Abstract
Background:
The standard triple therapy for the eradication of Helicobacter pylori consists of a combination of a proton pump inhibitor at a standard dose together with two antibiotics (amoxicillin 1000 mg plus either clarithromycin 500 mg or metronidazole 400 mg) all given twice daily for a period of 7-14 days. Recent reports have shown a dramatic decline in the rate of H. pylori eradication utilizing standard triple therapy from 95% down to 70-80%.
Aims:
Our study was designed to evaluate the effect of adding a probiotic as an adjuvant to common regimens used for H. pylori eradication.
Materials and Methods:
An open label randomized observational clinical study was designed to test three different regimens of H. pylori eradication treatment: Standard triple therapy with a concomitant probiotic added at the same time (n = 100), starting the probiotic for 2 weeks before initiating standard triple therapy along with the probiotic (n = 95), and the third regimen consists of the probiotic given concomitantly to sequential treatment (n = 76). The three arms were compared to a control group of patients treated with the traditional standard triple therapy (n = 106).
Results:
The eradication rate for the traditional standard therapy was 68.9%, and adding the probiotic “Bifidus infantis” to triple therapy, led to a successful rate of eradication of 83% (P < 0.001). Pre-treatment with 2 weeks of B. infantis before adding it to standard triple therapy increased the success rate of eradication to 90.5%. Similar improvement in eradication rate was noted when B. infantis was added as an adjuvant to the sequential therapy leading to an eradication rate of 90.8%.
Conclusion:
Adding B. infantis as an adjuvant to several therapeutic regimens commonly used for the eradication of H. pylori infection significantly improves the cure rates.
Keywords: Helicobacter pylori, probiotics, B. infantis, triple therapy, sequential therapy
The standard treatment for the eradication of Helicobacter pylori is the triple therapy. It includes a combination of a proton pump inhibitor (PPI) at a standard dose; together with two antibiotics (amoxicillin 1000 mg plus either clarithromycin 500 mg or metronidazole 400 mg) all given twice daily for a period of 7-14 days. Reported eradication cure rates used to be around 95% in the early nineties.[1] However, a meta-analysis in 2007 including over 53,000 patients showed that the eradication rate after standard triple treatment is currently below 80%.[2] Some European studies have reported even lower rates of eradication, of 60-65%.[3] Failure of eradication appears to be related to ingestion compliance, reinfection, or drug resistance of the H. pylori. In the UAE, there was a similar observation over the past 10 years where every day practice indicated that the eradication rate is noticeably decreasing and more failures are emerging.[4]
The sequential therapy is a novel therapeutic regimen for the eradication of H. pylori introduced in 2007 by an Italian group which consists of a 10-days treatment course of a PPI and three antibiotics given in sequence rather than all drugs given at the same time: (PPI, with amoxicillin 1000 mg given twice daily for the first 5 days) followed by (PPI, clarithromycin 500 mg and metronidazole 400 mg all given twice daily for the subsequent 5 days). This achieved a 95% eradication rate which is significantly superior to that achieved with the standard triple therapy, according to the Italian researchers.[5] Later on, these results were confirmed by many other authors with similar success rates.[6]
Probiotics are believed to have a role in eradicating and possibly preventing H. pylori infection as an adjunctive treatment. Literature has shown that lactobacilli or their cell-free cultures inhibit or kill H. pylori, prevent its adhesion to mammalian epithelial cells and prevent interleukin-8 release. In vivo models demonstrated that pre-treatment with a probiotic can prevent H. pylori infection and/or that administration of probiotics markedly reduced an existing infection.[7] A meta-analysis of 14 randomized clinical trials evaluating the role of supplemental probiotics in eradication therapy demonstrated that the cure rates for standard agents used alone and eradication co-therapy with probiotics, were 74.8% and 83.6%, respectively. The analysis revealed that the combined treatment, had not only increased the eradication rate, but had also decreased the occurrence of adverse effects due to antibiotics, like diarrhea.[8] Several studies also reported that the ingestion of fermented milk containing Lactobacillus had improved the symptoms of H. pylori infected gastritis. However, they did not affect eradication, and after stopping ingestion, the suppressing effect on H. pylori was lost.