Abstract
Purpose
To evaluate serum levels of YKL-40 in patients with pseudoexfoliation syndrome (PEX) in comparison with those of age- and sex-matched healthy subjects.
Methods
Forty patients with PEX (PEX group) and 40 age- and sex-matched control subjects (control group) were enrolled in the study. An enzyme immunoassay method using the commercially available test MicroVue YKL-40 was used to measure serum YKL-40 concentration. Systolic and diastolic blood pressures, serum levels of high sensitivity C-reactive protein (hsCRP), total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides were also examined.
Results
The mean age was 54.4±7.6 (ranging 41–65) years in each group. The mean serum YKL-40 level of the PEX group was significantly higher than that of the control group (P<0.001). In addition, the mean serum HsCRP, total cholesterol, LDL, and triglycerides levels were significantly higher, and mean serum HDL level was significantly lower in the PEX group than in the control group (all P<0.001, excluding both P=0.002 for triglycerides and HDL levels). Further, the mean systolic and diastolic blood pressures were significantly higher in the PEX group than in the control group (P1=0.001 and P2=0.01, respectively).
Conclusion
We have shown a relationship between PEX and elevated serum levels of YKL-40. We imply that a better understanding of the role of YKL-40 in the pathogenesis of endothelial dysfunction and atherosclerosis is necessary to develop new therapies for preventing or treating PEX. Further studies are warranted to clarify the clinical relevance of these findings.
Keywords: YKL-40, pseudoexfoliation syndrome, endothelial dysfunction
Introduction
Pseudoexfoliation syndrome (PEX) is an age-related disorder characterized with progressive accumulation of an abnormal extracellular fibrillar material on anterior structures of the eye and extraocular tissues, including periphery of blood vessels, skin, and visceral organs.1, 2 Although its physiopathology has not been documented, it has been thought to be a systemic biochemical process.2 PEX is the most common abnormal finding related with chronic open-angle glaucoma and it may lead to glaucoma in about half of the patients.3, 4, 5 It has been associated with many cardiovascular and cerebrovascular diseases2 and recent studies have suggested it as a marker of systemic vascular diseases.6, 7
YKL-40 is a recently discovered proinflammatory protein that has been demonstrated to have a role in the pathogenesis of endothelial dysfunction and atherosclerosis.8 Increased serum YKL-40 concentration has been found to be associated with cardiovascular morbidity.9, 10 It has been also displayed that YKL-40 could be used as a biomarker for atherosclerosis at very early stages of cardiovascular diseases.11 We hypothesized that YKL-40 could have a role in the pathogenesis of PEX. However, to our best knowledge, there is no study investigating the relationship between serum levels of YKL-40 and PEX.
Accordingly, the aim of this study was to evaluate serum levels of YKL-40 in patients with PEX in comparison with those of age- and sex-matched healthy subjects. Systolic and diastolic blood pressures, serum levels of high sensitivity C-reactive protein (hsCRP), total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides were also examined as predictors of cardiovascular diseases.
Materials and methods
A total of 80 subjects including 40 consecutive patients with PEX (PEX group) and 40 age- and sex-matched control subjects (control group) were enrolled in the study. The diagnosis of PEX was based on the presence of typical exfoliation material on the anterior lens capsule or pupillary margin in one or both eyes, with a normal optic disc and visual field findings in patients with an intraocular pressure (IOP) <21 mm Hg. Control subjects had no history of ocular disease (except for refractive error, strabismus, and cataract) and elevated IOP (>21 mm Hg), and no evidence of exfoliation material on the anterior lens capsule or pupillary margin. They had normal optic disc and visual fields. The study was conducted in accordance with the Declaration of Helsinki and it was approved by the local ethics committee. All subjects were informed about the study procedure and they consented to participate.
All subjects underwent a complete eye examination, including measurement of best-corrected visual acuity (Snellen charts), perimetry (Octopus 900; Haag Streit, Koeniz, Switzerland), slit-lamp examination of the anterior segment, gonioscopic evaluation of the anterior chamber angle, pachymetry, IOP measurement by Goldmann applanation tonometry, and fundoscopy. Subjects who had chronic or recurrent inflammatory eye disease, ocular trauma, ocular infection, severe retinal disease, corneal abnormality, intraocular surgery within the last 12 months, or laser surgery within the last 3 months, malignancy, asthma, collagen vascular disease, chronic kidney and hepatic failure, pulmonary embolism, and sepsis were excluded from the study.
Arterial blood pressure was measured with an appropriate-sized cuff after at least 10-min supine rest. Three consecutive measurements were obtained and the mean was used for analyses.
