Abstract
The presence of cilia in many vertebrate cell types and its function has been ignored for many years. Only in the past few years has its importance been rediscovered. In part, this was triggered by the realization that many gene products mutated in polycystic kidney diseases are localized to cilia and dysfunctional cilia result in kidney disease. Another breakthrough was the observation that the establishment of the left-right body axis is dependent on cilia function. Since then, many other developmental paradigms have been shown to rely on cilia-dependent signaling. In addition to mouse and Chlamydomonas, lower vertebrate model systems such as zebrafish, medaka and Xenopus have provided important new insights into cilia signaling and its role during embryonic development. This review will summarize those studies. We will also illustrate how these lower vertebrates are promising model systems for future studies defining the physiological function of cilia during organogenesis and disease pathophysiology.
Keywords: Cilia, Left-Right, Kidney, Zebrafish, Medaka, Xenopus, Kidney, PKD, ADPKD, ARPKD, NPHP, BBS, Review
2. INTRODUCTION
Flagella were initially described as a cellular organelle in Chlamydomonas and subsequently shown to be homologues to cilia present in most vertebrate cell types (1). Cilia are membrane extensions contiguous with the cell membrane characterized by the presence of nine peripheral doublets of microtubules. Most motile cilia also contain a central microtubule pair (9+2 pattern), while primary, sensory cilia lack this microtubule axoneme at its center and are referred to as 9+0 pattern. As first discovered in Chlamydomonas flagella (and cilia) are assembled by a process called intraflagella transport (IFT), in which large protein particles are carried along the ciliary microtubules by kinesin and dynein (2, 3). Studies in Chlamydomonas demonstrated that IFT particles are transport vehicles necessary for assembly, maintenance and function of flagella (4). Since then, IFT homologues have been identified in many different animal model systems, including C. elegans, Drosophila, zebrafish, medaka, Xenopus and mammals (5, 6, 7, 8) and mutations in the IFT genes showed that they are required for cilia assembly in Chlamydomonas (9), C. elegans (10, 11, 12), and zebrafish (13, 14, 15, 16). Interestingly, those studies not only show that IFTs are required for the structural integrity of cilia, but also implicated cilia as a possible common mechanism underlying several human pathologies, including left-right asymmetry defects, polycystic kidney disease, and retinal degeneration. Furthermore, recent studies suggest that the cilium participates in signal transduction pathways such as Hedgehog, Ca2+, Wnt, STATs, controlling cell proliferation and differentiation. There are many excellent, recent reviews discussing the role of cilia in those individual scenarios (17, 18, 19, 20, 21, 22, 23, 24). This review will summarize how the study of ciliogenesis has contributed to a better understanding of embryonic development in the lower, non-mammalian vertebrate model systems (zebrafish, medaka and Xenopus). A particular focus will be on how this information has generated a better understanding of how cilia-associated genes regulate human diseases.
3. NON-MAMMALIAN MODEL SYSTEMS TO STUDY CILIA FUNCTION
3.1. Zebrafish and medaka
The teleosts, zebrafish and medaka are very successful and widely used model systems. In a seminal paper published in 1981 (25), Streisinger described the use of zebrafish as a model system, which allowed genetic approaches to better understand vertebrate embryonic development. The Far East cousin to zebrafish, medaka has been used for the past 100 years. Systematic genetic analysis of medaka dates back to 1921 (26), and these studies represent the first vertebrate system that provided evidence of Y-linked inheritance. By 1975, a sufficient amount of information existed for publication of a book entitled “Medaka (Killifish)-Biology and Strains’ (27). Both zebrafish and medaka offer many experimental advantages to study vertebrate development and human disease. They produce large batches of offspring, which develop externally and are optically transparent. Furthermore, most organ systems develop within three days after fertilization and their development can easily be followed by simple morphological inspection or by GFP reporter strains (28). In addition, both zebrafish and medaka genome are sequenced and the sequence information is available through the Sanger Institute (http://www.ensembl.org/Danio_rerio/index.html, http://www.ensembl.org/Oryzias_latipes/index.html).
Zebrafish and medaka have been widely used as genetic models to screen for developmental defects (29, 30). Zebrafish and medaka mutants were generated by the chemical mutagen N-ethyl-N-nitrosourea (ENU) that induces base pair changes, as well as chemical and insertional mutagenesis (31, 32). These screens have identified mutations in many genes and have provided new insights in many signaling pathways. Furthermore, since the genes and proteins are well conserved between zebrafish/medaka and higher vertebrate systems, these mutagenesis screens have helped to better characterize pathogenic mechanisms and to identify new therapeutic targets in humans.
