Abstract
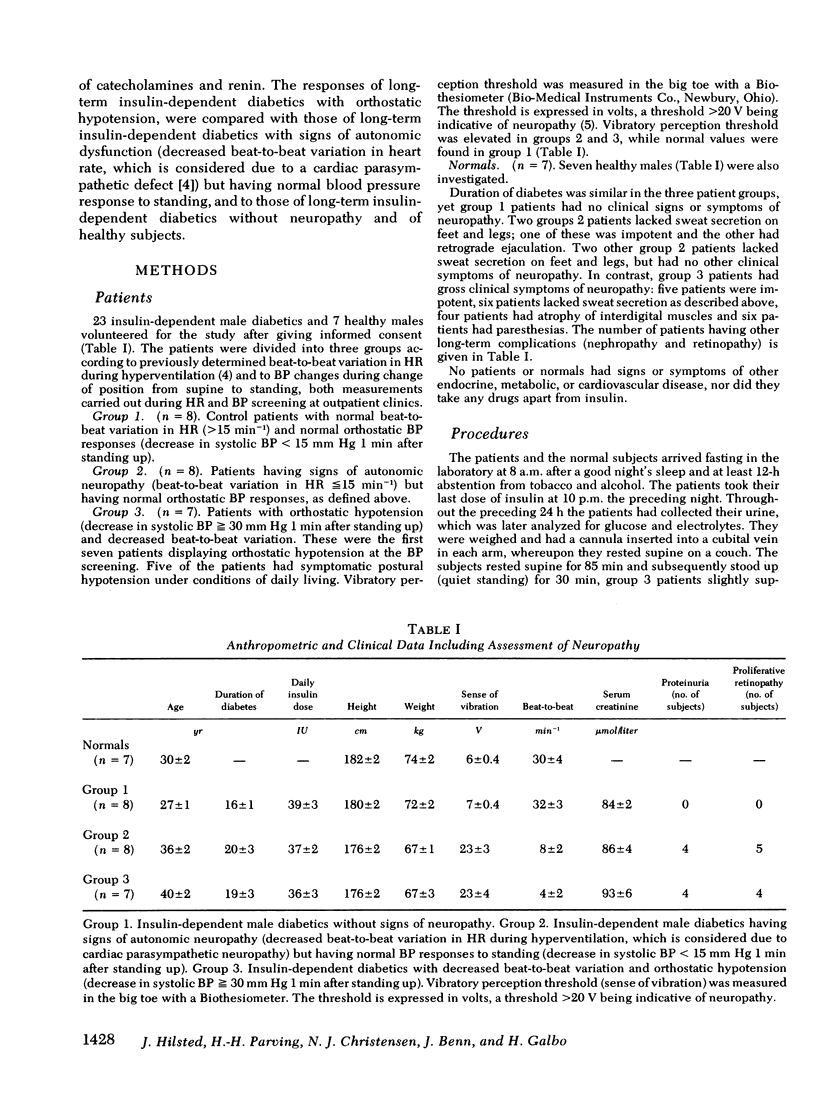
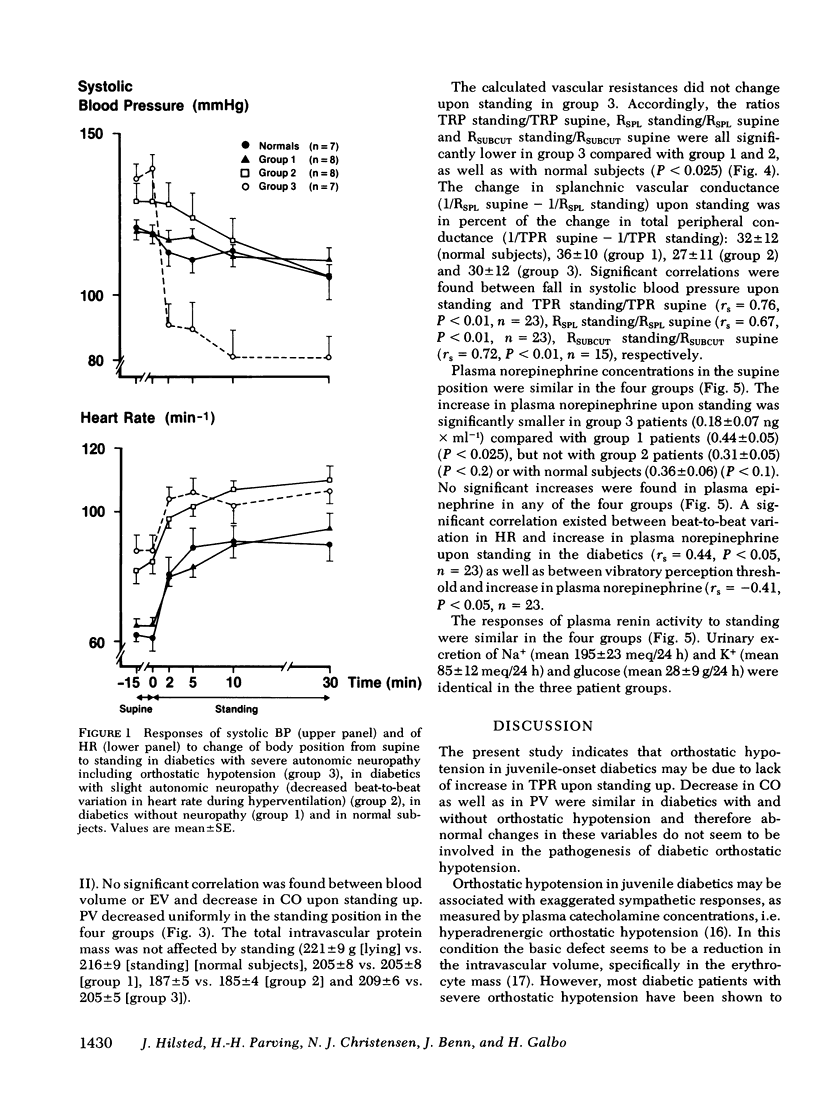
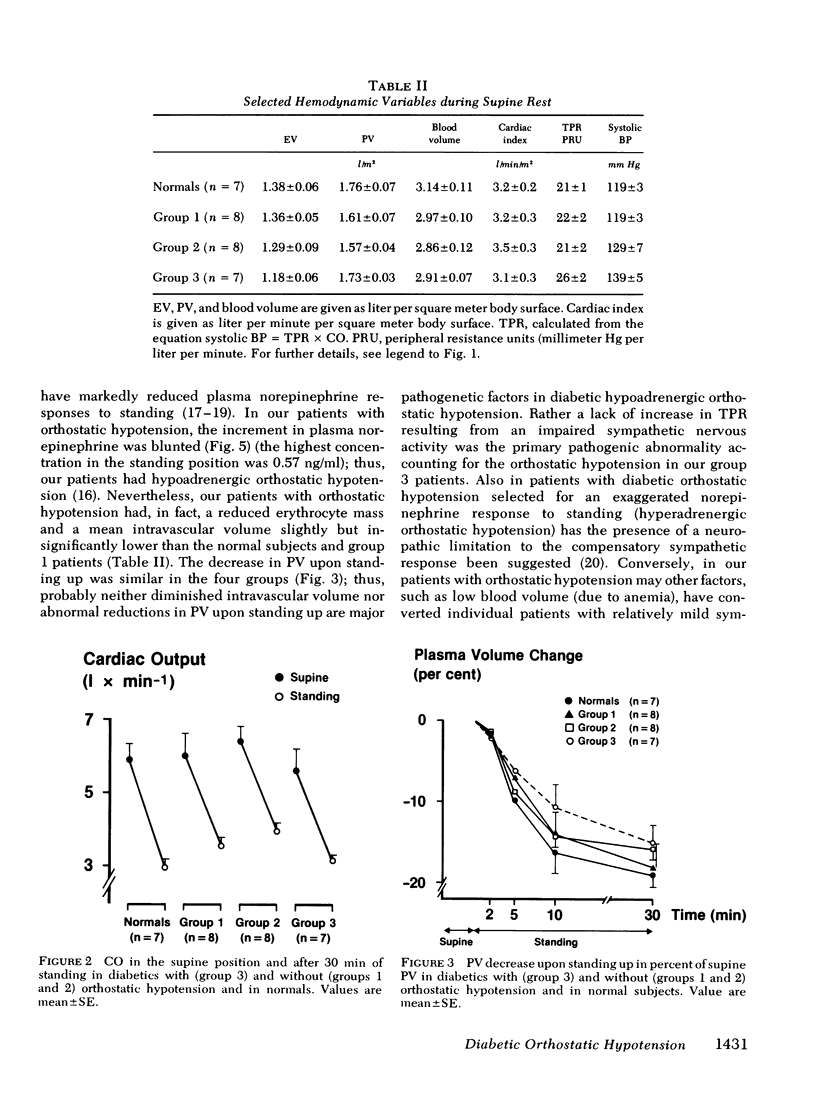
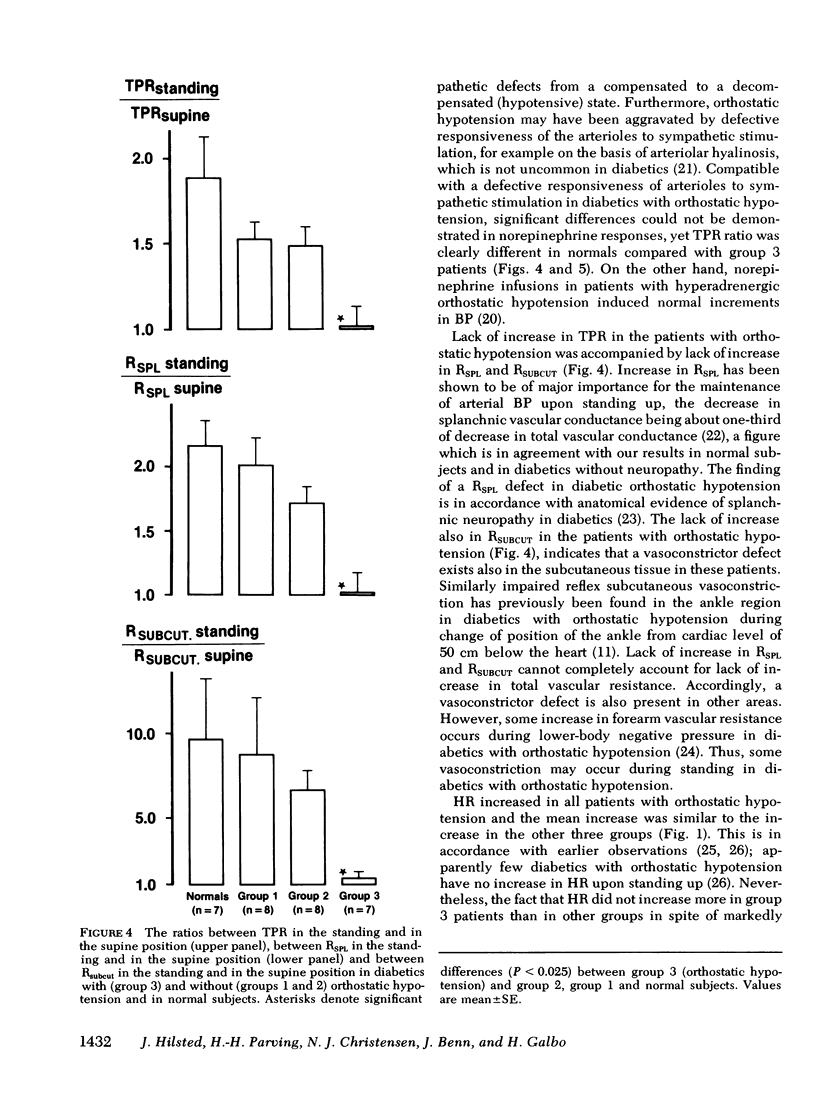
Hemodynamic variables (blood pressure, cardiac output, heart rate, plasma volume, splanchnic blood flow, and peripheral subcutaneous blood flow) and plasma concentrations of norepinephrine, epinephrine, and renin were measured in the supine position and after 30 min of quiet standing. This was done in normal subjects (n = 7) and in juvenile-onset diabetic patients without neuropathy (n = 8), with slight neuropathy (decreased beat-to-beat variation in heart rate during hyperventilation) (n = 8), and with severe neuropathy including orthostatic hypotension (n = 7). Blood pressure decreased precipitously in the standing position in the diabetics with orthostatic hypotension, whereas moderate decreases were found in the other three groups. Upon standing, heart rate rose and cardiac output and plasma volume decreased similarly in the four groups. The increases in total peripheral resistance, splanchnic vascular resistance and subcutaneous vascular resistance were all significantly lower (P less than 0.025) in the patients with orthostatic hypotension compared with the other three groups. The increase in plasma norepinephrine concentrations in the patients with orthostatic hypotension was significantly lower (P less than 0.025) than in the patients without neuropathy, whereas plasma renin responses to standing were similar in the four groups. We conclude that in diabetic hypoadrenergic orthostatic hypotension the basic pathophysiological defect is lack of ability to