[9] Sheu, et al., reported that pre-treatment with Lactobacillius and Bifidobacterium containing yogurt improved the efficacy of quadruple therapy after failed triple therapy. They also demonstrated a decreased bacterial load after pretreatment with yogurt.[10] Another study had shown that the eradication rates for two cohorts, one treated with yogurt plus triple therapy and the other with triple therapy only, were 82.6% and 69.3%, respectively for intention to treat analysis (P = 0.018) thus concluding that supplementation with yogurt containing Lactobacillus gasseri is effective for first-line eradication therapy.[11]
B. infantis is a probiotic that inhabits the intestines of both infants and adults. This type of bacteria is considered beneficial because of the acids it produces. The acids produced by B. infantis may help impede the growth or colonization of harmful bacteria within the gut. It has been reported to be effective in management of irritable bowel syndrome (IBS) and has beneficial action on the treatment of diarrhea of other causes. A multicenter, randomized, placebo controlled study demonstrated that Bifidobacterium infantis 35,624 can improve common IBS symptoms over a 4-week period.[12]
The effects of adding B. infantis when supplementing triple therapy for eradication of H. pylori, has not been adequately studied or investigated. Yang, et al., showed that using probiotic fortified yoghurt alongside antibiotic therapy, rendered the side effects fewer, and the rate of eradication of H. pylori significantly higher than in those following the medication therapy only.[10]
This paper presents a multi-center work that was done with a view to explore if modifying medicinal H. pylori therapy, by adding the probiotic B. infantis as an adjuvant with or before treatment will satisfactorily restore the previously achieved eradication rates.
MATERIALS AND METHODS
A prospective randomized open label clinical study was conducted to compare the efficacy of adding probiotics to different modifications of H. pylori treatment. The probiotic used was B. infantis 2036 and the regimens of eradication adopted were the standard triple therapy and the sequential therapy. Primary end point was to evaluate cure rates, at end of treatment. Secondary end point was to assess clinical response and impact on reducing adverse events.
Inclusion criteria
As per protocol of the study, all consenting patients, of 12-80 years of age who presented with upper gastrointestinal (GI) symptoms, and were diagnosed to have upper GI disease either clinically, or by endoscopy, were included when they had a solid evidence of H. pylori infection. Upon recruitment, H. pylori infection was to be concluded either from the Campylobacter Like organism Urease test (CLO urease), or by histologic proof for the patients who had endoscopy or by the 14C urea breath test (UBT) and/or by detecting pylori antigen in stool for those who did not have endoscopy. All patients however, should have a reference 14C UBT before starting treatment regardless of the diagnostic approach.
Exclusion criteria
Standard exclusion criteria were followed to assure that safety of treatment has been watched, and to exclude any inter-current illnesses that might have an impact on the response to medicines. These included: Pregnancy, patients known to be allergic to any of the medicines used, patients who would develop adverse reactions after starting therapy, use of other concomitant medications, chronic renal or hepatic disorders, and neoplastic disease. Patients who had received clarithromycin for any reason within the 6 months that preceded enrolment were also excluded and so was the case for patients who were treated on the basis of the office serology test for H. pylori alone.
The study extended over a period of one year and included four arms designed to cover four groups of patients treated with different regimens. The patients were either treated for the first time (naïve), or had a failed a previous attempt at least 6 months before enrolment (retreat). They were randomly assigned to one of the four arms of the study in six medical centers.
Each patient should have a documented follow-up visit at 6-8 weeks after completing the treatment with a check on H. pylori cure using 14C UBT. Endoscopy was repeated where indicated only (presence of new or persisting symptoms). The values obtained by the 14C UBT as determined by Heliprobe II, in counts per million were viewed as a reflection of the intensity of colonization, and the change in values after treatment were taken into consideration when assessing response.