Laboratory measurements
Blood samples were collected in all patients after fasting at least 8 h. Serum levels of lipid profiles including total cholesterol, LDL, HDL, and triglycerides were measured with the standard laboratory techniques. Serum levels of HsCRP were determined by an immunoturbidimetric method performed on the Abbott auto-analyzer (Architect C16000, Abbott, Abbott Park, IL, USA).
YKL-40 measurement
After 12 h of fasting, venous blood samples were drawn in the morning. Serum specimens were obtained after the samples were centrifuged at 2500 × g for 10 min. Serum specimens for YKL-40 were frozen at −80 °C until analysis. Serum YKL-40 level was determined with an enzyme immunoassay method using the commercially available test MicroVue YKL-40 (Quidel, San Diego, CA, USA) using streptavidin-coated microplate wells, a biotinylated-Fab monoclonal capture antibody, and an alkaline phosphatase-labelled polyclonal detection antibody. The intra-assay and inter-assay coefficients of variation were 6.0 and 6.6%, respectively. The assessments were calibrated by the calibrators inserted in the kit.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The distribution of the variables was checked with Kolmogorov–Smirnov test. As all variables were distributed normally, they were expressed as mean±SD. Paired t-test was used to compare the groups. Binary logistic regression model were used to obtain estimated adjusted odds ratio (OR) and their 95% confidence intervals (CIs) to measure the association between patients with PEX and YKL-40 levels. In this analyses, age, sex, triglycerides, and systolic blood pressure were included as independent variables. Statistical significance was set at P<0.05.
Results
The mean age was 54.4±7.6 (ranging 41–65) years in each group. There were 25 female (62.5%) and 15 males (37.5%) in each group. The mean IOP levels were 15.2±3.3 mm Hg in the PEX group and 14.9±2.9 mm Hg in the control group (P>0.05). Fifteen of 40 (37.5%) subjects had cataract reducing visual acuity in the PEX group. None of patients had diabetes mellitus or any inflammatory disease. There was no patient using non-sterodial anti-inflammatory disease. Thirteen patients (32.5%) in the PEX group and eight patients (20%) in the control group had arterial hypertension. However, there was no significant difference between the groups concerning rate of patients with arterial hypertension (P=0.204).
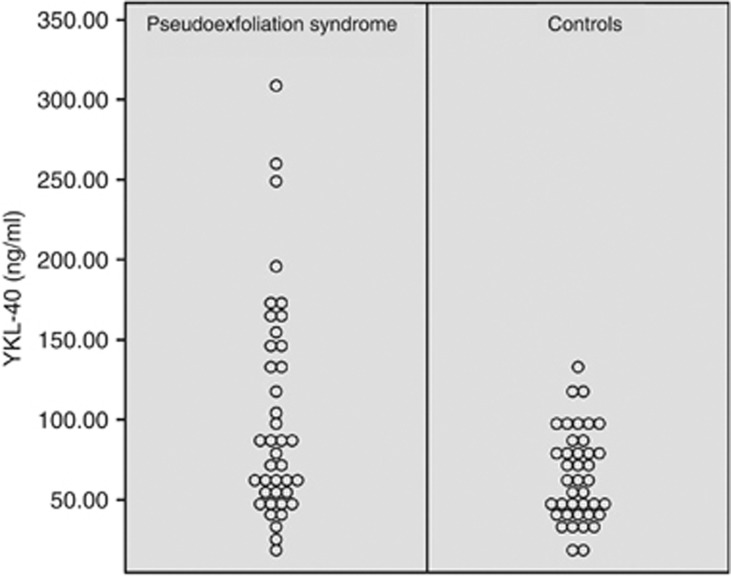
The clinical parameters of the groups are given in Table 1. The mean serum YKL-40 level of the PEX group was significantly higher than that of the control group (P<0.001, Figure 1). In addition, the mean serum HsCRP, total cholesterol, LDL, and triglycerides levels were significantly higher and mean serum HDL level was significantly lower in the PEX group than in the control group (all P<0.001, excluding P=0.002 for triglycerides and HDL levels). Further, the mean systolic and diastolic blood pressures were significantly higher in the PEX group than in the control group (P1=0.001 and P2=0.01, respectively).
