Abstract
Background/Aims:
Inflammatory bowel diseases (IBDs), Crohn's disease (CrD) and ulcerative colitis (UC), are chronic gastrointestinal inflammatory disorders. The precise etiology of IBD remains unclear, and it is thought that interactions among various factors, including, genetic factors, the host immune system and environmental factors, cause disruption of intestinal homeostasis, leading to dysregulated inflammatory responses of the gut. As inflammation is intimately related to formation of reactive intermediates, including, reactive oxygen species, oxidative stress has been proposed as a mechanism underlying the pathophysiology of IBD. The purpose of this study is to examine the lipid peroxidation, protein oxidation and anti-oxidative profile in Tunisian IBD.
Materials and Methods:
Malondialdehyde (MDA), conjugated dienes (CD), protein thiol levels, as well as the catalase (CAT) activity were evaluated in intestinal biopsies of 17 patients affected by IBD (12 CrD and 5 UC) and 12 healthy control individuals.
Results:
Oxidative stress was confirmed in these two types of disease biopsies as compared to controls. MDA and CD levels were significantly increased in both UC and CrD patients’ biopsies as compared to controls’ biopsies (P < 0.001). CAT activity was similar in UC and CrD biopsies’ and was not significantly increased in IBD patients’ biopsies compared with controls’ biopsies (P > 0.05). Anon-significant decrease in thiol (SH) level was observed in both UC and CrD patients’ biopsies compared with controls’ biopsies (P > 0.05).
Conclusion:
Increased levels of MDA and CD in IBD patients’ biopsies underline the implication of oxidative stress in the physiopathology of IBD.
Keywords: Catalase, conjugated dienes, malondialdehyde, protein thiol, superoxide, Tunisia
Crohn's disease (CrD) and ulcerative colitis (UC), known collectively as inflammatory bowel disease (IBD), are auto immune-like disorders characterized by chronic, idiopathic inflammation of the intestinal mucosal tissue, which causes arrange of symptoms including abdominal pain, severe diarrhea, rectal bleeding and wasting.[1,2] IBD tends to emerge in late childhood, occurs primarily in immuno competent individuals and is most prevalent in westernized regions of the world.[2,3] CrD and UC are distinguished by the tissues affected: CrD can affect any region of the gastrointestinal tract in a discontinuous and transmural manner, whereas pathology in UC is restricted to the surface mucosa of the colon, in particular the rectum.[1,2] Although, the exact etiology of IBD remains uncertain, dysfunctional immune regulation of the gut is believed to be the main culprit.[4] Amongst the immune regulatory factors, reactive oxygen species (ROS) are produced in abnormally high levels in IBD. Their destructive effects may contribute to the initiation and/or propagation of the disease.[5,6,7] ROS are highly reactive transient chemical molecules such as the super oxideanion (O2), hydrogen peroxide (H2 O2), the hydroxyl radical, and singlet oxygen. ROS are indispensable as mediators in many normal cellular processes, and under physiological condition, their generation is controlled by a large number of antioxidant systems, which act as protective mechanisms. These antioxidant control systems consist of enzymes and antioxidant substrates. Among antioxidant enzymes, the superoxide dismutase (SOD) catalyzed is mutation of O2 to H2 O2 and molecular oxygen. The decomposition of H2 O2 to nontoxic compounds is the main function of catalase (CAT), glutathione peroxidise (GPx) and peroxiredoxins.[8] Antioxidant substrates such as glutathione and α-to copherol also play a crucial role in scavenging ROS.[9] An imbalance between ROS and antioxidant defenses creates oxidative stress.[10] This disturbance of the oxidative status is the result of either an excessive ROS production or a deficiency in antioxidant system activities. Both parameters seem to be implicated in autoimmune diseases (systemic lupus erythematosus, pemphigus foliaceus, diabetes mellitus, primary Sjøgren's syndrome and IBD).[11,12,13,14,15]
In the light of these data, the present study aims to assess the oxidative damage seen in IBD biopsy, by studying the lipid peroxidation, the protein oxidation profile and CAT activity. Malondialdehyde (MDA) and conjugated dienes (CD) levels were measured as parameters of lipid peroxidation, protein thiol as parameter of protein oxidation. CAT activity was measured as parameters of antioxidant system evaluation.