Group A
Goup A was a control of patients (n = 106) that was meant to reflect the current status of response to the standard triple therapy. It was used as a comparison, against which the modified probiotic regimens would be evaluated. Ninety patients in this group were naïve to clarithromycin treatment while 16 others were retreat patients. They were seen again after 6-8 weeks after completion of the treatment for clinical assessment and to do the 14C UBT, which was considered as the standard for verifying eradication.
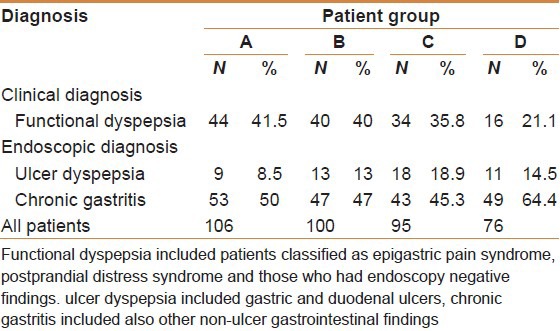
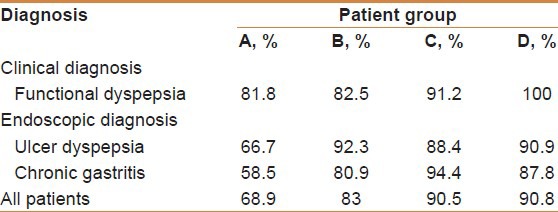
The clinical diagnosis and pathology found are included in Table 1.
Table 1.
The clinical findings of patients in all groups
Group B
This was a cohort of 100 patients who were randomly selected to be treated with B. infantis 2036 at 30 × 108 colonial forming unit (CFU) (twice daily) added as an adjuvant to the same triple therapy regimen given for group A. Sixty five patients in this group were naïve to clarithromycin treatment, while 35 were retreat patients.
Group C
This group consisted of a cohort of another 95 randomly selected patients, and were planned for a lead-in period of 2 weeks with the probiotic B. infantis 2036 at 30 × 108 CFU given twice daily alone, then followed by triple therapy combined with B. infantis as an adjuvant (same as in group B) for the subsequent 10 days. Fifty patients in this group were naïve to clarithromycin treatment, while 45 were retreat patients.
Group D
This group consisted of 76 patients treated with a sequential regimen of therapy together with B. infantis 2036 at 30 × 108 CFU twice daily for 10 days. Forty patients in this group were naïve to clarithromycin, while 36 were re-treat patients.
RESULTS
The results of the treatment of the four groups studied are discussed below individually and are summarized in Tables 1–5.
Table 5.
Main outcome of management for all patients
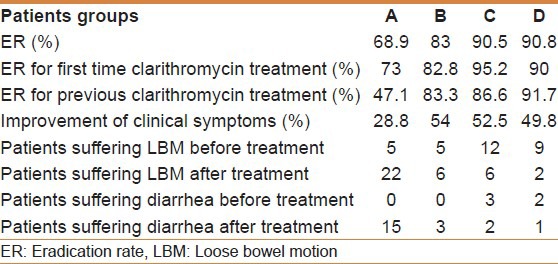
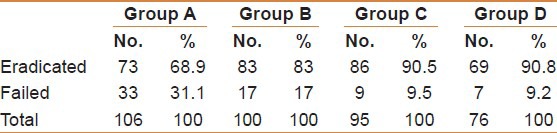
Table 3.
The results of therapy (eradication vs failed)
Group A
The mean age for this cohort was 37.2 years (17-64 years) with a normal distribution across the entire age group. Among all the patients, females were 51.9% and males 48.1%. The mean body mass index (BMI) was slightly increased (26.6 kg/m2) with 40.9% having normal body built and 59.1% were either overweight or frankly obese.
