Abstract
Liver X Receptor α is a nuclear transcription factor that regulates lipid metabolism. Recently, it has been shown that activation of LXRα with synthetic ligands has anti-inflammatory effects in atherosclerosis and chemical-induced dermatitis. Here, we investigated the effect of the LXRα agonist T0901317 on lung inflammation in a rodent model of hemorrhagic shock. Hemorrhagic shock was induced in male rats by withdrawing blood to a goal mean arterial blood pressure of 50 mmHg. Blood pressure was maintained at this level for 3 hours, at which point rats were rapidly resuscitated with shed blood. Animals were then treated with T0901317 (50 mg/kg) or vehicle intraperitoneally and sacrificed at 1, 2 and 3 hours after resuscitation. Treatment with T0901317 significantly improved the cardiac and stroke volume indices as well as heart rate of rats during the resuscitation period as compared to vehicle-treated rats. T0901317-treated animals showed significant improvement in the plasma level of lactate, while base deficit and bicarbonate levels both trended towards improvement. T0901317-treated animals also showed lower levels of the plasma cytokines and chemokines MCP-1, MIP-1α, TNF-α, KC and IL-6. Lung injury and neutrophil infiltration were reduced by treatment with T0901317 as evaluated by histology and myeloperoxidase assay. At molecular analysis, treatment with T0901317 increased nuclear LXRα expression and DNA binding while also inhibiting activation of NF-κB, a pro-inflammatory transcription factor, in the lung. Thus, our data suggest that LXRα is an important modulator of the inflammatory response and lung injury after severe hemorrhagic shock, likely through the inhibition of the NF-κB pathway.
Keywords: Hemorrhage, liver X receptor α, nuclear factor-κB, acute lung injury, T0901317
INTRODUCTION
Traumatic injury and subsequent hemorrhage continue to be a major worldwide health concern. Hemorrhage is the second-leading cause of mortality amongst all trauma victims, leading to 30–40% of all deaths in injured patients (1). While many deaths occur in the pre-hospital phase, some patients reach the hospital and enter the resuscitative phase of care. As a result of injury and hemorrhage, these patients show a massive inflammatory response. This inflammatory response results in the release of many cytokines and chemokines as well as activation of the innate immune system. The initiation of this cascade can quickly change from adaptive to maladaptive and lead to further injury including multi-organ failure (MOF) and death (2–4). Given the severity of the response and its consequences, down-regulation of this inflammatory cascade has been a focus of research in attempts to avoid the MOF and death often encountered after hemorrhage and resuscitation.
The Liver X Receptor (LXR) is a ligand-dependent nuclear transcription factor that is a regulator at the intersection between metabolism and inflammation (5–9). There are two sub-types of LXR, α and β. While LXRβ is expressed ubiquitously, LXRα expression is confined to select organs including the spleen, liver, intestine, adipose and lung. LXRα can be activated by natural ligands, mainly oxysterols, or synthetic ligands such as T0901317 or GW3965. Once activated, the nuclear receptor forms a heterodimer with Retinoid X Receptor (RXR). This heterodimer then binds to the LXR response element (LXRE) on DNA, leading to transcription of various genes important in cholesterol metabolism. (5–9).
Several in vitro studies have shown that LXR agonism not only regulates cholesterol metabolism, but also exerts anti-inflammatory effects. It has been shown that activation of LXRα decreases pro-inflammatory cytokine levels following endotoxin exposure. These cytokines include tumor necrosis factor α (TNFα), inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), macrophage inflammatory protein1α (MIP-1α), matrix metalloproteinase-9 (MMP-9) and monocyte chemoattractant protein 1(MCP-1)(10–13). In addition to these in vitro anti-inflammatory effects, LXRα agonism has also been shown to decrease inflammation in vivo. In models of contact dermatitis, tissue inflammation was markedly reduced with LXRα activation (11, 14). Further studies have also shown in vivo reduction of lung neutrophil infiltration after exposure to irritants and LPS (15, 16).
The exact mechanism through which LXRα activation reduces inflammation remains unclear, but many studies point to a regulation of the Nuclear Factor-κB (NF-κB) pathway as a possible mechanism. NF-κB is a pro-inflammatory transcription factor that initiates transcription of a multitude of pro-inflammatory mediators, including cytokines, chemokines and adhesion molecules (17). It has long been acknowledged that NF-κB is a key regulator in the inflammatory response and would be an ideal target for therapeutic intervention aimed at preventing the overwhelming inflammation seen after insults such as hemorrhagic shock (18). We have previously shown that some of the anti-inflammatory properties of LXRα activation may be secondary to reduction of NF-κB activity. Our in vitro study showed that LXR agonism prevented the production of pro-inflammatory cytokines by reducing IκB alpha degradation and subsequent NF-κB activation in macrophages (19).
These promising results suggest that LXRα activation could be a key component in reduction and control of the overwhelming inflammatory response following hemorrhagic shock. Our aim in this study was to explore the anti-inflammatory effects of the LXRα agonist T0901317 in a rodent model of hemorrhagic shock. We hypothesized that activation of LXRα by T0901317 would stem the systemic inflammatory response via inhibition of the NF-κB pathway and subsequently reduce hemodynamic abnormalities and hemorrhage-induced lung injury.
MATERIALS AND METHODS
Measurement of hemodynamic parameters
All aspects of this study complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23 revised 1996) and met approval of the Institutional Animal Care and Use Committee. Male Wistar rats (Charles River Laboratories, Wilmington MA) weighing between 240–310 grams were subjected to hemorrhagic shock. Each animal was anesthesized using intraperitoneal pentobarbital (80 mg/kg). Tracheostomy was then performed and the animal was ventilated at a respiratory rate of 60 breaths per minute, tidal volume of 7 mL/kg and FiO2 of 0.4 using a rodent ventilator (Harvard Apparatus, Holliston MA). Temperature was maintained at 37° C using a homeothermic blanket. The left carotid artery and right femoral artery were then cannulated with Polyethylene-50 tubing. For cardiac output measurement, polyethylene-10 tubing was inserted into the right internal jugular vein as well. Cardiac output (mL/min) was measured using a thermodilution technique (20). A thermistor was passed into the left carotid artery to the carotid arch. 0.15 mL of normal saline at room temperature was then rapidly injected into the right internal jugular vein. Heart rate (HR), mean arterial blood pressure (MABP) and cardiac output were measured using a Maclab A/D Converter and cardiac output pod (AD Instruments, Milford MA). The cardiac index (CI, mL/min/100g), total peripheral resistance index (TPRI, mmHg/mL/min/100g) and stroke volume index (SVI, mL/100g) were then calculated from computed integral values of thermodilution curves using standard arithmetic formulae.
Hemorrhagic shock model
After completion of the surgical procedure, rats were dosed with intravenous heparin to facilitate hemorrhage (100 IU/kg). Hemorrhagic shock was then induced using a pressure-controlled model as previously described (21). Blood was steadily withdrawn from the femoral arterial catheter until a MABP of 50 mmHg was obtained. This MABP was then maintained for a period of three hours by withdrawing or re-instilling small volumes of shed blood. After three hours of shock state, shed blood was rapidly re-infused over 5 minutes to resuscitate the animal. If re-transfusion of small volumes of blood were needed during the hypoperfusion period to maintain MABP at 50 mmHg, rapid resuscitation at the conclusion of hemorrhage was performed by transfusing the remaining shed blood supplemented with Ringer Lactate solution to a final volume of fluids equal to the initial total shed blood.