In addition, reverse genetic approaches using morpholino antisense oligos (MOs) have been instrumental to rapidly and economically test the function of those genes not identified in the genetic screens (33). MOs are synthetic molecules with standard nucleic acid bases, which are bound to morpholino instead of ribose rings. Replacement of anionic phosphates with the uncharged phosphorodiamidate groups eliminates ionization in the usual physiological pH range, so MOs in organisms or cells are uncharged. MOs are useful tools for reverse genetics by knocking down gene function or by modifying the splicing of pre-mRNA. Blocking the 5’-untranslated region of mRNA prevents translation of the coding region of the targeted transcript (“knocking down” gene expression). MOs can also interfere with pre-mRNA processing steps, by preventing splice-directing snRNP complexes from binding to targets at intron-exon junctions. In comparison with other molecular methods to assess gene function, MOs exhibit high sequence specificity, resistance to degradation by nucleases, and complete absence of non-specific side effects. Translational blockade or splicing donor targeting by MO are effective for at least the first 3 dpf (days post-fertilization) of development, which is within the time period of organogenesis.
Even though medaka is not as commonly used as zebrafish, it has several unique advantages: a smaller genome (about 800 Mb, half of the size of the zebrafish genome), the existence of polymorphism, highly fertile inbred strains and toleration of a wide temperature range (4 – 40 °C) during embryonic development (30). Medaka is an easily manipulated inbred fish model and potentially shows less gene redundancy than zebrafish. The genome has been sequenced by the National Institute of Genetics and the University of Tokyo (http://medaka.utgenome.org/) (34). All the sequence information is available via the ensembl website (http://www.ensembl.org/Oryzias_latipes/index.html). Similar to zebrafish, medaka has been used for genetic studies. In 1975, Tomita reported the first spontaneous medaka mutant collection (35). These studies with medaka mutants complement the zebrafish data and genetic screens in medaka (36, 37) have provided important additional information that will be useful in understanding human diseases.
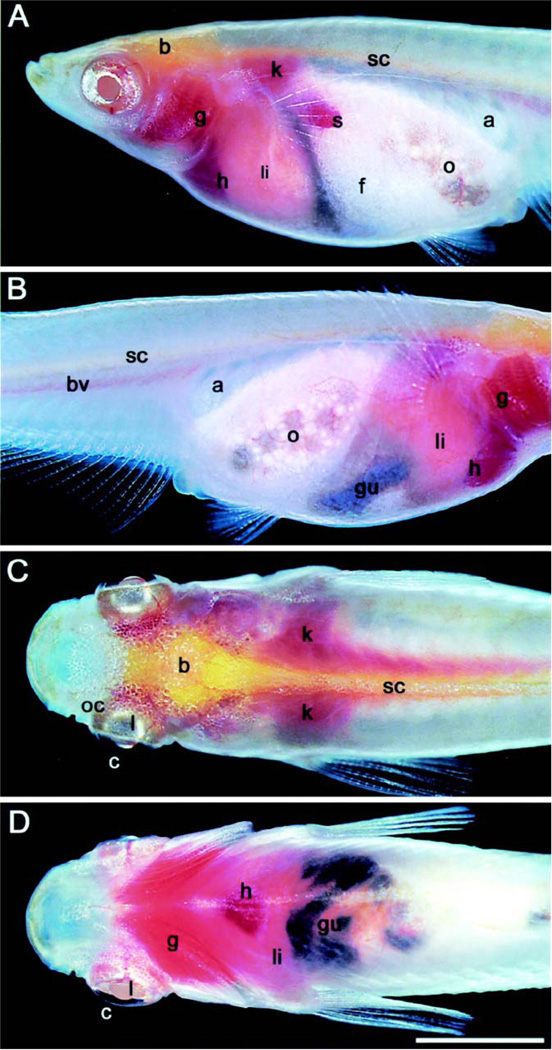
The bodies of most vertebrates are opaque, and internal organs are not visible from the outside. Both zebrafish and medaka have transparent bodies, and are therefore excellent models to follow vertebrate embryonic development under in vivo conditions. This advantage of transparency is lost after hatching because of the development of pigment cells in the skin. However, the Wakamatsu’s group has generated a “see-through” medaka strain by crossing color mutants, which shows defects in pigmentation (38) (Figure 1). The internal organs are visible through the body wall even in adult fish providing an opportunity to study late onset diseases such as Autosomal Dominant Polycystic Kidney Disease (ADPKD) (39).
Figure 1.