Table 1. Clinical characteristics of the groups (mean±SD).
| Characteristics | PEX group (n=40) | Control group (n=40) | P-value |
|---|---|---|---|
| YKL-40 (ng/ml) | 102.5±68.1 | 65.0±28.0 | <0.001 |
| HsCRP (mg/dl) | 0.7±0.6 | 0.3±0.2 | <0.001 |
| Total cholesterol (mg/dl) | 200.1±26.8 | 145.5±47.0 | <0.001 |
| LDL (mg/dl) | 128.3±24.1 | 104.2±29.0 | <0.001 |
| HDL (mg/dl) | 44.7±14.5 | 53.5±14.2 | 0.002 |
| Triglycerides (mg/dl) | 134.4±49.5 | 107.4±36.3 | 0.002 |
| Systolic blood pressure (mm Hg) | 127.5±9.6 | 121.8±8.2 | 0.001 |
| Diastolic blood pressure (mm Hg) | 70.1±11.4 | 65.2±6.9 | 0.010 |
Abbreviations: HDL, high-density lipoprotein cholesterol; HsCRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein cholesterol.
Figure 1.
Individual serum YKL-40 levels of PEX and control groups. There was significant difference between the mean YKL-40 levels of the groups (P<0.001).
Binary logistic regression model showed an association between patients with PEX and serum YKL-40 levels, even after additional adjustment for age, sex, triglycerides, and systolic blood pressure (OR: 1.015, 95% CI: 1.001–1.029, P=0.036) (Table 2).
Table 2. Binary logistic regression model for pseudoexfoliation syndrome (dependent variable).
| Independent variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Age (per year) | 0.958 (0.890–1.031) | 0.248 |
| Sex (male) | 0.645 (0.218–1.904) | 0.427 |
| YKL-40 (ng/ml) | 1.015 (1.001–1.029) | 0.036 |
| Triglycerides (mg/dl) | 1.009 (0.996–1.022) | 0.193 |
| SBP (mm Hg) | 1.056 (0.995–1.121) | 0.072 |
Abbreviations: CI, confidence interval; SBP, systolic blood pressure.
Conclusion
The present study demonstrated an association between elevated serum YKL-40 levels and PEX. In addition, we revealed that the mean serum HsCRP, total cholesterol, LDL and triglycerides levels, and the mean systolic and diastolic blood pressures were significantly higher whereas the mean serum HDL level was significantly lower in PEX patients than in control subjects.
The acute phase protein YKL-40 is a new potential biomarker of inflammation and vascular dysfunction in patients with coronary artery diseases.12 It is a 40-kDa heparin- and chitin-binding glycoprotein which is also known as human cartilage glycoprotein 39 (HCgp39), 38-kDa heparin-binding glycoprotein or chitinase-3-like protein 1.13, 14, 15 It is expressed by several cell types of the immune system.8, 12, 16, 17 The contribution of YKL-40 in inflammatory conditions and vascular processes implies that YKL-40 may have a role in endothelial dysfunction and atherosclerosis. In endothelial dysfunction, increased YKL-40 levels seem to be involved in relation to cell migration, reorganization, and tissue remodeling as a response to endothelial damage.14, 18, 19 Recently, several clinical studies have described an association between increased YKL-40 levels, and several cardiovascular conditions, myocardial infarction, atrial fibrillation, and mortality.10, 20, 21
PEX is a disorder of the extracellular matrix that is frequently related to severe chronic secondary open-angle glaucoma.2 Extraocular deposition of pseudoexfoliation material has been localized to the connective tissues or septa traversing the organ tissue. These deposits are associated with the presence of elastic fibers, collagen fibers, fibroblasts, and the walls of small blood vessels, suggesting the systemic nature of PEX. Previous studies have displayed that PEX was related to many cardiovascular and cerebrovascular diseases, such as transient ischemic attacks, myocardial infarction, stroke, aneurysms of the abdominal aorta, and asymptomatic myocardial dysfunction.7, 22, 23, 24 In a previous study, PEX was found to be positively associated with the risk for cardiovascular diseases among patients with age ≥50, and PEX was suggested as a risk factor for cardiovascular diseases.25 To our best knowledge, we are the first to show the elevated serum YKL-40 levels, a new potential biomarker of inflammation and vascular dysfunction, in patients with PEX.
In our study, PEX group had also elevated systolic and diastolic blood pressures, serum HsCRP, total cholesterol, LDL, and triglycerides levels, and reduced serum HDL levels, which were predictors for cardiovascular diseases. Yüksel et al26 evaluated the hsCRP levels in patients with PEX and pseudoexfoliation glaucoma in comparison with controls. Their results were conflicting with the present study. They found that there was no difference among the group concerning hsCRP levels. In addition, they found that the groups were similar regarding cholesterol, LDL, triglyceride, and systolic and diastolic blood pressures. We believe that this disagreement might be due to different study populations as Yüksel et al excluded patients with cardiovascular disease, serious hypertension, and hypercholesterolemia, which we didn't exclude. In accordance with our results, Miyazaki et al27 showed that hypertension was strongly correlated with PEX in patients aged 50 years or older.