Animals were then randomly divided into three groups: 1) Rats in the vehicle hemorrhagic shock group received vehicle (100% dimethyl sulfoxide) instead of T0901317 (N=18). 2) Rats in the treatment group received T0901317 at a 50 mg/kg dose (N=16). 3) Sham operated animals served as control at time=0 and underwent the same surgical procedure but were not bled (N=4). T0901317 and vehicle were delivered intraperitoneally (i.p.) as a bolus at the beginning of resuscitation (180 minutes) and every hour thereafter for a maximum of three doses. Rats were sacrificed at 1, 2 and 3 hours post-resuscitation. Plasma and lung samples were collected for histologic and biochemical studies.
Plasma lactate, base deficit and bicarbonate levels
Plasma levels of lactate, base deficit and bicarbonate were measured at times 0, 3 and 6 hours using a commercially available i-Stat system (Abbott Point of Care, Princeton, NJ).
Plasma levels of cytokines and chemokines
Plasma levels of MIP-1α, TNFα, IL-6, interleukin −10 (IL-10), KC, and MCP-1 were analyzed using a luminex multiplex system (Luminex Corporation, Austin TX) according to instructions from the manufacturer.
Plasma Cholesterol
Plasma levels of total cholesterol were measured by enzymatic procedures using a commercially available kit (Wako Diagnostics, Richmond VA).
Histology
Lung tissue was harvested and placed immediately in 10% neutral buffered formalin. The tissue was then embedded in formalin, sectioned and stained with hematoxylin and eosin. Light microscopy was used to evaluate cross-sections for tissue damage and inflammation.
Myeloperoxidase assay
Myeloperoxidase was measured as an indication of neutrophil infiltration in lung tissue following hemorrhagic shock. Lung tissue was homogenized in a buffer containing 0.5% HTAB in 10 mM MOPS. After homogenization, tissue was centrifuged for 30 min at 4,000 rpm at 4° C and supernatant was collected. Supernatant was then mixed with 3,3,5,5 tetramethylbenzidine and sodium phosphate buffer pH 5.5 in a 1:20 dilution. After 5 minutes of incubation, 0.1 mM H2O2 was added and the reaction was halted 3 minutes later by addition of 2 M acetic acid. Spectrophotometry was used to assess for rate of change of absorbance at 650nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 µmol hydrogen peroxide/min at 37°C and was expressed in units per 100 mg of tissue.
Subcellular fractionation and nuclear protein extraction
Lung tissue was homogenized using a polytron homogenizer (Brinkman Instruments, West Orange NY) in a buffer containing 0.32 M Sucrose, 10 mM Tris HCl, 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 50 mM NaF, 10 mM Mercaptoethanol, 20 µM Leupeptin, 0.15 µm Pepstatin A, 0.2 mM PMSF, 1 mM sodium orthovanadate and 0.4 nM microcystitin. Samples were then centrifuged at 1,000g for 10 min at 4°C. The supernatant was collected as cytosol extract. The pellet was washed in buffer and centrifuged at 3500 rpm for 10 min at 4°C. The supernatant was removed and the remaining pellet was washed in a buffer containing 50 mM Tris-HCl pH 7.5, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 1% Triton X-100, 0.5% NP40, 10% Glycerol, dH2O, 0.2 mM PMSF, 0.1 mM sodium orthovanadate and aprotinin. This solution was then centrifuged at 15,000 g for 30 min at 4°C. The supernatant was then collected as nuclear extract.
Western blot analysis
The nuclear content of LXRα was measured using immunoblot analysis on nitrocellulose membranes using primary antibodies against LXRα and secondary peroxidase-conjugated antibody. Membranes were also probed with primary antibody for β-Actin to ensure equal loading of samples. Immunoreaction was visualized via chemiluminescence on a photographic film. Densitometric analysis was performed using ImageQuant software (Molecular Dynamics, Sunnyvale CA.).
Electrophoretic mobility shift assay
Oligonucleotide probes corresponding to the NF-κB consensus sequence (5'-AGT TGA GGG GAC TTT CCC AGG C-3') or LXRα sequence (5'-GGC AAG AAG TAA CTG TCG GTC AAA TCC T-3') were labeled with γ-[32-P]ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (BioRad, Hercules CA). A standard amount of nuclear extract protein was incubated in EMSA buffer (12 mM HEPES (pH 7.9), 4 mM Tris-HCl (pH 7.9), 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 50 ng/ml poly(d(I-C)), 12% glycerol (v/v), and 0.2 mM PMSF) for 10 minutes. The radio-labeled probe was then added and incubated on ice for another 30 minutes. Samples were loaded into a polyacrylamide gel consisting of 5% acrylamide (29:1 ratio of acrylamide:bisacrylamide) and protein/nucleic acid complexes were resolved using electrophoresis at a constant current of 30 mA for 60 minutes in a buffer of 0.5X TBE (45 mM Tris-HCl, 45 mM Boric Acid, 1 mM EDTA). Gels were then transferred to Whatman 3M paper and dried under a vacuum at 80° C for one hour and exposed to photographic film at −70° C with an intensifying screen. Densitometric analysis was performed using ImageQuant software (molecular dynamics, Sunnyvale CA) (22, 23).
Materials
Primary antibodies directed against LXRα were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The primary anti-body directed against β-Actin was obtained from Abcam (Cambridge MA). The LXRα agonist T0901317 was obtained from Cayman Chemical Company (Ann Arbor MI). The oligonucleotide probes for NF-κB were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The oligonucleotide probe for LXRα corresponded to a previously published LXR consensus sequence (24) and was synthesized by Invitrogen Corporation (Carlsbad, CA).
Data analysis
Statistical analysis was performed using SigmaStat for Windows Version 3.10 (SysStat Software, San Jose CA). Data are represented as mean ± SEM of n observations, where n represents the number of animals in each group. Hemodynamic parameters were assessed using a general linear model for repeated measures allowing for missing data. For the remainder of the data, a two-way analysis of variance with Bonferroni or Holm-Sidak correction was used.