Materials and Methods
Patients and tissue samples
A total of 17 intestinal tissue samples from 12 CrD patients (7F, 5M), age 16-82 years and 5 UC patients (2F, 3M), age 25-60 years, undergoing colonoscopy were obtained for our study at the Gastroenterology Department of Hedi Chaker University Hospital (Sfax, Tunisia). Informed consent was obtained from all patients. Each patient had a confirmed diagnosis by standard endoscopic and histological criteria, and disease activity was graded according to the Harvey–Bradshawindex (HBI) or Mayoscore.[16,17] Twelve control samples were taken from healthy individuals with normal endoscopic findings and without macroscopic and microscopic evidence of inflammatory or neoplastic disease. Grading of mucosal inflammation (active disease) was performed by an expert gastroenterologist, who specializes in IBD, according the following criteria: CrD and UC active (HBI ≥ 8, Mayo score ≥ 6, respectively). This study was approved by the Ethical Committee of the Habib Bourguiba University-Hospital of Sfax, Tunisia.
Protein extraction
The biopsies were weighed and then placed in a buffersolution of tampon buffersaline (TBS; 50 mMTris, 150 mMNaCl, pH 7.4) at a temperature of 4°C. The biopsies were homogenized in TBS (1 mg/ml) in an ultrasound homogenizer (Vibracell®). Homogenates were centrifuged at 3.000 g at 4°C for 15 min and the supernatants were divided into aliquots. Biochemical estimation was carried out immediately.
MDA determination
MDA was evaluated using thiobarbituric acid-reactive species assay.[18] Biopsy supernatants (30 μl) were diluted in 500 μl distilled water and 2V of thiobarbituric acid agent was added (15% trichloroacetic acid and 0.8% thiobarbituric acid in 0.25 NHCl). The mixture was then heated at 95°C for 15 mins. After centrifugation at 3.000 rpm for 10 mn, optical density of the supernatant was determined at 532 nm. Values were reported to a calibration curve of 1,1,3,3-tetraethoxypropane.
Conjugated diene determination
The conjugated diene level was evaluated as described by Kurien and Scofield[11] with modification. Biopsy supernatants (25 μl) were extracted with 3 ml chloroform/methanol (2:1, v/v). After centrifugation at 1.000 g for 15 mins, 2 ml of organic phase was transferred into another tube and dried at 45°C. The dried lipids were dissolved in 2 ml of methanol and absorbance at 233 nm was determined. It corresponds to the maximum absorbance of the extracted compounds.
CAT activity determination
CAT activity was measured, as described previously by Aebi.[19] This method is based on the principle that the absorbance at 240 nm decreases because of H2O2 dismutation. The extinction coefficient of 43.6 l/mol/cm for H2O2 was used for calculation. One unit is defined as the amount of H2O2 converted into H2O and ½O2 in 1 mn under standard conditions, and the specific activity is reported as units per mg of protein.
Determination of protein thiol levels
Protein thiols were quantified spectro photometrically using 5,5-dithionitrobenzoic acid (DTNB); 250 ml of freshly prepared 10 m MDTN Bin 0.05 Mphosphate buffer pH8, were added to 50 ml of celly satin 1200 ml of 0.05 M phosphate buffer. After incubation in the dark for 15 min at room temperature, there lease of 5-thiobenzoic acid was quantified by measuring the absorbance at 412 nm and converted to absolute values using N-acetylcysteine as standard (0-0.1 mM). A correlation coefficient of r2 = 0.999 was obtained. The absorbance of sample slacking DTNB was subtracted to account for the background absorbance at 412 nm. Samples were analyzed in duplicate.
Protein quantification
The Protein levels were determined by using the Bio-Rad protein assay based on the Bradford dye procedure[20] and bovine serum albumin in served as standard.
Statistical analysis
Values of different parameters were expressed as the mean ± standard deviation (x ± SD). The one-way analysis of variance (ANOVA) was performed to evaluate the significance of differences between mean values. The post hoc test (Newman Kulls) was used to compare all paired means. A P value less than 0.05 were considered to be statistically significant. All statistical tests were performed using Graph-Pad Prism version 5 for Windows.
RESULTS
The study included 29 subjects in 3 groups. The CrD patient group consisted of 7 women and 5 men who were aged 16-82 years. The UC patient group consisted of 2 women and 3 men who were aged 25-60 years. The control group, consisted of 12 healthy volunteers.
