Abstract
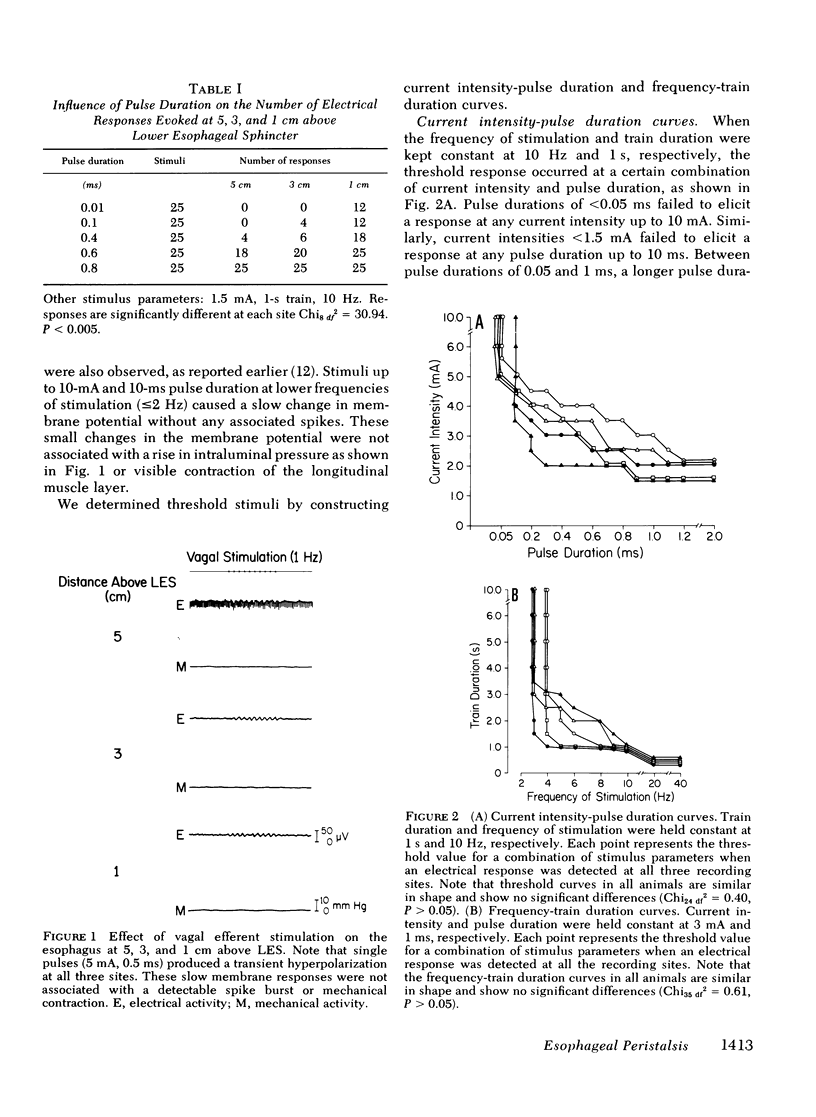
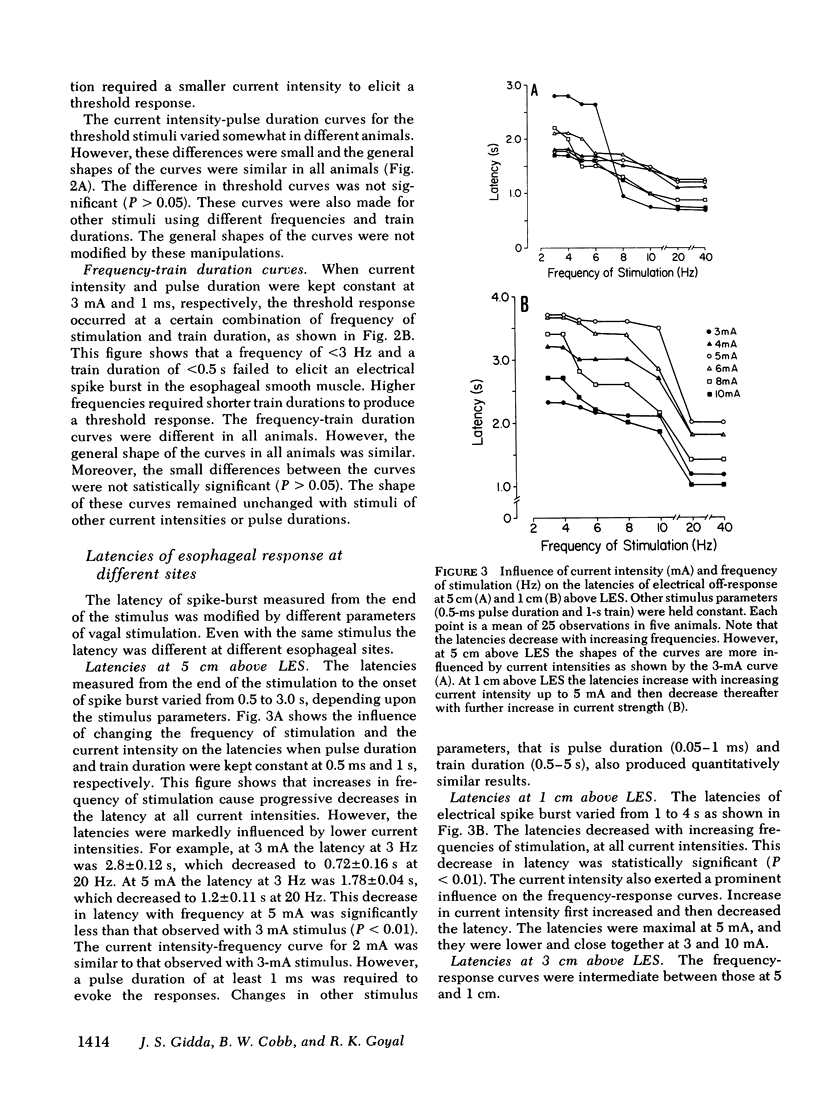
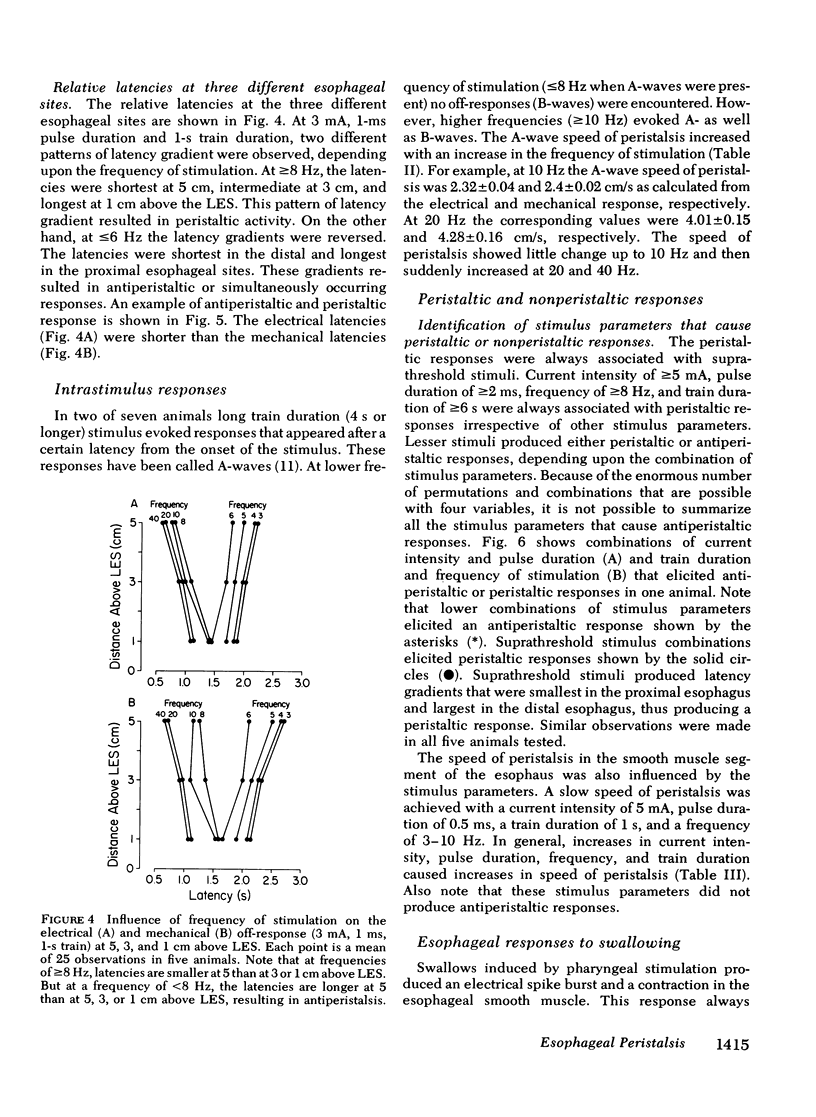
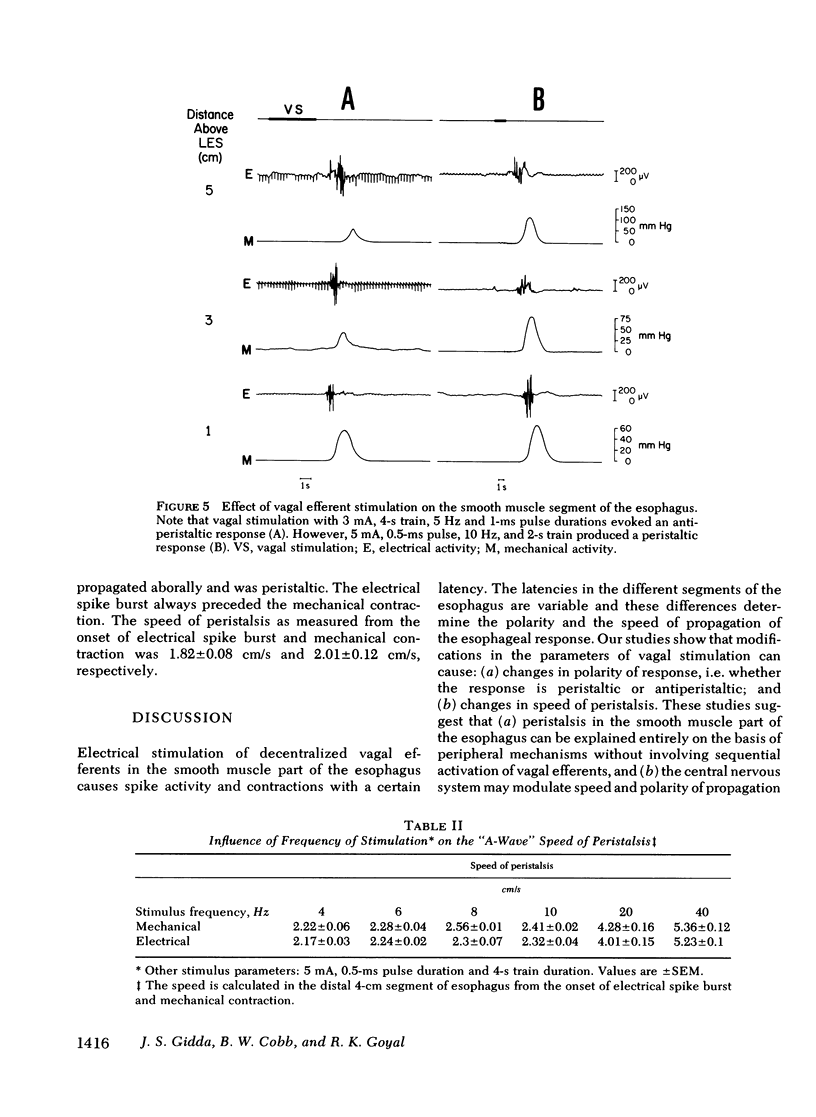
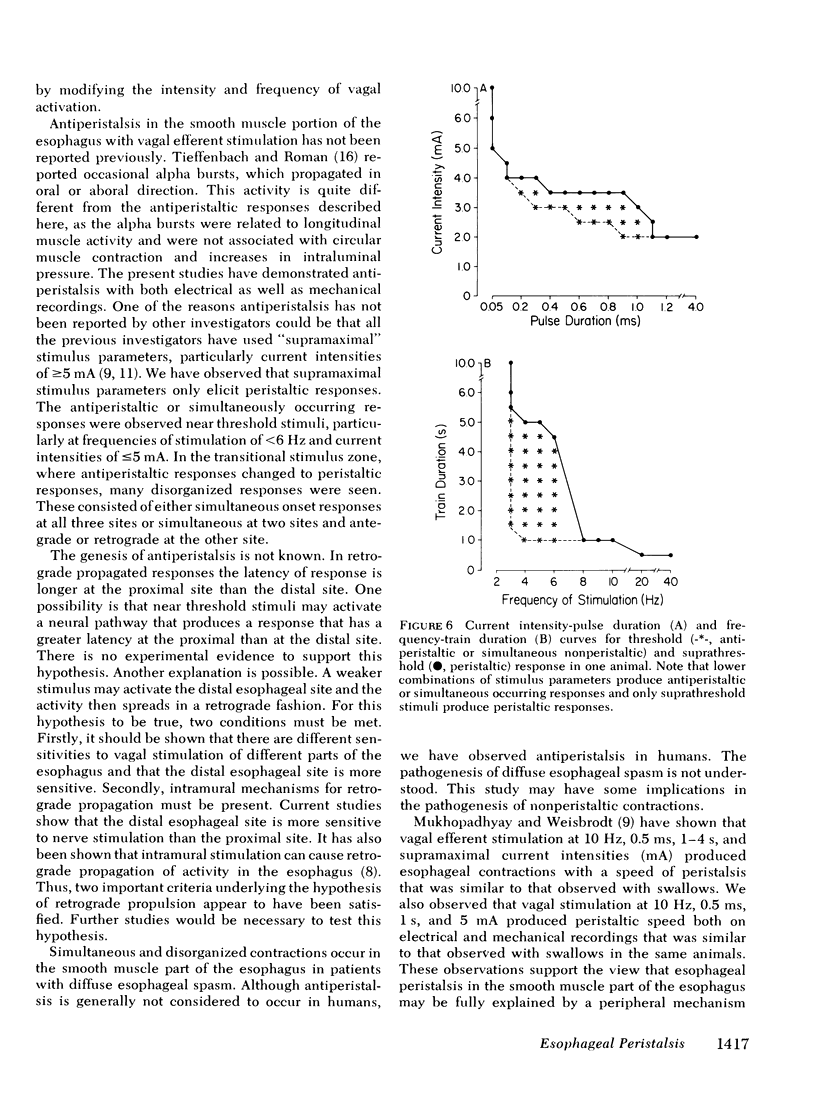
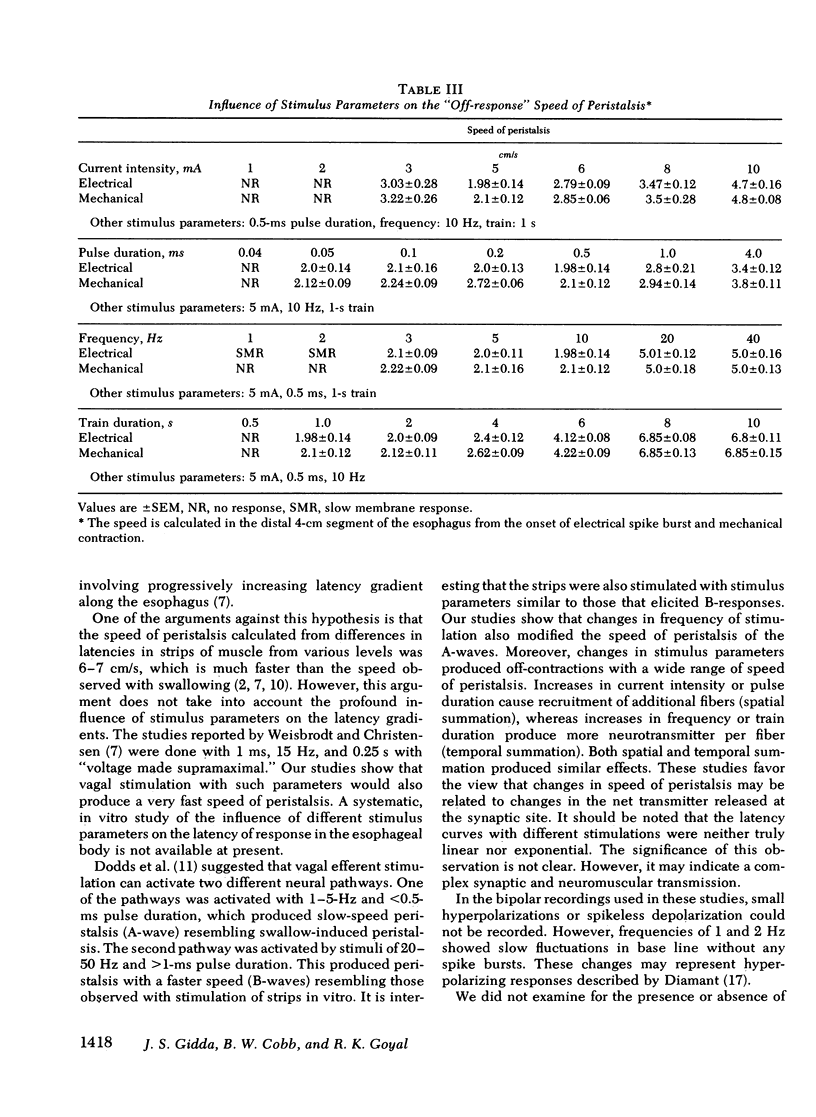
Experiments were performed on anesthetized opossums to study the influence of vagal efferent stimulation on peristalsis in the esophageal smooth muscle using various stimulus parameters. Current intensity, pulse duration, frequency, and train duration were varied systematically. Electrical and mechanical activities were recorded simultaneously at 5, 3, and 1 cm above the lower esophageal sphincter (LES). Vagal efferent stimulation produced a spike burst and contraction with a latency after the termination of the stimulus. This latency varied at different sites with the same stimulus parameters. For example, a stimulus of 5 mA, 0.5 ms, 10 Hz, and 1-s train produced latencies for the electrical response of 1.48 +/- 0.04, 2.2 +/- 0.12, and 3.5 +/- 0.09 s (+/- SEM) at 5, 3, and 