RESULTS
Effect of T0901317 on hemodynamics
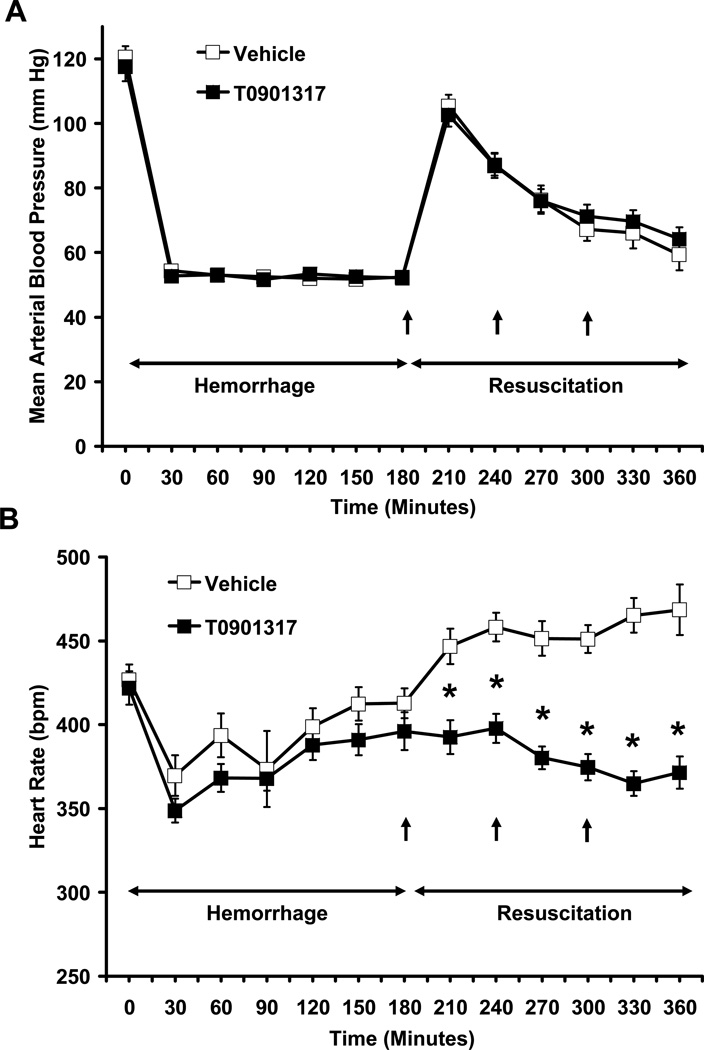
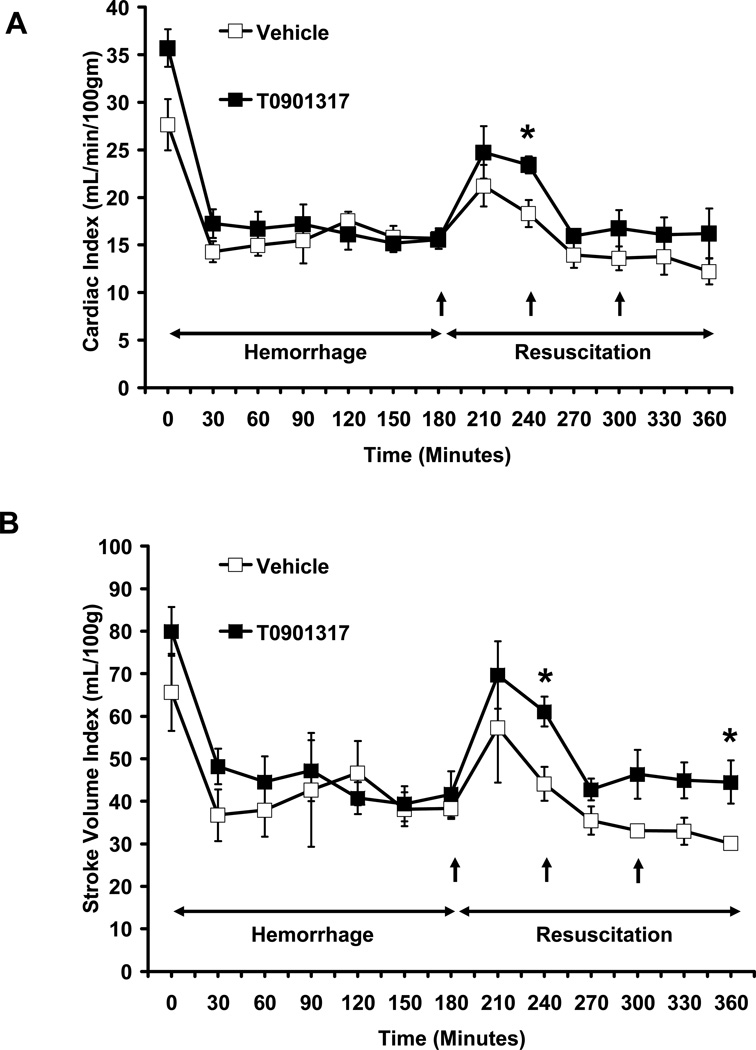
Rats selected for treatment with vehicle or T0901317 had similar heart rate (HR) (427 ± 9 bpm and 421.9 ± 10 bpm, respectively) and mean arterial blood pressure (MABP) (120.69 ± 3.3 mmHg and 117.7 ± 5 mmHg respectively) at baseline and had similar maximum volumes of blood and percentage of total blood volume withdrawn during the hemorrhagic shock phase (Fig 1, Table 1). The volume of Ringer Lactate solution delivered during resuscitation was also similar between the two groups (Table 1). Following resuscitation, MABP was similar in both vehicle and T0901317-treated groups (Fig. 1A). However, treatment with T0901317 significantly blunted hemorrhage-induced tachycardia at all measured time points when compared to vehicle treated animals (P < 0.001) (Fig. 1B). In addition, cardiac index (CI) and stroke volume index (SVI) were all increased in the T0901317-treated animals as compared to the vehicle group. While only certain time points showed significantly higher CI or SVI in T0901317-treated rats versus vehicle-treated rats, both CI and SVI were significantly higher in T0901317-treated rats as compared to vehicle-treated rats during the cumulative resuscitative period (P < 0.005 and P < 0.001, respectively) (Fig. 2).
FIG. 1. Effect of in vivo treatment with vehicle or T0901317 on the (A) mean arterial blood pressure and (B) heart rate of rats subjected to severe hemorrhage and resuscitation.
Each data point is represented as mean ± SEM of N = 12–18 in vehicle-treated rats or N = 10–16 in T0901317-treated rats. Asterisk (*) indicates P ≤ 0.001 versus vehicle-treated rats.
Table 1.
Body weight, total and percent blood removed, and Ringer Lactate solution supplementation.
| Parameter | Vehicle | T0901317 | P Value |
|---|---|---|---|
| Body Weight (g) | 274.8±5.8 | 283±6.4 | 0.349 |
| Total Blood Removed (mL) | 7.9±0.3 | 8.8±0.3 | 0.056 |
| Percent Blood Removed (% of estimated circulating blood volume)* | 41.6±1.7 | 44.4±1.3 | 0.202 |
| Ringer Lactate Solution Supplementation (mL) | 1.0±0.2 | 0.9±0.2 | 0.637 |
Values are means ± SEM of n = 18 rats in the vehicle-treated group and n = 16 in the T0901317-treated group.
Calculation of % blood removed = (blood removed (mL)/(weight (kg) × 0.7)) × 100
FIG. 2. Effect of in vivo treatment with vehicle or T0901317 on (A) Cardiac Index and (B) Stroke Volume Index of rats subjected to severe hemorrhage and resuscitation.
Data are represented as mean ± SEM of N= 3,4 rats for vehicle and T0901317-treated rats, respectively. Asterisk (*) indicates P ≤ 0.05 versus vehicle-treated rats.