Adult see-through medaka fish. The left (A) and right (B) sides of the body of a female. The dorsal (C) and ventral (D) views of a male. a, air bladder; b, brain; bv, blood vessels; c, conjunctiva; f, fat tissue; g, gill; gu, gut; h, heart; k, kidney; 1, lens; li, liver; o, ovary; oc, optic cup; s, spleen; sc, spinal cord. The dark color of the gut comes from ingested feed (the scale bar represents 4 mm.). Reproduced with permission from Y. Wakamatsu (38) and PNAS Copyright (2001) National Academy of Sciences, U.S.A..
3.2. Xenopus
The amphibian Xenopus laevis is an extensively used vertebrate model organism to study early embryonic development. Like zebrafish and medaka, Xenopus is a good experimental model due to year-round breeding, large litter sizes, and low-cost maintenance. Using microinjections of synthetic mRNAs, expression plasmids or MOs, gain- and loss-of-function phenotypes of individual genes can easily be analyzed. Moreover, the processes of embryogenesis and organogenesis have been largely conserved to higher vertebrates. Thus, Xenopus has been instrumental in identifying many concepts in modern developmental biology. In addition to the classically used species Xenopus laevis, the closely related Xenopus (Silurana) tropicalis has recently been a popular alternative. It shares most of the advantages of Xenopus laevis as an embryological system, but its shorter generation time (3–4 months) and diploid genome (instead of the allotetraploid genome of Xenopus laevis), allows genetic approaches and the generation of transgenic animals. Furthermore, the genome of Xenopus tropicalis is currently sequenced by the DOE Joint Genome Institute (JGI - http://genome.jgi-psf.org/Xentr4/Xentr4.home.html). Even though it is still incomplete, the available sequence information already has significant impact on studies using Xenopus laevis and Xenopus tropicalis.
4. CILIA SIGNALING IN LEFT-RIGHT ASYMMETRY
Left-right asymmetry was the first embryonic process shown to be associated with cilia function. It is highly conserved among vertebrates and regulates the switch from a bilateral symmetric embryo to an embryo with properly oriented left-right asymmetry (40, 41, 42). The core of the signaling cascade is the asymmetric activation of Nodal signaling on the ventral side of the node during late gastrulation, which is initiated by an asymmetric flow of extracellular fluid in the node. This “nodal flow” has been shown to be driven by a specialized type of primary cilia, which is motile but is of 9+0 organization. The formation and movement of these cilia are dependent on motor molecules, kinesins and dyneins, which interact with microtubule-based cytoskeleton. Mice lacking the kinesins, KIF3A (43) and KIF3B (44) do not form nodal cilia, while mice lacking the iv gene, which encodes the left-right dynein heavy chain (Ird; Dnahc11-Mouse Genome Informatics), results in non-motile cilia (45, 46). All these mice have a defect in left-right asymmetry supporting the notion that nodal flow is necessary to initiate the left-right axis specification. Interestingly, the node also contains a second population of non-motile cilia. These cilia are located around the periphery of the node and do not contain dynein. Instead, similar to the fluid-flow-sensing cilia in the kidney, these cilia contain polycystin-2 (PC2) and are thought to serve as a mechanosensor responsible for the asymmetric activation of a calcium signal on the left periphery of the node. Importantly, as first demonstrated by Essner et al. (47), the role of cilia in determining left-right asymmetry is conserved across a wide range of vertebrate species.
In zebrafish, Kupffer’s vesicle (KV) is the candidate organ for left-right axis specification. KV is a transient spherical organ conserved in all teleost fish (48), which was originally described by von Kupffer (49). Embryologically, it is formed by a cluster of cells, the forerunner cells. These cells are originally derived from the dorsal margin of the late blastula zebrafish embryo and migrate to a position within the tailbud close to the chordoneural hinge, where they form KV (50, 51, 52). At later stages during somitogenesis, KV reverts to forerunner progeny and is incorporated into notochord, muscle, and tail mesenchyme (51). While the function of KV was unknown for a long time, the observation by Essner et al. (47) that KV expresses lrd was the first clue that KV is required for the development of laterality in fish. Since then, it has been shown that the counterclockwise beating of the cilia present on the epithelial cells lining KV generate a counterclockwise flow pattern in KV (16). It is still unknown how this signal gets transformed into positional information.
