Abstract
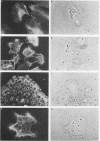
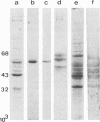
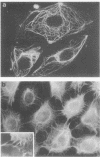
Cells cultured from second trimester human amniotic fluid were characterized in indirect immunofluorescence (IIF) microscopy using specific antibodies against the subunit proteins of different types of cytoskeletal intermediate filaments. Most of the amniotic fluid cell cultures contained only epithelial cells as indicated by the positive keratin-fluorescence in IIF. Five distinct types of keratin-positive cells could be characterized. A dominating cell type (E-1) in most cultures were rapidly proliferating epithelial cells, previously called amniotic fluid cells (AF-cells). These cells showed a fibrillar cytoplasmic fluorescence both with keratin antibodies and with antibodies against vimentin, the fibroblast type of intermediate filament protein. E-1 cells did not show the typical cell-to-cell arrangement of keratin fibrils between the adjacent cells, a characteristic previously found in most cultured epithelial cells. Most of the cultures also contained large epitheloid cells (E-2), showing a fine fibrillar cytoplasmic organization of both keratin- and vimentin filaments, clearly different from that seen in E-1 cells. Several cultures contained two additional epithelial cells both showing the typical cell-to-cell arrangement of keratin fibrils (E-3 and E-4). These two cell types could be distinguished because of their distinct difference in size. E-4 cells typically grew as small cell islands among other epitheloid cells. Amniotic fluid cell cultures occasionally contained also large multinucleated cells (E-5), which appeared to contain large amount of fibrillar keratin. Fibroblastic cells, identified by their decoration only with antibodies against vimentin, were rarely found in amniotic fluid cell cultures. Interestingly, in such cultures some cells with a fibroblastoid appearance were identified as epithelial cells on the basis of the positive keratin-fluorescence. The results show the suitability of IIF with cytoskeletal antibodies in characterization of heterogenous cell populations and indicate that normal amniotic fluid cell cultures mostly contain epithelial cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Lloyd C. W., Woods A., Carruthers L., Allcock C., Rees D. A. Mechanisms of cellular adhesion. III. Preparation and preliminary characterisation of adhesions. Exp Cell Res. 1978 Dec;117(2):231–244. doi: 10.1016/0014-4827(78)90136-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Crouch E., Balian G., Holbrook K., Duksin D., Bornstein P. Amniotic fluid fibronectin. Characterization and synthesis by cells in culture. J Cell Biol. 1978 Sep;78(3):701–715. doi: 10.1083/jcb.78.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Bornstein P. Characterization of a type IV procollagen synthesized by human amniotic fluid cells in culture. J Biol Chem. 1979 May 25;254(10):4197–4204. [PubMed] [Google Scholar]
- Crouch E., Bornstein P. Collagen synthesis by human amniotic fluid cells in culture: characterization of a procollagen with three identical proalpha1(I) chains. Biochemistry. 1978 Dec 12;17(25):5499–5509. doi: 10.1021/bi00618a027. [DOI] [PubMed] [Google Scholar]
- Crouch E., Sage H., Bornstein P. Structural basis for apparent heterogeneity of collagens in human basement membranes: type IV procollagen contains two distinct chains. Proc Natl Acad Sci U S A. 1980 Feb;77(2):745–749. doi: 10.1073/pnas.77.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix J. S., Littlefield J. W. Urinary tract epithelial cells cultured from human urine. Int Rev Cytol Suppl. 1979;(10):11–23. doi: 10.1016/s0074-7696(08)60609-9. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Gerbie A. B., Melancon S. B., Ryan C., Nadler H. L. Cultivated epithelial-like cells and fibroblasts from amniotic fluid: their relationship to enzymatic and cytologic analysis. Am J Obstet Gynecol. 1972 Oct 1;114(3):314–320. doi: 10.1016/0002-9378(72)90608-4. [DOI] [PubMed] [Google Scholar]
- Gosden C. M., Brock D. J. Morphology of rapidly adhering amniotic-fluid cells as an aid to the diagnosis of neural-tube defects. Lancet. 1977 Apr 30;1(8018):919–922. doi: 10.1016/s0140-6736(77)92221-8. [DOI] [PubMed] [Google Scholar]
- Hoehn H., Bryant E. M., Fantel A. G., Martin G. M. Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. III. The fetal urine as a potential source of clonable cells. Humangenetik. 1975 Oct 7;29(4):285–290. doi: 10.1007/BF00394190. [DOI] [PubMed] [Google Scholar]
- Hoehn H., Bryant E. M., Karp L. E., Martin G. M. Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. I. Clonal morphology and growth potential. Pediatr Res. 1974 Aug;8(8):746–754. doi: 10.1203/00006450-197408000-00003. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lehto V. P., Virtanen I. Keratin filaments of mouse epithelial cells are rapidly affected by epidermal growth factor. J Cell Biol. 1981 Aug;90(2):537–541. doi: 10.1083/jcb.90.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- Martin A. O. Characteristics of amniotic fluid cells in vitro and attempts to improve culture techniques. Clin Obstet Gynaecol. 1980 Apr;7(1):143–173. [PubMed] [Google Scholar]
- Megaw J. M., Priest J. H., Priest R. E., Johnson L. D. Differentiation in human amniotic fluid cell cultures: II: Secretion of an epithelial basement membrane glycoprotein. J Med Genet. 1977 Jun;14(3):163–167. doi: 10.1136/jmg.14.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon S. B., Lee S. Y., Nadler H. L. Histidase activity in cultivated human amniotic fluid cells. Science. 1971 Aug 13;173(3997):627–628. doi: 10.1126/science.173.3997.627. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Priest R. E., Marimuthu K. M., Priest J. H. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest. 1978 Aug;39(2):106–109. [PubMed] [Google Scholar]
- Priest R. E., Priest J. H., Laundon C. H., Snider P. W. Multinucleate cells in cultures of human amniotic fluid form by fusion. Lab Invest. 1980 Aug;43(2):140–144. [PubMed] [Google Scholar]
- Priest R. E., Priest J. H., Moinuddin J. F., Keyser A. J. Differentiation in human amniotic fluid cell cultures: I: Collagen production. J Med Genet. 1977 Jun;14(3):157–162. doi: 10.1136/jmg.14.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest R. E., Priest J. H., Moinuddin J. F., Sgoutas D. S. Differentiation in human amniotic fluid cell cultures: chorionic gonadotropin production. In Vitro. 1979 Feb;15(2):142–147. doi: 10.1007/BF02618111. [DOI] [PubMed] [Google Scholar]
- Schmid E., Tapscott S., Bennett G. S., Croop J., Fellini S. A., Holtzer H., Franke W. W. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation. 1979;15(1):27–40. doi: 10.1111/j.1432-0436.1979.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Screening for congenital hypothyroidism. Br Med J. 1980 Jul 5;281(6232):1–2. [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Liem R. K. Neurofilaments. J Neurochem. 1979 Jul;33(1):5–13. doi: 10.1111/j.1471-4159.1979.tb11699.x. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLE M., PENTTINEN K. Routine culture of human amnion cells. Ann Med Exp Biol Fenn. 1962;40:342–351. [PubMed] [Google Scholar]