Effect of T0901317 on total metabolic acidosis
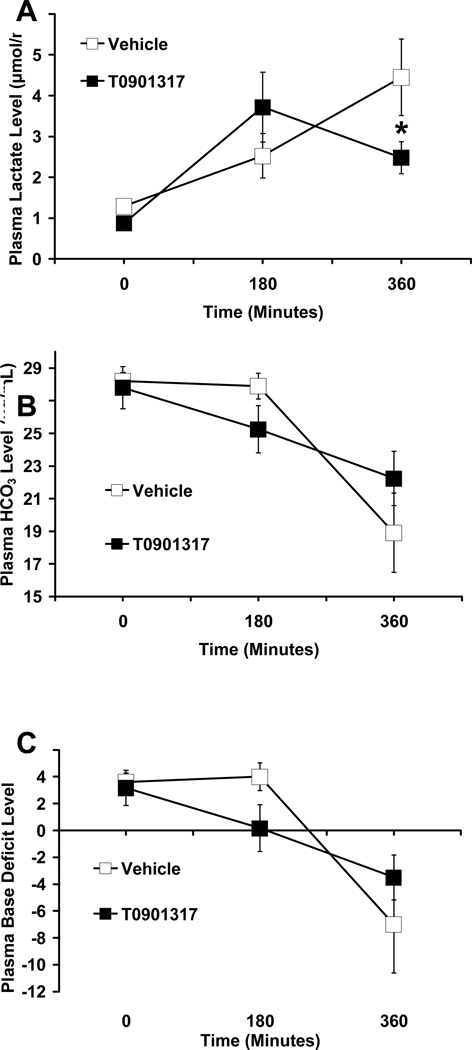
T0901317-treated animals showed fewer signs of metabolic acidosis compared to vehicle-treated animals at 180 minutes post-resuscitation. Although not statistically significant, plasma bicarbonate levels were higher (22.23 ±1.66 µg/mL vs 18.9 ± 2.42 µg/mL) and base deficit was less negative (−3.5 ± 1.67 versus −7 ± 3.62); while plasma lactate levels were significantly lower (2.5 ± 0.4 µmol/mL vs. 4.4 ± 0.94µmol/mL, P < 0.05) in T0901317-treated animals compared to vehicle-treated animals (Fig. 3).
FIG. 3. Effect of in vivo treatment with vehicle or T0901317 on the plasma levels of (A) lactate, (B) bicarbonate and (C) base deficit in rats subjected to severe hemorrhage and resuscitation.
All values are displayed as mean ± SEM for N= 6, 5 for T0901317-treated animals and vehicle-treated animals, respectively. Asterisk (*) indicates P < 0.05 versus vehicle-treated rats.
Effect of T0901317 on lung injury and neutrophil infiltration
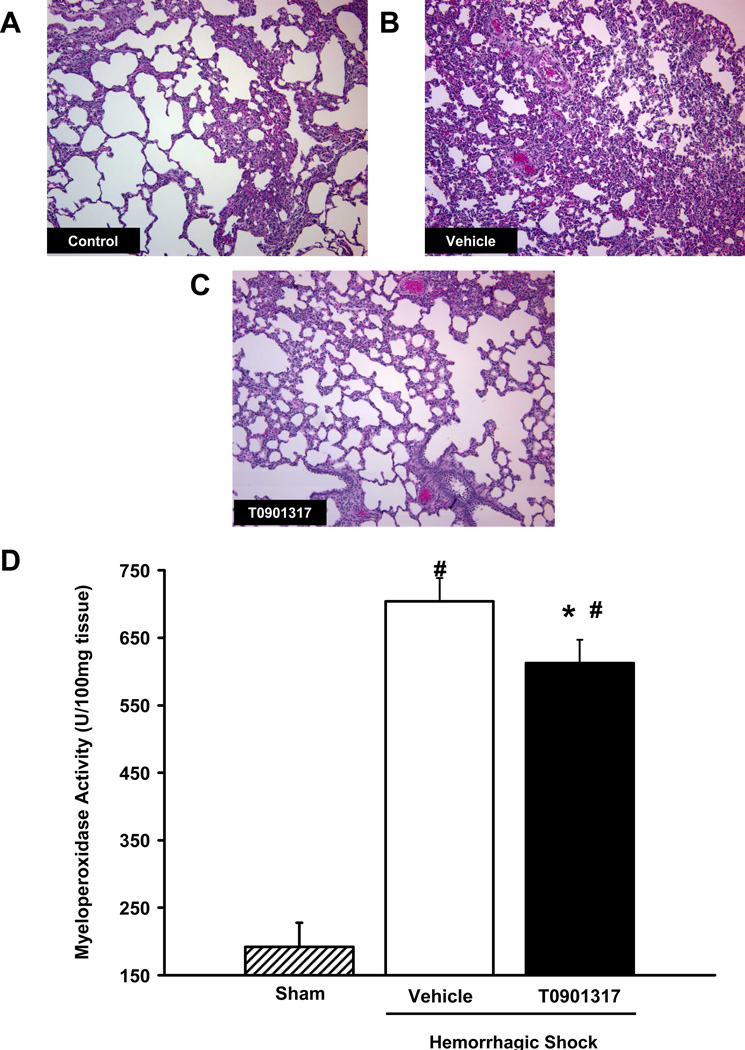
At histological examination, hemorrhagic shock resulted in severe lung injury characterized by reduced alveolar space and accumulation of inflammatory cells in vehicle-treated rats. On the contrary, histological images of rats treated with T0901317 (Fig. 4C) showed preservation of lung architecture and a reduction of neutrophil infiltration when compared to vehicle treated rats at 180 minutes post-resuscitation (Fig. 4B). The degree of neutrophil infiltration in the lungs was further confirmed by measurement of myeloperoxidase activity. At 180 minutes post-resuscitation, there was significantly less myeloperoxidase activity in the lungs of rats treated with T0901317 as compared to vehicle-treated rats (613±34 U/100mg tissue versus 728±35 U/100mg tissue, respectively; P < 0.05) (Fig. 4D).
FIG. 4. Effect of in vivo treatment with vehicle or T0901317 on lung injury and neutrophil infiltration in rats subjected to severe hemorrhage and resuscitation.
A-C are representative histological images of rat lung tissue after staining with hematoxylin and eosin. (A) Cross section of lung tissue from a control rat; (B) cross section of lung tissue from a vehicle-treated rat at 180 minutes post-resuscitation; (C) cross section of lung tissue from a T0901317-treated rat at 180 minutes post-resuscitation. All images are shown at 100x magnification. (D) Lung myeloperoxidase activity in vehicle and T0901317-treated rats at 180 minutes post-resuscitation. Data are represented as mean ± SEM for N = 4– 7 animals for each group. Asterisk (*) indicates P < 0.05 versus vehicle-treated rats, # indicates P < 0.05 versus sham rats.