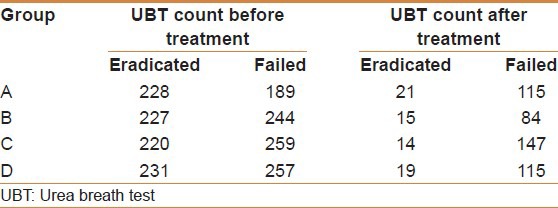
At the end of treatment, 73 subjects were successfully cured from H. pylori infection with an eradication rate of 68.9%. The mean count of colonization of H. pylori as determined by Heliprobe 2 before treatment for all patients was 222 count per million (CPM), ranging from 65 to 556. After treatment with the triple therapy this reduced to a mean value of 50 CPM for all patients (normal value < 50 CPM). The mean drop in H. pylori colonization for all patients was 165 CPM being more noticeable with the successfully eradicated group of patients (206 CPM). In the patients who failed to respond to treatment they still could demonstrate a drop of the UBT count (mean 74 CPM).
The eradication rate for patients who were grouped as functional dyspepsia was 81.8%. The eradication rate for patients with ulcerative disease was 66.7% and for those with non-ulcerative chronic active gastritis was 58.5%. Post-treatment, clinical evaluation indicated that 28.8% were said to have shown improvement of symptoms whereas 68.5% of all the patients reported no change and in 2.7% cases the symptoms worsened regardless of the results of eradication. Naïve patients to clarithromycin had a successful eradication rate of 73%, and retreat patients had only a 47.1% success rate.
Five patients had loose bowel motions at the beginning. After treatment 22 patients reported loose bowel motions and 15 developed diarrhea of variable severity [Table 5].
Group B
The mean age in this group was 37.3 years (17-62 years) with a normal distribution across the age groups. Male to female ratio was (1.04:1). The mean BMI was 26.3 kg/m2 with 44% of the subjects having a normal body weight, and 56% overweight.
At the end of treatment 83 subjects were successfully cured from H. pylori with an eradication rate of 83%. The mean count of colonization of H. pylori before treatment for all patients was 229 per million, ranging from 65 to 442. After treatment with the triple therapy and B. infantis as an adjuvant this has reduced to a mean value of 26.7 CPM for all patients (normal value < 50 CPM). The mean drop in H. pylori colonization for all patients was 203 CPM being more noticeable with the successfully eradicated group of patients (mean: 206 CPM) than with the failed group who could still demonstrate a drop in the UBT count (mean: 160 CPM).
The eradication rate for patients who were grouped as functional dyspepsia was (82.5%), for those with ulcerative disease (92.3%) and for those with non-ulcerative chronic active gastritis (80.9%). Post-treatment, clinical evaluation indicated that 54% were said to have shown improvement of symptoms where as 46% remained the same or had worsened. Naïve patients to clarithromycin had a successful eradication of 82.8% and retreat patients had an eradication rate of 83.3%. This indicated that the regimen used in this cohort produced the same response whether patient was treated before with clarithromycin or not.
Five patients of this group had loose bowels before initiating therapy. After treatment, six patients only had the same complaint and three patients developed diarrhea at the end of treatment [Table 5].
Group C
The mean age for patients in this cohort was 37.3 years (19-61 years) with a normal distribution across the age groups. Male/female ratio was the same. 43% had a normal body built (mean BMI 25.8 kg/m2) and 57% were overweight.
At the end of treatment 86 subjects were successfully cured from H. pylori with an eradication rate of 90.5% [Table 1]. The mean 14C UBT count of colonization of H. pylori before treatment for all patients was 223 per million ranging from 96 to 390. After treatment with a lead in period with B. infantis for 2 weeks followed by the co-therapy with B. infantis as an adjuvant the mean value reduced to 26 CPM. The mean drop in H. pylori 14C UBT count was 174 per million [Table 2] being more noticeable with the successfully eradicated patients (mean: 206 CPM) than in the failed patients who demonstrated a drop of the UBT count to a mean of 112 CPM.
Table 2.
The results of treatment (eradication rates)
Patients who were grouped as functional dyspepsia had an eradication rate of 91.2%, and those with ulcerative disease had 88.4% and non-ulcerative chronic active gastritis had 94.4%. Clinically, 52.6% of the successfully eradicated patients reported improvement of clinical symptoms and 47.4% remained the same or had worsened after treatment. Patients naïve to clarithromycin treatment had a successful eradication rate of 95.2% and retreat patients had a rate of 86.6%.