increase vascular resistance, probably due to impaired sympathetic activity in the autonomic nerves innervating resistance vessels; cardiac output and plasma volume responses to standing are similar to those found in normal subjects and in diabetics without neuropathy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Hosking D. J., Hampton J. R. Cardiovascular responses to graded reductions of central blood volume in normal subjects and in patients with diabetes mellitus. Clin Sci (Lond) 1980 Mar;58(3):193–200. doi: 10.1042/cs0580193. [DOI] [PubMed] [Google Scholar]
- Bonde-Petersen F., Norsk P., Suzuki Y. A comparison between freon and acetylene rebreathing for measuring cardiac output. Aviat Space Environ Med. 1980 Nov;51(11):1214–1221. [PubMed] [Google Scholar]
- Burden A. C., Thurston H. Plasma renin activity in diabetes mellitus. Clin Sci (Lond) 1979 Mar;56(3):255–259. doi: 10.1042/cs0560255. [DOI] [PubMed] [Google Scholar]
- Campbell I. W., Ewing D. J., Anderton J. L., Thompson J. H., Horn D. B., Clarke B. F. Plasma renin activity in diabetic autonomic neuropathy. Eur J Clin Invest. 1976 Sep 10;6(5):381–385. doi: 10.1111/j.1365-2362.1976.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Catecholamines and diabetes mellitus. Diabetologia. 1979 Apr;16(4):211–224. doi: 10.1007/BF01221946. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma catecholamines in long-term diabetics with and without neuropathy and in hypophysectomized subjects. J Clin Invest. 1972 Apr;51(4):779–787. doi: 10.1172/JCI106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. J. Plasma noradrenaline and adrenaline in patients with thyrotoxicosis and myxoedema. Clin Sci Mol Med. 1973 Aug;45(2):163–171. doi: 10.1042/cs0450163. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R., Munichoodappa C., Braaten J. T. Decreased response of plasma renin activity to orthostasis in diabetic patients with orthostatic hypotension. Diabetes. 1974 Oct;23(10):835–840. doi: 10.2337/diab.23.10.835. [DOI] [PubMed] [Google Scholar]
- Cryer P. E. Disorders of sympathetic neural function in human diabetes mellitus: hypoadrenergic and hyperadrenergic postural hypotension. Metabolism. 1980 Nov;29(11 Suppl 1):1186–1189. doi: 10.1016/0026-0495(80)90028-1. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Silverberg A. B., Santiago J. V., Shah S. D. Plasma catecholamines in diabetes. The syndromes of hypoadrenergic and hyperadrenergic postural hypotension. Am J Med. 1978 Mar;64(3):407–416. doi: 10.1016/0002-9343(78)90220-6. [DOI] [PubMed] [Google Scholar]
- Engelman K., Portnoy B. A sensitive double-isotope derivative assay for norepinephrine and epinephrine. Normal resting human plasma levels. Circ Res. 1970 Jan;26(1):53–57. doi: 10.1161/01.res.26.1.53. [DOI] [PubMed] [Google Scholar]
- Hilsted J. Decreased sympathetic vasomotor tone in diabetic orthostatic hypotension. Diabetes. 1979 Nov;28(11):970–973. doi: 10.2337/diab.28.11.970. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Galbo H., Christensen N. J. Impaired cardiovascular responses to graded exercise in diabetic autonomic neuropathy. Diabetes. 1979 Apr;28(4):313–319. doi: 10.2337/diab.28.4.313. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Jensen S. B. A simple test for autonomic neuropathy in juvenile diabetics. Acta Med Scand. 1979;205(5):385–387. doi: 10.1111/j.0954-6820.1979.tb06069.x. [DOI] [PubMed] [Google Scholar]