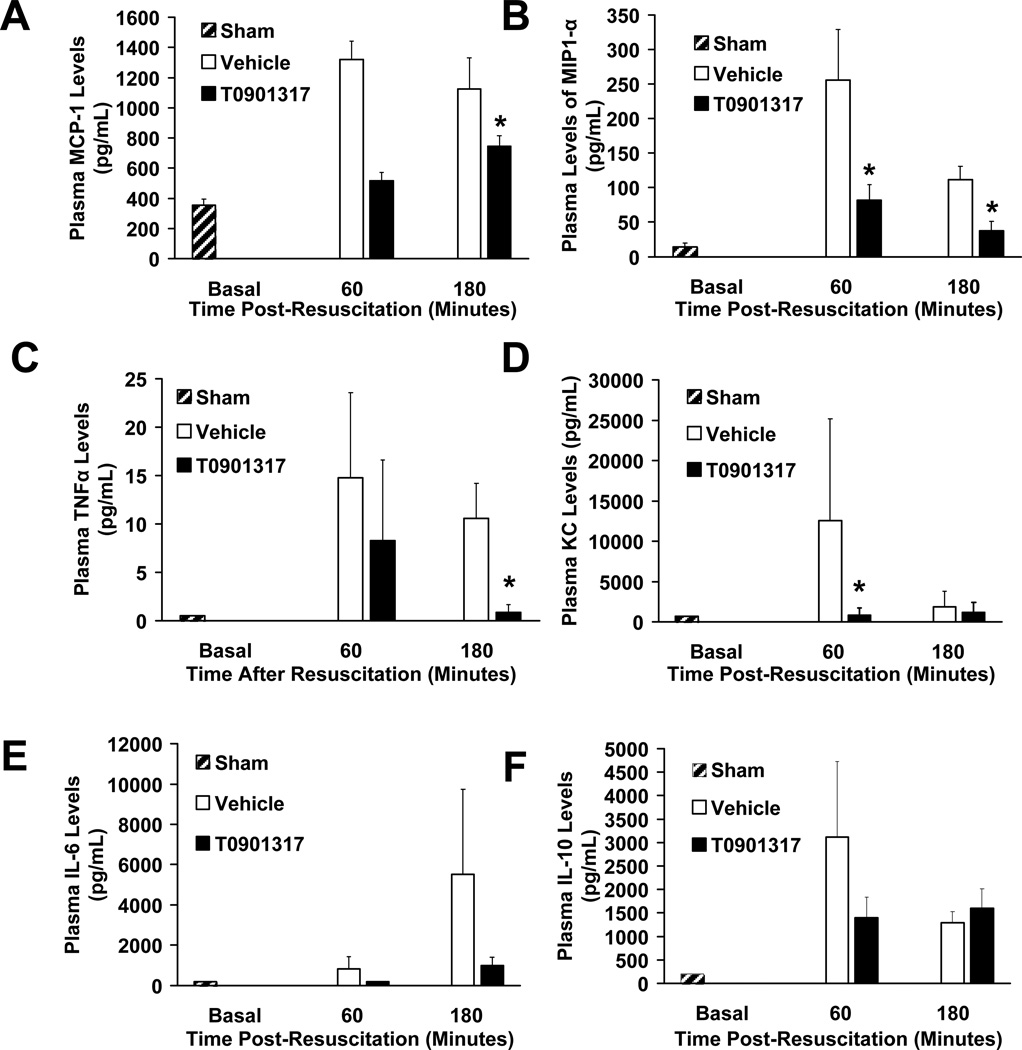
Effect of T0901317 on plasma cytokine and chemokine levels
Plasma levels of cytokines and chemokines were measured at 60 and 180 minutes post-resuscitation. There was a significant decrease in plasma concentrations of MCP-1, MIP-1α, TNFα and KC in T0901317-treated animals when compared to vehicle-treated rats. Although lower than vehicle-treated rats, plasma levels of IL-6 were not significantly different in T0901317-treated rats. Levels of IL-10 were similar in both groups (Fig. 5).
FIG. 5. Effect of in vivo treatment with vehicle or T0901317 on plasma levels of (A) MCP-1, (B) MIP-1α, (C) TNF-α, (D) KC, (E) IL-6 and (F) IL-10 (pg/mL) in rats subjected to severe hemorrhage and resuscitation.
Data are represented as mean value ± SEM for N= 3 (60 minutes) and 10 (180 minutes) in T0901317 treated animals; N = 3 (60 Minutes) and 11 (180 minutes) for vehicle-treated animals. Asterisk (*) indicates P < 0.05 versus vehicle-treated animals.
Effect of T0901317 on plasma cholesterol
Since LXRα is a crucial regulator of cholesterol metabolism, we also measured plasma levels of cholesterol. There was a significant increase of plasma total cholesterol concentration in vehicle-treated rats (74.1 ± 6 mg/dL) at 180 minutes post-resuscitation when compared to basal levels of sham control rats (55.2 ±4.8 mg/dL). Treatment with T0901317 did not significantly change plasma concentration of total cholesterol (77.8 ± 5.1 mg/dL) at 180 minutes post-resuscitation when compared to treatment with vehicle alone (P = 0.65
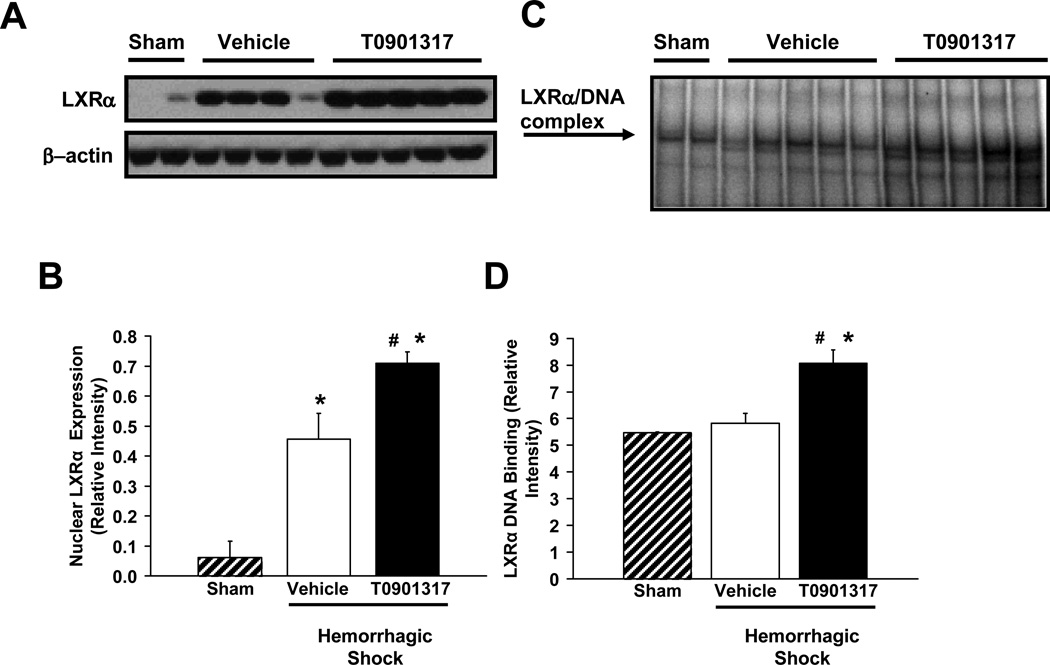
Effect of T0901317 on LXRα expression and activation
To confirm the mechanism of action of T0901317 on the LXRα pathway, we evaluated both the expression of the receptor as well as its DNA binding at 180 minutes post-resuscitation in lung nuclear extracts. At basal condition, a faint constitutive band was observed in sham animals. After resuscitation, there was an increase in the nuclear expression and DNA binding of LXRα in both vehicle-treated and T0901317-treated rats. However, treatment with T0901317 significantly increased both the nuclear expression and activity of the receptor when compared to vehicle-treated rats (P < 0.05) (Fig. 6).
