mandl I. Antigenicity of a papillary serous cystadenocarcinoma tissue homogenate and its fractions. Am J Obstet Gynecol. 1969 Nov 15;105(6):856–861. doi: 10.1016/0002-9378(69)90091-x. [DOI] [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Mendes N. F., Tolnai M. E., Silveira N. P., Gilbertsen R. B., Metzgar R. S. Technical aspects of the rosette tests used to detect human complement receptor (B) and sheep erythrocyte-binding (T) lymphocytes. J Immunol. 1973 Sep;111(3):860–867. [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Schlossman S. F. A monoclonal antibody defining a lymphoma-associated antigen in man. J Immunol. 1980 Aug;125(2):570–577. [PubMed] [Google Scholar]
- Order S. E., Donahue V., Knapp R. Immunotherapy of ovarian carcinoma. An experimental model. Cancer. 1973 Sep;32(3):573–579. doi: 10.1002/1097-0142(197309)32:3<573::aid-cncr2820320309>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Order S. E., Kirkman R., Knapp R. Serologic immunotherapy: results and probable mechanism of action. Cancer. 1974 Jul;34(1):175–183. doi: 10.1002/1097-0142(197407)34:1<175::aid-cncr2820340127>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Order S. E., Rosenshein N. B., Klein J. L., Lichter A. S., Ettinger D. S., Dillon M. B., Leibel S. A. New methods applied to the analysis and treatment of ovarian cancer. Int J Radiat Oncol Biol Phys. 1979 Jun;5(6):861–873. doi: 10.1016/0360-3016(79)90071-3. [DOI] [PubMed] [Google Scholar]
- Order S. E., Thurnston J., Knapp R. Ovarian tumor antigens: a new potential for therapy. Natl Cancer Inst Monogr. 1975 Oct;42:33–43. [PubMed] [Google Scholar]
- Parker L. M., Griffiths C. T., Yankee R. A., Canellos G. P., Gelman R., Knapp R. C., Richman C. M., Tobias J. S., Weiner R. S., Frei E., 3rd Combination chemotherapy with adriamycin-cyclophosphamide for advanced ovarian carcinoma. Cancer. 1980 Aug 15;46(4):669–674. doi: 10.1002/1097-0142(19800815)46:4<669::aid-cncr2820460407>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rao B., Wanebo H. J., Ochoa M., Jr, Lewis J. L., Jr, Oettgen H. F. Intravenous Corynebacterium parvum: an adjunct to chemotherapy for resistant advanced ovarian cancer. Cancer. 1977 Feb;39(2):514–526. doi: 10.1002/1097-0142(197702)39:2<514::aid-cncr2820390220>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Nadler L. M., Sallan S. E., Schlossman S. F. Subset derivation of T-cell acute lymphoblastic leukemia in man. J Clin Invest. 1979 Aug;64(2):392–397. doi: 10.1172/JCI109474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber J., Lazarus H., Rosen F. S. Bone marrow-derived lymphoid cell lines from patients with agammaglobulinemia. J Clin Invest. 1978 Aug;62(2):302–310. doi: 10.1172/JCI109130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Tobias J. S., Griffiths C. T. Management of ovarian carcinoma. Current concepts and future prospects (first of two parts). N Engl J Med. 1976 Apr 8;294(15):818–822. doi: 10.1056/NEJM197604082941506. [DOI] [PubMed] [Google Scholar]
- Webb H. E., Oaten S. W., Pike C. P. Treatment of malignant ascitic and pleural effusion with Corynebacterium parvum. Br Med J. 1978 Feb 11;1(6109):338–340. doi: 10.1136/bmj.1.6109.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh M. Y., Hellström I., Brown J. P., Warner G. A., Hansen J. A., Hellström K. E. Cell surface antigens of human melanoma identified by monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2927–2931. doi: 10.1073/pnas.76.6.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Chabner B. A., Hubbard S. P., Fisher R. I., Bender R. A., Anderson T., Simon R. M., Canellos G. P., DeVita V. T., Jr Advanced ovarian adenocarcinoma. A prospective clinical trial of melphalan (L-PAM) versus combination chemotherapy. N Engl J Med. 1978 Dec 7;299(23):1261–1266. doi: 10.1056/NEJM197812072992301. [DOI] [PubMed] [Google Scholar]
- van Nagell J. R., Jr, Donaldson E. S., Gay E. C., Sharkey R. M., Rayburn P., Goldenberg D. M. Carcinoembryonic antigen in ovarian epithelial cystadenocarcinomas: the prognostic value of tumor and serial plasma determinations. Cancer. 1978 Jun;41(6):2335–2340. doi: 10.1002/1097-0142(197806)41:6<2335::aid-cncr2820410636>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]