Before treatment, 12 patients in this group, reported loose bowel motions, and 3 had diarrhea. After treatment, six patients only had loose bowels and two had diarrhea [Table 5].
Group D
The mean age for patients in this cohort (n = 76) was 38.3 years (17-58 years) with a slightly skewed distribution across the age groups and a peak at the age group 30-39 (36.8). Males and females were equal in number. The mean BMI was 27.49 kg/m2 (range 18-34) with 30.3% of the subjects having a normal body built, 69.7% were overweight.
At the end of treatment 69 subjects were successfully cured from H. pylori infection with an eradication rate of 90.8% [Table 1]. The mean H. pylori count for all patients was 233 per million with a range from 96 to 373. After treatment with Sequential therapy together with B. infantis as an adjuvant this was reduced to a mean value of 28 CPM (normal values < 50 CPM). The mean drop in H. pylori achieved was 187.2 CPM, being more noticeable in the eradicated group (191 CPM) than in the failed group (150 CPM).
All patients with functional dyspepsia were cured. Patients with ulcerative disease had an eradication rate of 90.9% and those with non-ulcerative chronic active gastritis had 87.8%. Clinically, 49.3% of patients reported improvement in symptoms at 6-8 weeks from treatment and 51.7% reported no change in symptoms or that they may have worsened regardless of the result whether the infection was successfully eradicated or not.
Patients naïve to clarithromycin had a successful eradication rate of 90%, whereas retreat patients had 91.7%.
In this group, nine patients were having loose bowel motions and two had reported diarrhea before starting therapy. At the end of treatment only one patient had diarrhea and 2 had loose bowel motions [Table 5].
DISCUSSION
In the early years of H. pylori treatment the results of the standard triple therapy were satisfactorily successful, yielding eradication rates of 95-96%.[13,14,15] Several reports had indicated lately, a serious drop in the eradication rate with the triple therapy.[2,3] This has alarmed researchers to find an alternative way of treatment either by changing the antibiotics, or by adopting new systems and schedules of therapy, or by adding adjuvants that may help enhance the response to the standard treatment.
In this study, the demographic details indicated homogeneity in the four arms with no significant differences in age, gender, ethnicity, BMI or personal habits.
The first arm of this study had revealed an eradication rate of 68.9%. This indicated clearly that the global trend of decline in success of response to triple therapy applies to our areas as well. Naïve patients who did not receive clarithromycin before had a better eradication rate (73%), than retreat patients (47.1%). Clinical improvement was minimal in this group being achieved in 28.8% of the patients treated regardless of the results of eradication. In the post-treatment it was noted that loose bowel motions and diarrhea had appeared in 9.4% and 15.1% of patients, respectively, which was believed to be an adverse event to the antibiotics used. In this work, two factors had possibly contributed to the increase in H. pylori eradication failure, namely the repeated retreat events with same medications which are an unfortunate common practice in the area, that patients are unjustifiably exposed to, and the increase in resistance to clarithromycin.