FIG. 6. Effect of treatment with vehicle or T0901317 on the expression and DNA binding of LXRα in rodent lung after hemorrhage and resuscitation.
(A) Representative western blot of LXRα and β-Actin from nuclear extract of rodent lung tissue at 180 minutes post-resuscitation. (B) Image analysis of representative western blot (Fig 6A) of LXRα content in nuclear extract of rodent lung tissue 180 minutes post-resuscitation as evaluated by western blot. N= 2, 4, 5 for sham, vehicle and T0901317 treated animals, respectively. (C) Representative EMSA probed for LXRα in nuclear extract of rodent lung tissue at 180 minutes post-resuscitation. (D) Image analysis of representative EMSA (Fig. 6C) showing LXRα binding to DNA in nuclear extract of rodent lung tissue at 180 minutes post-resuscitation. N= 2, 5, 5 for sham, vehicle and T0901317 treated animals, respectively. Asterisk (*) indicates P < 0.05 versus sham rats; pound (#) indicates P < 0.05 versus vehicle-treated rats.
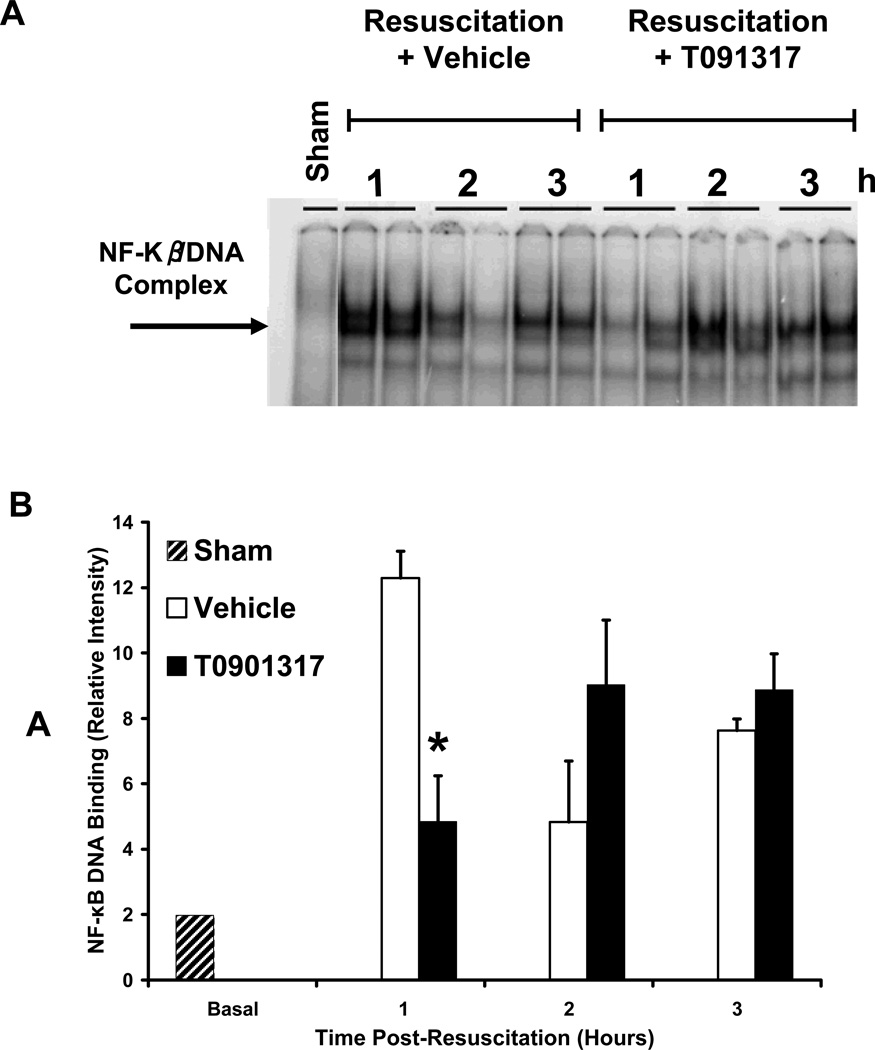
Effect of T0901317 on NF-κB activation
To explore the role of LXRα activation in the NF-κB pathway, we investigated the amount of NF-κB bound to the DNA in rat lung nuclear extracts after hemorrhage and resuscitation. Sham animals showed a faint constitutive band at basal condition. Hemorrhage and resuscitation increased DNA-binding in both vehicle and T0901317-treated animals. However, at 60 minutes post-resuscitation, we found that T0901317-treated rats had significantly decreased binding of NF-κB in lung nuclear extracts as compared to vehicle-treated rats at the same time point (Fig. 7).
FIG. 7. Effect of in vivo treatment with vehicle or T0901317 on DNA binding of NF-κB in rodent lung after hemorrhage and resuscitation.
(A) Representative EMSA probed for NF-κB in nuclear extract of rodent lung tissue at 60, 120 and 180 minutes post-resuscitation in rats treated with T0901317 or vehicle. (B) Image analysis of representative EMSA (Fig. 7A) showing NF-κB binding to DNA in nuclear extract of rodent lung tissue at 60, 120 and 180 minutes post-resuscitation. N= 1 for sham and N= 2 for both vehicle and T0901317 treated animals at each respective time point. Asterisk (*) indicates P < 0.05 versus vehicle-treated animals.
DISCUSSION
Our study has shown that activation of LXRα with the synthetic ligand T0901317 improves the hemodynamic profile of rats subjected to severe hemorrhage and resuscitation. Furthermore, lung injury and neutrophil infiltration are reduced with LXRα activation. This pulmonary protection is seen in conjunction with a decrease in plasma cytokine and chemokines levels. These anti-inflammatory effects are likely due to an inhibition of the NF-κB pathway. These findings all suggest that LXRα regulates inflammation after a hemorrhagic insult on a systemic scale, though its effects on organs other than the lungs remains to be explored.
In our study, we used a pressure-controlled model of hemorrhagic shock to standardize the level of hypoperfusion during the hemorrhage phase. After resuscitation, LXRα activation significantly decreased heart rate while also increasing stroke volume and cardiac index. Interestingly, there was no significant change in the MABP post-resuscitation despite these positive findings. One possible explanation for this is the interaction between LXRα and the Renin-Angiotensin System (RAS). Several studies have shown that activated LXRα interacts with the RAS, leading to decreased renin levels and a decreased response to Angiotensin II (25–27). This interaction would limit the ability of animals to vasoconstrict and increase their blood pressure. Concordant with this hypothesis, we did not observe any changes in total peripheral resistance (data not shown) in rats treated with T0901317, despite improvements in other hemodynamic parameters.