Evaluation of lipid peroxidation in the biopsies
To determine lipid peroxidation, MDA and CD levels were assessed in CrD and UC biopsies in each patient, as well as in control subjects. The results, depicted in Figure 1, show that MDA and CD levels were significantly increased in CrD and UC biopsies of patients compared with those of control subjects (MDA: P < 0.001; CDP < 0.001).
Figure 1.
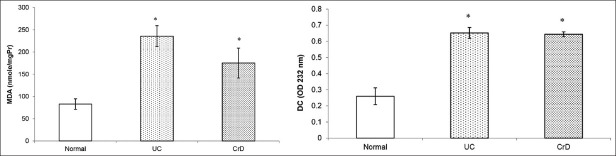
Box plots showing the levels of malondialdehyde and conjugated dienes in biopsies of UC and CrD patients and biopsies of control subjects. MDA and CD levels were significantly increased in UC and CrD biopsies of patients compared with those of control subjects (MDA: P < 0.001; CD P < 0.001). *P < 0.05 as compared with control subjects
Evaluation of CAT activity in the biopsies
CAT activity in biopsies of CrD and UC patients as well as in control subjects’ biopsies are shown in Figure 2. Our data show a non-significant rise in CAT activity in CrD and UC patients’ biopsies compared with controls’ biopsies (P > 0.05), however, CrD and UC patients showed higher activity of CAT than control subjects.
Figure 2.
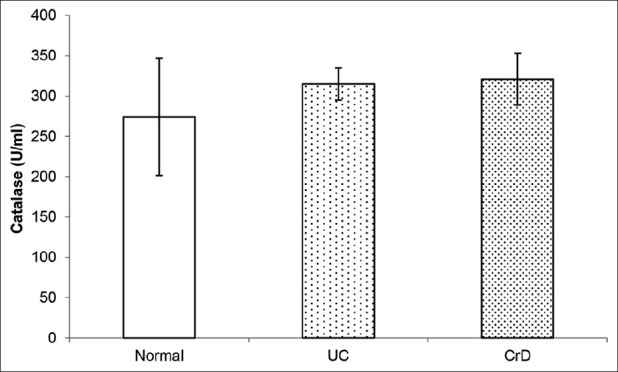
Box plots showing catalase activity in biopsies of UC and CrD patients and biopsies of control subjects. Our data show a non significant rise in catalase activity in UC and CrD patients’ biopsies compared with controls’ biopsies (P > 0.05)
Evaluation of protein oxidation
Thiol (SH) levels in CrD and UC biopsies of patients as well as in control subjects’ biopsies are shown in Figure 3. A non-significant decrease in SH level was observed in patients’ biopsies compared with controls’ biopsies (P > 0.05).
Figure 3.
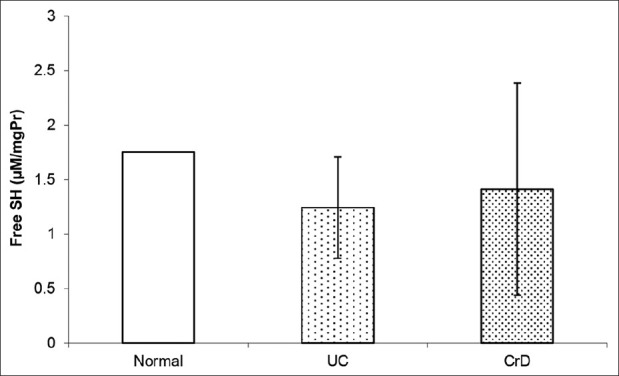
Box plots showing the levels of thiol SH in biopsies of UC and CrD patients and biopsies of control subjects, non significant decrease in SH level was observed in patients’ biopsies compared with controls’ biopsies (P > 0.05)
DISCUSSION
Auto immune process induces an inflammatory reaction and ROS are among its products. ROS are formed as normal metabolic products and are important in normal cellular functioning, but their production can be increased under pathological conditions and cause damage.[21,22]
As far as we know, the present study is the first to assess the oxidative stress markers in North African IBD patients in general and Tunisian IBD patients in particular.
In this study, the measurement of lipid peroxidation showed increased levels of MDA and CD markers in IBD biopsies of patients compared with control subjects, indicating the presence of oxidative stress. This was in agreement with the report of Alzoghaibi et al.,[23] who found a high MDA level in the plasma of CrD and UC patients. In contrast, Durak et al.,[24] have reported that tissue MDA levels in UC patients was significantly low indicating that the mucosa was not under oxidative stress and the defense mechanism was not impaired and Tüzün et al.,[25] have demonstrated that plasma MDA levels were not different between IBD patients and control group.