The long-term use of clarithromycin based treatment and the continued widespread use of long-acting macrolides in general practice, led to 10-15% of H. pylori strains to acquire resistance to clarithromycin. This had consequently reduced the cure rates by 20-30% when patients are treated by a clarithromycin based regimen. Clarithromycin failure appears to be on the rise worldwide as concluded from several studies. This appeared partly to be due to the injudicious use of clarithromycin by repeated trials of eradication of H. pylori but, mainly due to drug resistance as well. Drug resistance is likely to be associated with the increased activity of the efflux pump of H. pylori, and/or to the emergence of the 23S r-RNA point mutations of the microbe. Efflux pumps appear to play a major role so much that pharmacologic research is targeting it to achieve reversing of drug resistance in H. pylori.[16,17,18,19,20]
Sequential therapy is a novel schedule of treatment that emerged as a possible solution to the decline in H. pylori eradication rates. Vaira, et al., in 2007, showed in a large prospective controlled study a 90% cure rate for this new regimen of treatment versus 80% for the old standard treatment. Jafri, et al., performed a meta-analysis of several clinical trials involving sequential treatment and concluded a favorable response confirming the efficacy of this regimen. The restoration of successful eradication rates by sequential therapy is expected to reduce the need to follow-up proof of cure and to the need to re-instate repeated attempts at eradication with the reported ever-decreasing success rates.[5,6]
Probiotics had an in vitro inhibitory effect on H. pylori. Animal studies had demonstrated that probiotic treatment is effective in reducing H. pylori associated gastric inflammation. Seven of 9 human studies showed an improvement of H. pylori associated gastritis and decrease in colonization after the administration of probiotics. On the other hand the addition of probiotics to the standard antibiotic treatment had improved H. pylori eradication rates (81% with combination treatment vs. 71%, for H. pylori eradication treatment alone). Probiotics also remarkably reduced the antibiotic associated side effects (incidence of side-effects: 23% with combination therapy versus 46%, for H. pylori eradication treatment alone). No study, could demonstrate the eradication of H. pylori infection by using probiotics alone. Therefore, probiotics should not be considered as an alternative to the standard treatment but could definitely present a low cost, large scale alternative solution when used as an adjunctive agent to prevent or decrease H. pylori colonization.[21]
B. infantis is a member of the Bifidobacteria family, a strain of bacteria that is normally found in the human intestines. Because these bacteria do not normally cause infections, they can be used in probiotic supplements, which can aid in promoting intestinal health and help prevent infections. B. infantis work within the digestive system to restore intestinal balance and maintain normal digestive health. In a previous study, B. infantis was shown to be beneficial to those who suffer from symptoms of IBS like bloating, gas movement, diarrhea, constipation, urgency, and abdominal discomfort.[12] However, its effect on eradication of H. pylori has not been adequately investigated.
According to the World Gastroenterology Organization Practice Guidelines published in May 2008, probiotics may reduce the GI side-effects of antibiotic therapy and improve patient compliance.[22] There is currently insufficient evidence to support the concept that a probiotic used alone, without concomitant antibiotic therapy, would be effective, but the indication from literature review suggest that certain probiotics may be helpful as an adjuvant to antibiotics in the eradication of H. pylori infection.
The use of probiotics to modify the conventional treatment regimens is based on sound biological and physiological principles. Probiotics modulate the intestinal ecosystem by stimulating mucosal immune mechanisms and by stimulating non-immune mechanisms through antagonism with potential pathogens. These phenomena are thought to mediate most beneficial effects, including reduction of the incidence and severity of diarrhea. In addition to the therapeutic effects, probiotics reduce the common side effects of GI upset during conventional antibiotic treatment. It was demonstrated that in symptom free, H. pylori positive subjects Bacillus clausii bacteriotherapy reduced the incidence of the most common side-effects related to anti-H. pylori antibiotic therapy compared with placebo. Probiotics may especially, be helpful in patients with recurrent H. pylori infection and a history of GI adverse effects with antibiotics.[23,24,25]
A recent meta-analysis of 14 randomized trials suggested that supplementation of anti-H. pylori antibiotic regimens with certain probiotics may also be effective in increasing eradication rates and may be considered helpful for patients with eradication failure.[8] Further clinical studies will be necessary to establish better guidelines in supplementation of anti-H. pylori antibiotic regimens.
It is reported that B. infantis produced better epithelial tight junctions and is thus of particular importance in alleviating inflammatory bowel diseases such as Crohns or ulcerative colitis.[26] Similarly, it may confer epithelial protection of gastric mucosa and prevent H. pylori from causing mucosal damage and subsequent inflammation. Furthermore, Bifidobacteria allows better opportunities for antibiotics when given together to eradicate H. pylori infection.