Improvement of hemodynamic parameters such as CI and SVI implies that systemic oxygen demand has decreased. However, systemic markers of acidosis are used in conjunction with hemodynamic parameters to clinically assess cellular hypoxia (28). Among the values used are plasma lactate, base deficit and bicarbonate. In our study, we found that activation of LXRα significantly improved plasma lactate and showed a trend towards improvement of bicarbonate and base deficit as compared to vehicle-treated animals. This indicates that therapy with T0901317 improved end organ perfusion and abrogated cellular hypoxia, leading to less metabolic acidosis. However, the role of LXRα activation in glucose utilization cannot be ignored. Several studies have shown that activation of LXRα leads to increased pancreatic insulin secretion, decreased hepatic gluconeogensis and improved peripheral glucose uptake (7). This improved glucose utilization could lead to improved cellular metabolism and improvement in metabolic parameters of acidosis, especially lactate. In our study, we monitored glucose levels during hemorrhage and resuscitation and found elevated plasma glucose levels after hemorrhage, with reduction of these levels to baseline 180 minutes after resuscitation. However, there was no significant difference in these plasma glucose levels at any time point in rats treated with T0901317 versus vehicle-treated rats (data not shown). This implies that LXRα activation did not significantly change peripheral glucose utilization and the improvement in lactate was indeed a reflection of reduced cellular hypoxia in those rats treated with T0901317.
Decreased concentrations of total cholesterol occur early in course of critical illness, including major trauma and hemorrhage. Low cholesterol levels correlate with the severity of the systemic inflammatory response and high concentrations of pro-inflammatory cytokines (29, 30). Since LXRα is a master regulator of cholesterol synthesis, transport and catabolism (31), we also assessed plasma total cholesterol levels in rats subjected to severe hemorrhage and resuscitation. In contrast to clinical data, which report occurrence of hypocholesterolemia in critically ill patients, we observed that there was an increase of total cholesterol after hemorrhage and resuscitation as compared to basal levels of sham control rats. However, we found no significant impact on total cholesterol levels as a result of treatment with T0901317. This suggests that the anti-inflammatory effects of T0901317 are independent of its cholesterol regulating properties. The increase in total plasma cholesterol seen after hemorrhage and resuscitation in vehicle-treated rats is likely a stress response to severe hemorrhage, aimed at increasing the pre-cursors of cortisol biosynthesis. In support of this hypothesis, a previous study by Daull et al (32) showed a similar elevation of plasma cholesterol during the immediate resuscitative period following autologous blood transfusion amongst rats subjected to hemorrhage and resuscitation. Furthermore, a study by Abarca et al. (33) also showed an increase in plasma cholesterol levels of rats during the early phase of reversible circulatory shock induced by endotoxin injection.
Hemorrhage and resuscitation induces an inflammatory response that includes the infiltration of neutrophils into tissue (2). This infiltration is followed by the release of reactive oxygen species and subsequent tissue damage, which can ultimately lead to acute lung injury and even death (34, 35). Reduction of leukocyte adherence and infiltration into lung tissue has been found to improve survival after severe hemorrhage and resuscitation (36). In our study, we have shown a reduction in hemorrhage-induced neutrophil infiltration and lung injury as assessed by both histology and myeloperoxidase assay. Multiple studies have previously shown LXRα activation to reduce lung injury and neutrophil infiltration following infectious, LPS, or chemical insult to rodent lung (15, 16, 37, 38). Our study is the first report that LXRα activation affords lung protection following hemorrhagic shock. Thus, our findings suggest that LXRα activation not only limits inflammation in lung-specific models, but also exerts pulmonary protective properties during sterile non-infectious systemic inflammation.
Pro-inflammatory cytokine and chemokine release is a hallmark of the inflammatory response after hemorrhage and resuscitation (2, 4). This elevation leads to a systemic inflammatory response and organ dysfunction, including acute lung injury (34). Reduction of cytokine levels are therefore a therapeutic goal aimed at reduction of organ injury, especially pulmonary injury. Several previous studies have shown that LXRα activation reduces levels of these pro-inflammatory cytokines and chemokines after LPS or irritant insult both in vivo and in vitro. A recent study by Crisafulli et al. also shows the same reduction after splanchnic ischemia and reperfusion (11, 16, 19, 39). Our study confirms these results in a model of rodent hemorrhagic shock, with significant reductions in plasma levels of MCP-1, MIP-1α, TNF-α and KC. High levels of TNF-α have been associated with increased acute lung injury (34, 35). MCP-1, MIP-1α and KC are chemokines that induce chemotaxis of inflammatory cells (40–42). In our study, treatment with T0901317 induced a significant decrease of TNFα and several chemokines, in particular KC, which correlates with the observed reduction of lung neutrophilia and injury. Therefore, we hypothesize that LXRα activation abrogates neutrophil infiltration into the lung and subsequently limits parenchymal damage following hemorrhagic shock by limiting the production of crucial pro-inflammatory cytokines and chemokines.
NF-κB is a nuclear transcription factor that regulates the expression of a multitude of pro-inflammatory genes and has been implicated as a factor in the development of acute lung injury (17, 35). Amongst the pro-inflammatory cytokines up-regulated by NF-κB activation are TNF-α, KC, MIP-1α, MCP-1 and IL-6. In a previous study, we demonstrated that LXRα activation inhibits the LPS-induced inflammatory response of murine macrophages by inhibiting the NF-κB pathway (19). Recent in vivo models of spinal cord trauma and splanchnic ischemia and reperfusion have also shown a reduction in NF-κB activation after treatment with a synthetic LXRα agonist (39, 43). In our current model of hemorrhagic shock and resuscitation, we have demonstrated that treatment with T0901317 inhibits the early onset (i.e. at 60 minutes post-resuscitation) of NF-κB binding to DNA in the lung. The exact mechanism by which LXRα inhibits NF-κB activation remains unclear and appears complex. Activated LXRα seems to inhibit NF-κB/DNA binding while also inhibiting the transactivation of NF-κB already bound to DNA. For example, earlier in vitro studies have shown that activation of the NF-κB pathway in LPS-challenged macrophages is inhibited by LXRα activation, but failed to show a decrease in NF-κB binding to DNA (10, 11, 15, 44). Ghisletti et al. support this finding in their study, showing that LXRα activation of macrophages prevents the clearance of co-repressors in a SUMOylation dependent manner, thus preventing the transcription of several pro-inflammatory genes (45). This trans-repression would decrease the transcriptional activity of NF-κB without altering its binding profile. However, in vivo studies of splanchnic ischemia and reperfusion did show a reduction of NF-κB binding to DNA, similar to the findings we report in this study (39). This suggests that LXRα does block NF-κB binding to DNA and subsequent transcription of pro-inflammatory genes in in vivo models or in models of ischemia and reperfusion. However, the exact mechanism remains unclear and further investigation is needed to define the precise role activated LXRα plays in inhibition of the NF-κB pathway in vivo.
In conclusion, we have shown that activation of LXRα with the synthetic agonist T0901317 improves the hemodynamic profile and reduces lung inflammation after hemorrhage and resuscitation in a rodent model. The reduction of inflammation is consistent with inhibition of NF-κB binding during the early phase of shock. Further studies using inhibitors of LXRα, such as GSK 2033 (46), or transgenic mice deprived of the functional gene for LXRα (14) will establish the precise mechanisms of LXRα and NF-κB interaction.
Acknowledgements
This investigation was supported by the National Institutes of Health (R01AG-027990 to Dr. Basilia Zingarelli). Dr. Patrick Solan was supported by a National Institutes of Health training grant (T32 GM-008478).