Moreover in our study, we have demonstrated an increased CAT activity in the biopsies of UC and CrD patients as compared with controls, but this result was not significant. This increase in CAT activity may represent a compensatory response to ROS production, which is known to enhance CAT expression.[26] SOD and CAT enzymes usually act in a synergetic manner. SOD, the first line of defense against ROS, catalyzes the dismutation of the O2 into H2O2. H2O2 can then be transformed into H2O and O2 by CAT, GPx and peroxiredoxins.[27] However, Krzystek-Korpacka et al.,[28] reported a decrease in CAT and GPx activities in IBD. This corroborates findings on several plasma antioxidants, decreased concentration, and/or activity of which has been confined to active IBD.[29,30] This discrepancy can be linked to the difference in the study design. In the previous studies, antioxidant enzyme activities were assessed in plasma of IBD patients; but in the current study, for the first time they were measured in IBD biopsies.
There lease of various inflammatory mediators has been reported in UC and CrDbiopsy.[31,32] It is well evidenced that inflammatory cells produce, in an inflammatory environment, large quantities of O2 radical and other ROS, which could be responsible for the oxidative stress observed in CrD and UC biopsy. It is also well-known that NADPH oxidase expressed in neutophiles is a strong candidate as a source of ROS production.[33,34]
The measurement of protein oxidation showed a non-significant decrease of SH level in UC and CrD biopsies of patients than in controls’ biopsies. Our data showed for the first time protein oxidation in IBD patients’ biopsies. Oxidized proteins cause major physiological perturbations including loss of structure and function.[10,35,36] In auto immune diseases, oxidatively modified proteins are responsible for additional perturbations because they represent potential targets for the immune system by breaking the B-cell tolerance.[37] Indeed, oxidatively modified proteins lead to the genesis of new epitopes that incite auto antibody production.
Proteinoxidation causes conformational changes which include the exposure of the hydrophobic regions of the proteins, explaining the increased interaction between auto antibodies and autoantigens after oxidation.[10,38] In a previous study we had demonstrated that oxidative modification of proteins could lead to an increase of reactivity of auto-antibody of auto-antibody of Lupus erythematosus patients patients, when compared with native proteins.[39]
CONCLUSION
This study shows that oxidative stress is involved in the pathogenesis of UC and CrD. The administration of antioxidants such as vitamins[40] could be a therapeutic management to stop the spread of IBD in the biopsy of patients by increased lipid peroxidation, protein oxidation, and modulation of antioxidant defense system.
Footnotes
Source of Support: This work was supported by grants from DGRST (Direction Générale de la Recherche Scientifique et Technique), Tunisie.
Conflict of Interest: None declared
REFERENCES
- 1.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH. The genetics and immune pathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–66. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: Genetics and immunobiology. Curr Gastroenterol Rep. 2008;10:568–75. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 6.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–84. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karp SM, Koch TR. Oxidative stress and antioxidants in inflammatory bowel disease. Dis Mon. 2006;52:199–207. doi: 10.1016/j.disamonth.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 8.MacMillan-Crow LA, Crow JP. Does more MnSOD mean more hydrogen peroxide? Anticancer Agents Med Chem. 2011;11:178–80. doi: 10.2174/187152011795255939. [DOI] [PubMed] [Google Scholar]
- 9.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–64. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 10.Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991;69:965–8. doi: 10.1007/BF01645140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73:1655–66. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 12.de M Bandeira S, da Fonseca LJ, da S Guedes G, Rabelo LA, Goulart MO, Vasconcelos SM. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14:3265–84. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abida O, Gargouri B, Ben Mansour R, Mseddi-Djemal M, Masmoudi A, Ben Ayed M, et al. Biomarkers of oxidative stress in epidermis of Tunisian pemphigus foliaceus patients. J Eur Acad Dermatol Venereol. 2012 doi: 10.1111/j.1468-3083.2012.04626.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Norheim KB, Jonsson G, Harboe E, Hanasand M, Gøransson L, Omdal R. Oxidative stress, as measured by protein oxidation, is increased in primary Sjøgren's syndrome. Free Radic Res. 2012;46:141–6. doi: 10.3109/10715762.2011.645206. [DOI] [PubMed] [Google Scholar]