Previous studies emphasized that inflammatory mechanisms might have a significant role in the pathogenesis of PEX.28, 29 Cumurcu et al30 displayed that serum α 1-antitrypsin, an acute phase protein associated with inflammatory processes, was significantly higher in PEX patients than in controls. Fiore et al31 found that eyes with PEX had a greater post-laser inflammatory reaction than eyes without PEX. Elevated homocysteine levels in PEX and its association with chronic inflammation have also been revealed.32, 33, 34 Increased homocysteine stimulates proinflammatory markers such as interleukin (IL)-1β, IL-6, IL-12, IL-18, and CRP.35, 36, 37 We believe that chronic and systemic inflammation in PEX syndrome may be related with increased serum YKL-40 levels.
It has been reported that in cases of established PEX, plasma homocysteine levels were elevated.38 Therefore, hyper-homocysteinemi has been thought to have a role in the pathogenesis of PEX. Homocysteine, a sulphur-containing amino acid synthesized during the metabolism of methionine, is a well-known independent risk factor for vascular diseases, and may contribute to ischemic alterations and oxidative stress.39 In this regard, in the present study patients with PEX had elevated YKL-40 levels, so it can be said that vascular disorders may have a role in the pathogenesis of PEX. Accordingly, we can speculate that both YKL-40 and homocysteine may have an indirect role in the pathogenesis of PEX by causing vascular disorders.
Bojesen et al40 investigated YKL-40 in 3130 Danish people with no known disease, aged 20–80 years. They demonstrated that plasma YKL-40 increased with age within and across healthy individuals from the general population. They suggested using age-stratified or age-adjusted reference levels for evaluating YKL-40 test results. Therefore, in the present study, we compared age-matched groups to prevent influence of age on mean YKL-40 levels. Further, after additional adjustment for age, we found that there was an extant association between patients with PEX and serum YKL-40 levels.
In conclusion, we have shown a relationship between PEX and elevated serum levels of YKL-40. We imply that a better understanding of the role of YKL-40 in the pathogenesis of endothelial dysfunction and atherosclerosis is necessary to develop new therapies for preventing or treating PEX. Further studies are warranted to clarify the clinical relevance of these findings.
The authors declare no conflict of interest.
References
- Streeten BW, Li Z-Y, Wallace RN, Eagle RC, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophtalmol. 1992;110:1757–1762. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Vesti E, Kivela T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res. 2000;19:345–368. doi: 10.1016/s1350-9462(99)00019-1. [DOI] [PubMed] [Google Scholar]
- Ritch R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3:176–177. [PubMed] [Google Scholar]
- Hietanen J, Soisalon-Soininen S, Kivela T, Tarkkanen A. Evaluation of the clinical association between exfoliation syndrome and abdominal aortic aneurysm. Acta Ophthalmol Scand. 2002;80:617–619. doi: 10.1034/j.1600-0420.2002.800611.x. [DOI] [PubMed] [Google Scholar]
- Schumacher S, Schlotzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001;357:359–360. doi: 10.1016/s0140-6736(00)03645-x. [DOI] [PubMed] [Google Scholar]
- Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. 2006;55:221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143:35–42. doi: 10.1016/j.ijcard.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, et al. High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J. 2009;30:1066–1072. doi: 10.1093/eurheartj/ehp049. [DOI] [PubMed] [Google Scholar]
- Boot RG, van Achterberg TA, van Aken BE. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–694. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270:13076–13083. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39(CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem. 1995;270:26252–26256. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical veinendothelial cells. Exp Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbruchel DA, Friis T, et al. YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J. 2008;42:295–302. doi: 10.1080/14017430802220567. [DOI] [PubMed] [Google Scholar]