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamroz-Wisniewska A, Wojcicka G, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part II: non-lipid effects, role in pathology, and therapeutic implications. Postepy Hig Med Dosw (Online) 2007;61:760–785. [PubMed] [Google Scholar]
- 6.Wojcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online) 2007;61:736–759. [PubMed] [Google Scholar]
- 7.Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008;59(Suppl 7):31–55. [PubMed] [Google Scholar]
- 8.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 9.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 10.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 11.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 12.Myhre AE, Agren J, Dahle MK, Tamburstuen MV, Lyngstadaas SP, Collins AJ, Foster SJ, Thiemermann C, Aasen AO, Wang JE. Liver X receptor is a key regulator of cytokine release in human monocytes. Shock. 2008;29:468–474. doi: 10.1097/shk.0b013e31815073cb. [DOI] [PubMed] [Google Scholar]
- 13.Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ, Collins JL, Thiemermann C, Aasen AO, Wang JE. Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock. 2006;25:141–146. doi: 10.1097/01.shk.0000191377.78144.d9. [DOI] [PubMed] [Google Scholar]
- 14.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 15.Birrell MA, Catley MC, Hardaker E, Wong S, Willson TM, McCluskie K, Leonard T, Farrow SN, Collins JL, Haj-Yahia S, Belvisi MG. Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses. J Biol Chem. 2007;282:31882–31890. doi: 10.1074/jbc.M703278200. [DOI] [PubMed] [Google Scholar]
- 16.Crisafulli C, Mazzon E, Paterniti I, Galuppo M, Bramanti P, Cuzzocrea S. Effects of Liver x receptor agonist treatment on signal transduction pathways in acute lung inflammation. Respir Res. 11:19. doi: 10.1186/1465-9921-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingarelli B. Nuclear factor-kappaB. Crit Care Med. 2005;33:S414–S416. doi: 10.1097/01.ccm.0000186079.88909.94. [DOI] [PubMed] [Google Scholar]
- 18.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–S111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 19.Piraino G, Cook JA, O'Connor M, Hake PW, Burroughs TJ, Teti D, Zingarelli B. Synergistic effect of peroxisome proliferator activated receptor-gamma and liver X receptor-alpha in the regulation of inflammation in macrophages. Shock. 2006;26:146–153. doi: 10.1097/01.shk.0000223121.03523.69. [DOI] [PubMed] [Google Scholar]
- 20.Zingarelli B, Hake PW, Mangeshkar P, O'Connor M, Burroughs TJ, Piraino G, Denenberg A, Wong HR. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock. 2007;28:554–563. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 21.Zingarelli B, Hake PW, O'Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor gamma. Crit Care Med. 2009;37:1978–1987. doi: 10.1097/CCM.0b013e31819feb4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849–2857. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zingarelli B, Sheehan M, Hake PW, O'Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–6837. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 24.Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C, Feingold KR. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism. 2007;56:267–279. doi: 10.1016/j.metabol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JA, Lopez-Ilasaca M, Pratt RE, Dzau VJ. Liver X receptors alpha and beta regulate renin expression in vivo. J Clin Invest. 2005;115:1913–1922. doi: 10.1172/JCI24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leik CE, Carson NL, Hennan JK, Basso MD, Liu QY, Crandall DL, Nambi P. GW3965, a synthetic liver X receptor (LXR) agonist, reduces angiotensin II-mediated pressor responses in Sprague-Dawley rats. Br J Pharmacol. 2007;151:450–456. doi: 10.1038/sj.bjp.0707241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuipers I, van der Harst P, Kuipers F, van Genne L, Goris M, Lehtonen JY, van Veldhuisen DJ, van Gilst WH, de Boer RA. Activation of liver X receptor-alpha reduces activation of the renal and cardiac renin-angiotensin-aldosterone system. Lab Invest. 90:630–636. doi: 10.1038/labinvest.2010.7. [DOI] [PubMed] [Google Scholar]
- 28.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441–453. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannini I, Boldrini G, Chiarla C, Giuliante F, Vellone M, Nuzzo G. Pathophysiologic correlates of hypocholesterolemia in critically ill surgical patients. Intensive Care Med. 1999;25:748–751. doi: 10.1007/s001340050940. [DOI] [PubMed] [Google Scholar]
- 30.Bonville DA, Parker TS, Levine DM, Gordon BR, Hydo LJ, Eachempati SR, Barie PS. The relationships of hypocholesterolemia to cytokine concentrations and mortality in critically ill patients with systemic inflammatory response syndrome. Surg Infect (Larchmt) 2004;5:39–49. doi: 10.1089/109629604773860291. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 32.Daull P, Blouin A, Cayer J, Beaudoin M, Belleville K, Sirois P, Nantel F, Chang TM, Battistini B. Profiling biochemical and hemodynamic markers using chronically instrumented, conscious and unrestrained rats undergoing severe, acute controlled hemorrhagic hypovolemic shock as an integrated in-vivo model system to assess new blood substitutes. Vascul Pharmacol. 2005;43:289–301. doi: 10.1016/j.vph.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Abarca S, Garcia R. Cholesterol metabolism in rat adrenal gland during reversible endotoxic shock. Eur J Biochem. 1993;211:829–834. doi: 10.1111/j.1432-1033.1993.tb17615.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 35.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 36.Vedder NB, Winn RK, Rice CL, Chi EY, Arfors KE, Harlan JM. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988;81:939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smoak K, Madenspacher J, Jeyaseelan S, Williams B, Dixon D, Poch KR, Nick JA, Worthen GS, Fessler MB. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J Immunol. 2008;180:3305–3312. doi: 10.4049/jimmunol.180.5.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong H, He J, Lee JH, Mallick E, Gao X, Li S, Homanics GE, Xie W. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J Biol Chem. 2009;284:30113–30121. doi: 10.1074/jbc.M109.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crisafulli C, Di Paola R, Mazzon E, Paterniti I, Galuppo M, Genovese T, Bramanti P, Cappellani A, Cuzzocrea S. Liver X receptor agonist treatment reduced splanchnic ischemia and reperfusion injury. J Leukoc Biol. 87:309–321. doi: 10.1189/jlb.0609438. [DOI] [PubMed] [Google Scholar]
- 40.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 43.Paterniti I, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Galuppo M, Bramanti P, Cuzzocrea S. Liver X receptor agonist treatment regulates inflammatory response after spinal cord trauma. J Neurochem. 112:611–624. doi: 10.1111/j.1471-4159.2009.06471.x. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda T, Kanno M, Kawamoto M, Yuge O, Ninomiya Y. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 22(R)-hydroxycholesterol requires de novo protein synthesis in activated macrophages. J Steroid Biochem Mol Biol. 2005;97:376–383. doi: 10.1016/j.jsbmb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 45.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuercher WJ, Buckholz RG, Campobasso N, Collins JL, Galardi CM, Gampe RT, Hyatt SM, Merrihew SL, Moore JT, Oplinger JA, Reid PR, Spearing PK, Stanley TB, Stewart EL, Willson TM. Discovery of tertiary sulfonamides as potent liver X receptor antagonists. J Med Chem. 53:3412–3416. doi: 10.1021/jm901797p. [DOI] [PubMed] [Google Scholar]