Our work shows that when B. infantis was added to the standard triple therapy as a supplement the success of eradication increased significantly from 68.9% by the triple therapy alone to 83% (P < 0.001). Priming the GI tract with a lead-in period of 2 weeks with B. infantis alone followed by triple therapy with the probiotic yielded better results than triple therapy, and achieved a statistically significant eradication rate of 90.5% (P < 0.001 vs. triple therapy alone). Adding B. infantis, to a sequential regimen of treatment had shown an eradication rate of 90.8% which is almost similar to group C. There was no significant difference between groups B, C, and D [Figure 1]. In the standard triple therapy group, eradication rates for naïve patients to clarithromycin exhibited a better response than the retreat patients, (73% vs. 47.1%), respectively, implicating a possible role for clarithromycin resistance to account for this (P < 0.001). This phenomenon was not encountered in the B. infantis treated groups (groups B, C, and D) where no difference was noted between naïve and retreat patients. This favorable finding could be attributed to a positive role for B. infantis on the H. pylori-clarithromycin resistant strains, rendering them more susceptible to treatment [Table 4].
Figure 1.
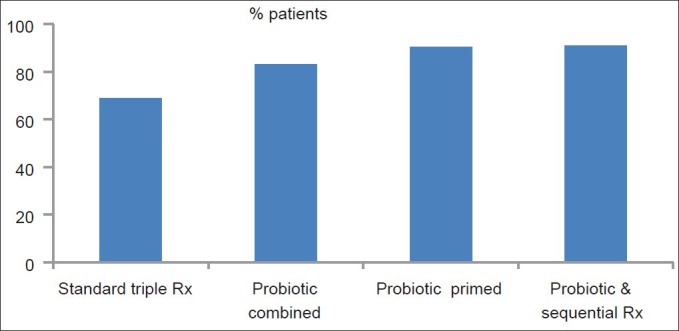
Eradication rates of Helicobacter pylori by different regimens used
Table 4.
The eradication rates (cure percentage) as per their clinical condition
Clinical improvement was another advantage of adding B. infantis to the standard triple therapy. Improvement and clinical satisfaction was reported by 28.8%, 54%, 52.6%, and 49.3% of all patients treated with triple therapy, co-therapy, primed co-therapy, and sequential co-therapy, respectively. This observation had demonstrated a significant difference of clinical response for all modified regimens than triple therapy alone (P < 0.001) regardless of the result whether the H. pylori infection was eradicated or not.
Another secondary endpoint of this study is the remarkable improvement in bowel activity in those who were treated with B. infantis. The number of patients is small to establish solid statistical conclusions, however, it was apparent that patients who were treated with triple therapy alone had either loose bowels or frank diarrhea after initiating therapy seven times more than pretreatment status. This observation was encountered rarely in the B. infantis treated groups (twice for co-therapy, less than half for the primed co-therapy and less than a third for the sequential co-therapy) [Table 4].
Yuan[27] reported that B. infantis helped regeneration of the colonic mucosa after a course of the chemotherapeutic agent 5-Flouro Uracil which is commonly used for colonic carcinoma with an associated suppression of underlying inflammation. This may explain the present observation that B. infantis alleviated symptoms of side effects of antibiotics used in this study.
CONCLUSION
This study was done to explore an alternative to the standard triple therapy for the eradication of H. pylori, which has witnessed a significantly remarkable decline of eradication to 68.9% than previously obtained by most researchers more than a decade ago (95%). The response to the therapeutic regimen of standard triple therapy was affected negatively by previous exposure to clarithromycin.
The probiotic B. infantis appeared to be a sound alternative to triple therapy, when added as an adjuvant: Concurrently with, or after priming the GI tract for 2 weeks or with sequential treatment. A successful eradication rate of 83%, 90.5%, and 90.8% was obtained respectively. Each was significantly better than triple therapy alone (68.9%, P < 0.001).
It is also note-worthy that B. infantis led to better results of eradication for people who had been previously treated with clarithromycin, that is, reduced the negative effect of clarithromycin resistance. B. infantis also improved significantly the clinical symptoms and the incidence of antibiotic induced diarrhea with only few and milder incidents of diarrhea reported.