- Leveston S. A., Shah S. D., Cryer P. E. Cholinergic stimulation of norepinephrine release in man. Evidence of a sympathetic postganglionic axonal lesion in diabetic adrenergic neuropathy. J Clin Invest. 1979 Aug;64(2):374–380. doi: 10.1172/JCI109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Walsh J. C., Huang C. Y., McLeod J. G. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain. 1975 Sep;98(3):341–356. doi: 10.1093/brain/98.3.341. [DOI] [PubMed] [Google Scholar]
- Mathias C. J., Christensen N. J., Frankel H. L., Peart W. S. Renin release during head-up tilt occurs independently of sympathetic nervous activity in tetraplegic man. Clin Sci (Lond) 1980 Oct;59(4):251–256. doi: 10.1042/cs0590251. [DOI] [PubMed] [Google Scholar]
- P ARVING H. H., Rasmussen S. M. Transcapillary escape rate of albumin and plasma volume in short- and long-term juvenile diabetics. Scand J Clin Lab Invest. 1973 Aug;32(1):81–87. doi: 10.3109/00365517309082454. [DOI] [PubMed] [Google Scholar]
- Page M. M., Watkins P. J. The heart in diabetes: autonomic neuropathy and cardiomyopathy. Clin Endocrinol Metab. 1977 Jul;6(2):377–388. doi: 10.1016/s0300-595x(77)80043-1. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Rossing N., Nielsen S. L., Lassen N. A. Increased transcapillary escape rate of albumin, IgG, and IgM after plasma volume expansion. Am J Physiol. 1974 Aug;227(2):245–250. doi: 10.1152/ajplegacy.1974.227.2.245. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Smidt U. M., Friisberg B., Bonnevie-Nielsen V., Andersen A. R. A prospective study of glomerular filtration rate and arterial blood pressure in insulin-dependent diabetics with diabetic nephropathy. Diabetologia. 1981 Apr;20(4):457–461. doi: 10.1007/BF00253407. [DOI] [PubMed] [Google Scholar]
