Abstract
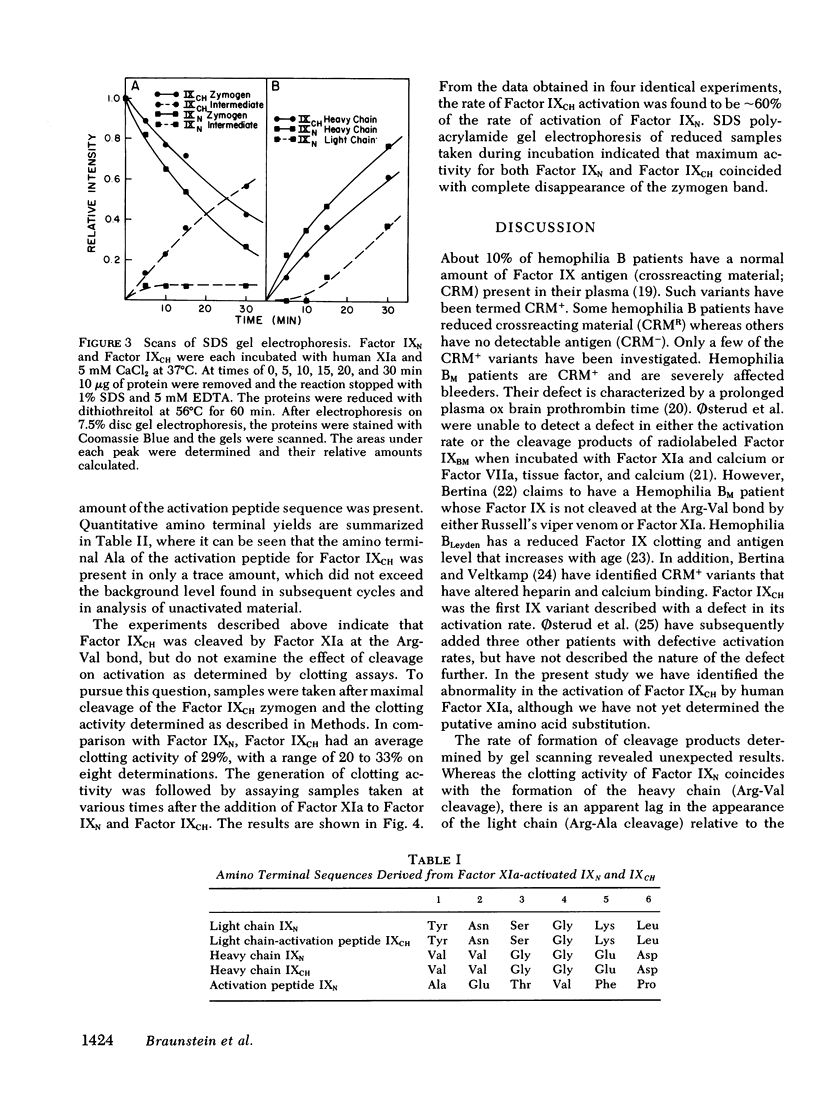
Factor IXChapel Hill (Factor IXCH), an abnormal Factor IX molecule isolated from the plasma of a patient with mild hemophilia B, has previously been shown to exhibit delayed activation by Factor XIa and calcium. In this study, we have found that Factor IXCH is cleaved upon incubation with human Factor XIa and calcium; however, cleavage of this protein is not observed by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis under nonreducing conditions. Under reducing conditions, the rate of disappearance of the zymogen parallels both the appearance of the heavy chain and the generation of clotting activity. In addition, a protein band that migrates with an apparent molecular weight of 45,000 also increases in parallel with clotting activity. Factor IXCH and normal Factor IX (Factor IXN), after incubation with Factor XIa and calcium, were subjected to amino terminal sequence analysis. Activated Factor IXN is cleaved at an arginine-alanine (Arg-Ala) bond and an arginine-valine (Arg-Val) bond as demonstrated by formation of the three amino terminal sequences corresponding to the amino terminal of the light chain, heavy chain, and activation peptide. However, activated Factor IXCH has only two amino terminal sequences, corresponding to the original amino terminal sequence and the heavy chain (formed by cleavage at the Arg-Val bond). It is concluded that the major defect in Factor IXCH is the inability of Factor XIa to cleave the Arg-Ala bond at a significant rate. The rate of formation of clotting activity of Factor IXCH is approximately 60% of the rate of formation of clotting activity of Factor IXN. The specific clotting activity of activated Factor IXCH is between 20 and 33% of activated Factor IXN.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertina R. M., Veltkamp J. J. The abnormal factor IX of hemophilia B+ variants. Thromb Haemost. 1978 Oct 31;40(2):335–349. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- CATTAN A. D., DENSON K. W. THE INTERACTION OF CONTACT PRODUCT AND FACTOR IX. Thromb Diath Haemorrh. 1964 Apr 15;11:155–166. [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S., SIMPSON M. E. The chromatography of growth hormone on cellulose derivatives. J Biol Chem. 1956 Jun;220(2):939–949. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Furie B. C., Furie B. Coagulant protein of Russell's viper venom. Methods Enzymol. 1976;45:191–205. doi: 10.1016/s0076-6879(76)45019-x. [DOI] [PubMed] [Google Scholar]