- Nojgaard C, Host NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, et al. Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coron Artery Dis. 2008;19:257–263. doi: 10.1097/MCA.0b013e3282f40dd5. [DOI] [PubMed] [Google Scholar]
- Repo LP, Teräsvirta ME, Koivisto KJ. Generalized transluminance of the iris and the frequency of the pseudoexfoliation syndrome in the eyes of transient ischemic attack patients. Ophthalmology. 1993;100:352–355. doi: 10.1016/s0161-6420(93)31642-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124:685–687. doi: 10.1016/s0002-9394(14)70908-0. [DOI] [PubMed] [Google Scholar]
- Bojic L, Ermacora R, Polic S, Ivanisevic M, Mandic Z, Rogosic V, et al. Pseudoexfoliation syndrome and asymptomatic myocardial dysfunction. Graefes Arch Clin Exp Ophthalmol. 2005;243:446–449. doi: 10.1007/s00417-004-1074-9. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos GK, Mela EK, Georgakopoulos CD, Papadopoulos GE, Damelou AN, Alexopoulos DK, et al. Pseudoexfoliation syndrome prevalence in Greek patients with cataract and its association to glaucoma and coronary artery disease. Eye. 2009;23:442–447. doi: 10.1038/sj.eye.6702992. [DOI] [PubMed] [Google Scholar]
- Yüksel N, Pirhan D, Altintaş O, Cağlar Y. Systemic high-sensitivity C-reactive protein level in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Glaucoma. 2010;19:373–376. doi: 10.1097/IJG.0b013e3181bdb570. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Kubota T, Kubo M, Kiyohara Y, Iida M, Nose Y, et al. The prevalence of pseudoexfoliation syndrome in a Japanese population: the Hisayama study. J Glaucoma. 2005;14:482–484. doi: 10.1097/01.ijg.0000185436.15675.b3. [DOI] [PubMed] [Google Scholar]
- Damji KF, Bains HS, Stefansson E, Loftsdottir M, Sverrisson T, Thorgeirsson E, et al. Is pseudoexfoliation syndrome inherited? A review of genetic and nongenetic factors and a new observation. Ophthalmic Genet. 1998;19:175–185. doi: 10.1076/opge.19.4.175.2310. [DOI] [PubMed] [Google Scholar]
- Janciauskiene S, Krakau T. Alzheimer's peptide and serine proteinase inhibitors in glaucoma and exfoliation syndrome. Doc Ophthalmol. 2003;106:215–223. doi: 10.1023/a:1022949121078. [DOI] [PubMed] [Google Scholar]
- Cumurcu T, Ozyurt H, Demir HD, Yardim H. Serum alpha-1-antitriypsin levels in patients with pseudoexfolative syndrome. Curr Eye Res. 2008;33:159–162. doi: 10.1080/02713680701861752. [DOI] [PubMed] [Google Scholar]
- Fiore PM, Melamed S, Epstein DL. Trabecular precipitates and elevated intraocular pressure following argon laser trabeculoplasty. Ophthalmic Surg. 1989;20:697–701. [PubMed] [Google Scholar]
- Cumurcu T, Sahin S, Aydin E. Serum homocysteine, vitamin B12 and folic acid levels in different types of glaucoma. BMC Ophthalmol. 2006;6:6. doi: 10.1186/1471-2415-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puustjarvi T, Blomster H, Kontkanae M, Punnonen K, Terasvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:749–754. doi: 10.1007/s00417-004-0918-7. [DOI] [PubMed] [Google Scholar]
- Yxfeldt A, Wallberg-Jonsson S, Hultdin J, Rantapaa-Dahlqvist S. Homocysteine in patients with rheumatoid arthritis in relation to inflammation and B-vitamin treatment. Scand J Rheumatol. 2003;32:205–210. doi: 10.1080/03009740310003686. [DOI] [PubMed] [Google Scholar]
- Su SJ, Huang LW, Pai LS, Liu HW, Chang KL. Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition. 2005;21:994–1002. doi: 10.1016/j.nut.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Holven KB, Aukrust P, Retterstol K, Hangve TA, Morkid L, Ose L, et al. Scand J Clin Lab Invest. 2006. pp. 45–54. [DOI] [PubMed]
- Tso TK, Huang WN, Huang HY, Chang CK. Relationship of plasma interleukin-18 concentrations to traditional and non-traditional cardiovascular risk factors in patients with systemic lupus erythematosus. Rheumatology. 2006;45:1148–1153. doi: 10.1093/rheumatology/kel082. [DOI] [PubMed] [Google Scholar]
- Vasavada RM, Ritch R, Liebmann JM, Jole M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol. 2003;136:41–46. doi: 10.1016/s0002-9394(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Tranchina L, Centofanti M, Oddone F, Tanga L, Roberti G, Liberatoscioli L, et al. Levels of plasma homocysteine in pseudoexfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:443–448. doi: 10.1007/s00417-010-1487-6. [DOI] [PubMed] [Google Scholar]
- Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta. 2011;412:709–712. doi: 10.1016/j.cca.2011.01.022. [DOI] [PubMed] [Google Scholar]