Footnotes
Source of Support: None
Conflict of Interest: None declared.
REFERENCES
- 1.Dajani AI, Awad S, Ukabam S, Nounou MA, Abdul Rasheed Z, Gautam S, et al. One-week triple regime therapy consisting of pantoprazole, amoxicillin and clarithromycin for cure of Helicobacter pylori-associated upper gastrointestinal diseases. Digestion. 1999;60:298–304. doi: 10.1159/000007675. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou Hammour A, Dajani AI, Nounou M, Zakaria M, Mohammed N, Al Badri S. Current stand of classical triple therapy for the eradication of H. pylori in the UAE. Proceedings of the 6th Emirates Gastroenterology and Hepatology Conference, oral presentation in H. pylori plenary session. 2010 Apr [Google Scholar]
- 5.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann Intern Med. 2007;146:556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jafri NS, Hornung CA, Howden CW. Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton-Miller JM. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. 2003;22:360–6. doi: 10.1016/s0924-8579(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 8.Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: The effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–68. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 9.Pantoflickova D, Corthésy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F, et al. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805–13. doi: 10.1046/j.1365-2036.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 10.Sheu BS, Cheng HC, Kao AW, Wang ST, Yang YJ, Yang HB, et al. Pretreatment with Lactobacillus- and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy. Am J Clin Nutr. 2006;83:864–9. doi: 10.1093/ajcn/83.4.864. [DOI] [PubMed] [Google Scholar]
- 11.Deguchi R, Nakaminami H, Rimbara E, Noguchi N, Sasatsu M, Suzuki T, et al. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol. 2012;27:888–92. doi: 10.1111/j.1440-1746.2011.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 13.Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O’Morain C, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: The MACH I Study. Helicobacter. 1996;1:138–44. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Adamek RJ, Szymanski C, Pfaffenbach B, Opferkuch W, Ricken D, Wegener M. Short-term triple therapy with pantoprazole, clarithromycin and metronidazole for the healing of Helicobacter pylori infection. Dtsch Med Wochenschr. 1995;120:358–60. doi: 10.1055/s-2008-1055353. [DOI] [PubMed] [Google Scholar]
- 15.Labenz J, Peitz U, Tillenburg B, Becker T, Börsch G, Stolte M. Short-term triple therapy with pantoprazole, clarithromycin and metronidazole in eradication of Helicobacter pylori. Leber Magen Darm. 1995;25:122. [PubMed] [Google Scholar]
- 16.Prazeres Magalhães P, De Magalhães Queiroz DM, Campos Barbosa DV, Aguiar Rocha G, Nogueira Mendes E, Santos A, et al. Helicobacter pylori primary resistance to metronidazole and clarithromycin in Brazil. Antimicrob Agents Chemother. 2002;46:2021–3. doi: 10.1128/AAC.46.6.2021-2023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006;28:6–13. doi: 10.1016/j.ijantimicag.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088–94. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, et al. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol. 2010;25:S75–9. doi: 10.1111/j.1440-1746.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuwayama H, Asaka M, Sugiyama T, Fukuda Y, Aoyama N, Hirai Y, et al. Rabeprazole-based eradication therapy for Helicobacter pylori: A large-scale study in Japan. Aliment Pharmacol Ther. 2007;25:1105–13. doi: 10.1111/j.1365-2036.2007.03298.x. [DOI] [PubMed] [Google Scholar]
- 21.Lesbros-Pantoflickova D, Corthésy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr. 2007;137:812S–8. doi: 10.1093/jn/137.3.812S. [DOI] [PubMed] [Google Scholar]
- 22.Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46:468–81. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 23.Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: Randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20:1181–8. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: The role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother. 2011;45:960–6. doi: 10.1345/aph.1Q104. [DOI] [PubMed] [Google Scholar]
- 25.Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: A parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–9. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 26.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–34. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 27.Yuan KT. China: Department of Surgery, First Affliated Hospital of Sun Yat-Sen University; 2012. PhD Thesis., Guangzhou; pp. 42–58. [Google Scholar]