- Hougie C., Twomey J. J. Haemophilia Bm: a new type of factor-IX deficiency. Lancet. 1967 Apr 1;1(7492):698–700. doi: 10.1016/s0140-6736(67)92179-4. [DOI] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- NOSSEL H. L. THE ACTIVATION AND CONSUMPTION OF FACTOR IX. Thromb Diath Haemorrh. 1964 Dec 31;12:505–509. [PubMed] [Google Scholar]
- Neal W. R., Tayloe D. T., Jr, Cederbaum A. I., Roberts H. R. Detection of genetic variants of haemophilia B with an immunosorbent technique. Br J Haematol. 1973 Jul;25(1):63–68. doi: 10.1111/j.1365-2141.1973.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Osterud B., Kasper C. K., Lavine K. K., Prodanos C., Rapaport S. I. Purification and properties of an abnormal blood coagulation factor IX (factor IXBm)/kinetics of its inhibition of factor X activation by factor VII and bovine tissue factor. Thromb Haemost. 1981 Feb 23;45(1):55–59. [PubMed] [Google Scholar]
- Osterud B., Kasper C. K., Prodanos C. Factor IX variants of hemophilia B. The effect of activated factor XI and the reaction product of factor VII and tissue factor on the abnormal factor IX molecules. Thromb Res. 1979;15(1-2):235–243. doi: 10.1016/0049-3848(79)90069-0. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFMAN S., RAPAPORT S. I., PATCH M. J. THE IDENTIFICATION AND SYNTHESIS OF ACTIVATED PLASMA THROMBOPLASTIN COMPONENT (PTC'). Blood. 1963 Dec;22:733–749. [PubMed] [Google Scholar]
- Veltkamp J. J., Meilof J., Remmelts H. G., van der Vlerk D., Loeliger E. A. Another genetic variant of haemophilia B: haemophilia B Leyden. Scand J Haematol. 1970;7(2):82–90. doi: 10.1111/j.1600-0609.1970.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]
- YIN E. T., DUCKERT F. The formation of intermediate product I in a purified system. The role of factor IX or its precursor and of a Hageman factor-PTA fraction. Thromb Diath Haemorrh. 1961 Sep 1;6:224–234. [PubMed] [Google Scholar]
- Zur M., Nemerson Y. Kinetics of factor IX activation via the extrinsic pathway. Dependence of Km on tissue factor. J Biol Chem. 1980 Jun 25;255(12):5703–5707. [PubMed] [Google Scholar]