- ROWELL L. B., BLACKMON J. R., BRUCE R. A. INDOCYANINE GREEN CLEARANCE AND ESTIMATED HEPATIC BLOOD FLOW DURING MILD TO MAXIMAL EXERCISE IN UPRIGHT MAN. J Clin Invest. 1964 Aug;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell L. B., Detry J. M., Blackmon J. R., Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol. 1972 Feb;32(2):213–220. doi: 10.1152/jappl.1972.32.2.213. [DOI] [PubMed] [Google Scholar]
- SCHMIDT F. H. [Enzymatic determination of glucose and fructose simultaneously]. Klin Wochenschr. 1961 Dec 1;39:1244–1247. doi: 10.1007/BF01506150. [DOI] [PubMed] [Google Scholar]
- Sackner M. A., Markwell G., Atkins N., Birch S. J., Fernandez R. J. Rebreathing techniques for pulmonary capillary blood flow and tissue volume. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):910–915. doi: 10.1152/jappl.1980.49.5.910. [DOI] [PubMed] [Google Scholar]
- Tohmeh J. F., Shah S. D., Cryer P. E. The pathogenesis of hyperadrenergic postural hypotension in diabetic patients. Am J Med. 1979 Nov;67(5):772–778. doi: 10.1016/0002-9343(79)90733-2. [DOI] [PubMed] [Google Scholar]
- Triebwasser J. H., Johnson R. L., Burpo R. P., Campbell J. C., Reardon W. C., Blomqvist C. G. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977 Mar;48(3):203–209. [PubMed] [Google Scholar]
- Ziegler M. G. Postural hypotension. Annu Rev Med. 1980;31:239–245. doi: 10.1146/annurev.me.31.020180.001323. [DOI] [PubMed] [Google Scholar]