- 15.Sklyarov AY, Panasyuk NB, Fomenko IS. Role of nitric oxide-synthase and cyclooxygenase/lipooxygenase systems in development of experimental ulcerative colitis. J Physiol Pharmacol. 2011;62:65–73. [PubMed] [Google Scholar]
- 16.Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw index. Inflamm Bowel Dis. 2006;12:304–10. doi: 10.1097/01.MIB.0000215091.77492.2a. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–6. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargouri B, Lassoued S, Ayadi W, Karray H, Masmoudi H, Mokni N, et al. Lipid peroxidation and antioxidant system in the tumor and in the blood of patients with nasopharyngeal carcinoma. Biol Trace Elem Res. 2009;132:27–34. doi: 10.1007/s12011-009-8384-z. [DOI] [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Alzoghaibi MA, Al Mofleh IA, Al-Jebreen AM. Lipid peroxides in patients with inflammatory bowel disease. Saudi J Gastroenterol. 2007;13:187–90. doi: 10.4103/1319-3767.36750. [DOI] [PubMed] [Google Scholar]
- 24.Durak I, Yasa MH, Bektas A, Kaçmaz M, Cimen MY, Oztürk HS. Mucosal antioxidant defense is not impaired in ulcerative colitis. Hepato gastroenterology. 2000;47:1015–7. [PubMed] [Google Scholar]
- 25.Tüzün A, Erdil A, Inal V, Aydin A, Bağci S, Yeşilova Z, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–72. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 26.Lassoued S, Gargouri B, El FekiAel F, Attia H, Van Pelt J. Transcription of the epstein-barr virus lytic cycle activator BZLF-1 during oxidative stress induction. Biol Trace Elem Res. 2010;137:13–22. doi: 10.1007/s12011-009-8555-y. [DOI] [PubMed] [Google Scholar]
- 27.Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free RadicBiol Med. 1994;17:235–48. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 28.Krzystek-Korpacka M, Neubauer K, Berdowska I, Zielinski B, Paradowski L, Gamian A. Impaired erythrocyte antioxidant defense in active inflammatory bowel disease: Impact of anemia and treatment. Inflamm Bowel Dis. 2010;16:1467–75. doi: 10.1002/ibd.21234. [DOI] [PubMed] [Google Scholar]
- 29.D’Odorico A, Bortolan S, Cardin R, D’Inca’ R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 30.Boehm D, Krzystek-Korpacka M, Neubauer K, Matusiewicz M, Berdowska I, Zielinski B, et al. Paraoxonase-1 status in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:93–9. doi: 10.1002/ibd.20582. [DOI] [PubMed] [Google Scholar]
- 31.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens C, Walz G, Singaram C, Lipman ML, Zanker B, Muggia A, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–26. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 33.Abreu MT, Sparrow MP. Translational research in inflammatory bowel disease. Mt Sinai J Med. 2006;73:1067–73. [PubMed] [Google Scholar]
- 34.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arutyunova EI, Danshina PV, Domnina LV, Pleten AP, Muronetz VI. Oxidation of glyceraldehyde-3-phosphate dehydrogenase enhances its binding to nucleic acids. Biochem Biophys Res Commun. 2003;307:547–52. doi: 10.1016/s0006-291x(03)01222-1. [DOI] [PubMed] [Google Scholar]
- 36.Shacter E, Williams JA, Levine RL. Oxidative modification of fibrinogen inhibits thrombin-catalyzed clot formation. Free Radic Biol Med. 1995;18:815–21. doi: 10.1016/0891-5849(95)93872-4. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh Z, Ahmad R, Sheikh N, Ali R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity. 2007;40:512–20. doi: 10.1080/08916930701574331. [DOI] [PubMed] [Google Scholar]
- 38.Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multi catalytic proteinase complex, proteasome. J BiolChem. 1993;268:15405–11. [PubMed] [Google Scholar]
- 39.Ben Mansour R, Lassoued S, Elgaied A, Haddouk S, Marzouk S, Bahloul Z, et al. Enhanced reactivity to malondialdehyde-modified proteins by systemic lupus erythematosus autoantibodies. Scand J Rheumatol. 2010;39:247–53. doi: 10.3109/03009740903362511. [DOI] [PubMed] [Google Scholar]
- 40.Aghdassi E, Wendland BE, Steinhart AH, Wolman SL, Jeejeebhoy K, Allard JP. Antioxidant vitamin supplementation in Crohn's disease decreases oxidative stress. A randomized controlled trial. Am J Gastroenterol. 2003;98:348–53. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]