1 cm above LES, respectively. The differences in latency were statistically significant (P less than 0.01). The latency of response at any one site also changed with different stimulus parameters; e.g. at 1 cm above LES, the latency of electrical response at 10 Hz was 3.5 +/- 0.09 s, but at 20 Hz the latency was 2.01 +/- 0.06 s when current intensity, pulse, and train duration remained at 5 mA, 0.5 ms, and 1 s. This decrease in latency with increasing frequency was statistically significant (P less than 0.01). By changing stimulus parameters, antiperistalsis or peristalsis with different speeds of propagation could be induced. Antiperistalsis or simultaneous responses occurred near threshold stimulus parameters. Suprathreshold stimuli produced peristaltic responses. Speed of peristalsis in the distal esophagus was 1.82 +/- 0.08 cm/s with swallowing, which was not different from 1.98 +/- 0.14 cm/s (P greater than 0.05) with vagal stimulation of 5 mA, 0.5 ms, 10 Hz, and 1-s train. These studies suggest that: (a) peristalsis in the smooth muscle part of the esophagus can be explained entirely on the basis of peripheral mechanisms, and (b) the central nervous system may modulate the occurrence, polarity, and speed of propagation by modifying the intensity and frequency of vagal activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen J., Arthur C., Conklin J. L. Some determinants of latency of off-response to electrical field stimulation in circular layer of smooth muscle of opossum esophagus. Gastroenterology. 1979 Oct;77(4 Pt 1):677–681. [PubMed] [Google Scholar]
- Christensen J., Lund G. F. Esophageal responses to distension and electrical stimulation. J Clin Invest. 1969 Feb;48(2):408–419. doi: 10.1172/JCI105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. Patterns and origin of some esophageal responses to stretch and electrical stimulation. Gastroenterology. 1970 Dec;59(6):909–916. [PubMed] [Google Scholar]
- Diamant N. E., El-Sharkawy T. Y. Neural control of esophageal peristalsis. A conceptual analysis. Gastroenterology. 1977 Mar;72(3):546–556. [PubMed] [Google Scholar]
- Dodds W. J., Christensen J., Dent J., Wood J. D., Arndorfer R. C. Esophageal contractions induced by vagal stimulation in the opossum. Am J Physiol. 1978 Oct;235(4):E392–E401. doi: 10.1152/ajpendo.1978.235.4.E392. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Gidda J. S. Relation between electrical and mechanical activity in esophageal smooth muscle. Am J Physiol. 1981 Apr;240(4):G305–G311. doi: 10.1152/ajpgi.1981.240.4.G305. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Genesis of basal sphincter pressure: effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology. 1976 Jul;71(1):62–67. [PubMed] [Google Scholar]
- Mukhopadhyay A. K., Weisbrodt N. W. Neural organization of esophageal peristalsis: role of vagus nerve. Gastroenterology. 1975 Mar;68(3):444–447. [PubMed] [Google Scholar]
- Rattan S., Goyal R. K. Neural control of the lower esophageal sphincter: influence of the vagus nerves. J Clin Invest. 1974 Oct;54(4):899–906. doi: 10.1172/JCI107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C. Contrôle nerveux du péristaltisme oesophagien. J Physiol (Paris) 1966 Jan-Feb;58(1):79–108. [PubMed] [Google Scholar]
- Roman C., Tieffenbach L. Enregistrement de l'activité unitaire des fibres motrices vagales destinées à l'oesophage du babouin. J Physiol (Paris) 1972;64(5):479–506. [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E., Waterfall W. E. Myogenic and neural control systems for esophageal motility. Gastroenterology. 1977 Dec;73(6):1345–1352. [PubMed] [Google Scholar]
- Tieffenbach L., Roman C. Rle de l'innervation extrinsèque vagale dans la motricité de l'oesophage à musculeuse lisse: Etude électromyographique chez le chat et le babouin. J Physiol (Paris) 1972;64(3):193–226. [PubMed] [Google Scholar]
- Weisbrodt N. W., Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 1972 Jun;62(6):1159–1166. [PubMed] [Google Scholar]
- Weisbrodt N. W. Neuromuscular organization of esophageal and pharyngeal motility. Arch Intern Med. 1976 May;136(5):524–531. [PubMed] [Google Scholar]