A particular advantage of zebrafish is that mutagenesis and knockdown experiments using MOs have identified several steps required for the inductive activity of KV. The Yost group also developed a unique technique to deliver MOs specifically to dorsal forerunner cells (DFCs), by injecting into the yolk cells between the 512-cell and 1000-cell stages (52). This approach has yielded data about KV formation, ciliogenesis and cilia-mediated signaling. In respect to the formation of KV, Essner et al. (52) demonstrated that interfering with the cellular architecture of KV either by laser ablation experiments eliminating the DFCs or by surgical disruption of KV interfered with left-right patterning. Zebrafish mutants affecting endoderm development, such as casanova, a member of the SoxF group of HMG domain transcription factors, lack forerunner cells, do not form KV and thus develop left-right asymmetry defects (54, 55). Similarly, specifically interfering with the translation of no tail (the zebrafish homologue of brachyury) in the DFCs results in impaired morphogenesis of KV (53). Although the ciliated DFCs were present in the region where KV should form, they were disorganized and could not generate the directed fluid-flow required for left-right development.
Both NCX and Na, K-ATPase cooperates to maintain a low intracellular Ca2+ level. MO knock down of Na, K-ATPase a2 induced cardiac laterality and its functional partner Ncx4a, are involved in a very early step of left-right patterning in zebrafish. The Chen group recently discovered that down regulation of Na, K-ATPase a2 and Ncx4a in DFCs and KV is sufficient to immobilize KV cilia, perturb directional flow in Kupffer vesicle, disrupt the asymmetric expression patterns of zebrafish laterality genes in the brain and lateral plate, and randomize the placement of all internal organs (56).
In Xenopus, Essner et al. (47) showed that lrd is expressed on the ventral side of the dorsal blastopore lip. Subsequent analyses have extended observation and now suggest that the entire gastrocoel roof plate (GRP) is the left-right organizer in Xenopus. The GRP is derived from the superficial mesoderm and forms an elongated triangular structure located at the posterior end of the notochord, anterior to the blastopore lip facing the gastrocoel (57, 58). The GRP cells can be detected between stage 13 and 19 and subsequently ingresses into the underlying mesoderm. Four criteria argue that the GRP is the Xenopus equivalent of the mouse node and KV in zebrafish (59, 60): First, bilateral expression of Nodal mRNA is detected along the border to the GRP and this expression precedes the asymmetric expression of Nodal in the left lateral plate. Second, the GRP itself is characterized by the presence of monociliated epithelial cells that express classical markers of cilia involved in left-right determination, i.e. Lrd, Inversin and PC2. Third, time-lapse imaging could detect ciliary beating and a leftward fluid flow. Finally, interfering with the fluid flow by injecting methylcellulose into the archenteron resulted in Xenopus embryos with laterality defects.
Interestingly, in addition to the expression in the GRP, many cilia-associated genes such as pkd2 or Inversin can be detected at early cleavage stage Xenopus embryos in a left-right asymmetric fashion (15, 61, 123). Loss- and gain- of function studies have suggested that these early stages contribute to the formation of a left-right body axis (24). One of the challenges in the future will be to connect those events and demonstrate that such an early patterning event also plays a role in other vertebrate species.
5. CILIA AND KIDNEY DEVELOPMENT
The realization that cilia play an important role in the kidney was initially proposed from the study of polycystic kidney diseases (PKD). These diseases are characterized by the formation of fluid-filled cysts derived from the epithelial lining of the nephron, and caused by extensive proliferation and apoptosis of renal epithelial cells. Mutations in two genes, PKD1 and PKD2, have been identified as the cause of ADPKD (62, 63, 64, 65). The two proteins associated with human ADPKD, Polycystin1 (PC1) and Polycystin2 (PC2), have been detected in the cilia present on the apical surface of the tubular epithelial cells (66, 67). Subsequently, most of the other genes involved in PKD such as PKHD1 for autosomal recessive PKD (ARPKD), BBS1, BBS2, BBS4, BBS7 and BBS8 for Bardet-Biedl syndrome (BBS) (20, 21) and NPH1, NPHP2, NPHP3, NPHP4 and NPHP5 for nephronophthisis (NPHP) have also been shown to be associated to the cilia or the basal body (22). Interestingly, neither polycystin-1 (PC1) nor PC2 are required for ciliogenesis, but for downstream signaling (18, 68, 69). PC1 is an 11 transmembrane domain protein, while PC2 is a member of the transient receptor potential (TRP) super family, which is cation channels involved in mechanosensation and triggering Ca2+ influx into cells. Both proteins regulate differentiation and proliferation of kidney epithelial cells. However, the precise cellular and molecular mechanism how the cilia localization of PC1 and PC2 leads to ADPKD is still poorly understood.
5.1. Cilia in the pronephric kidney
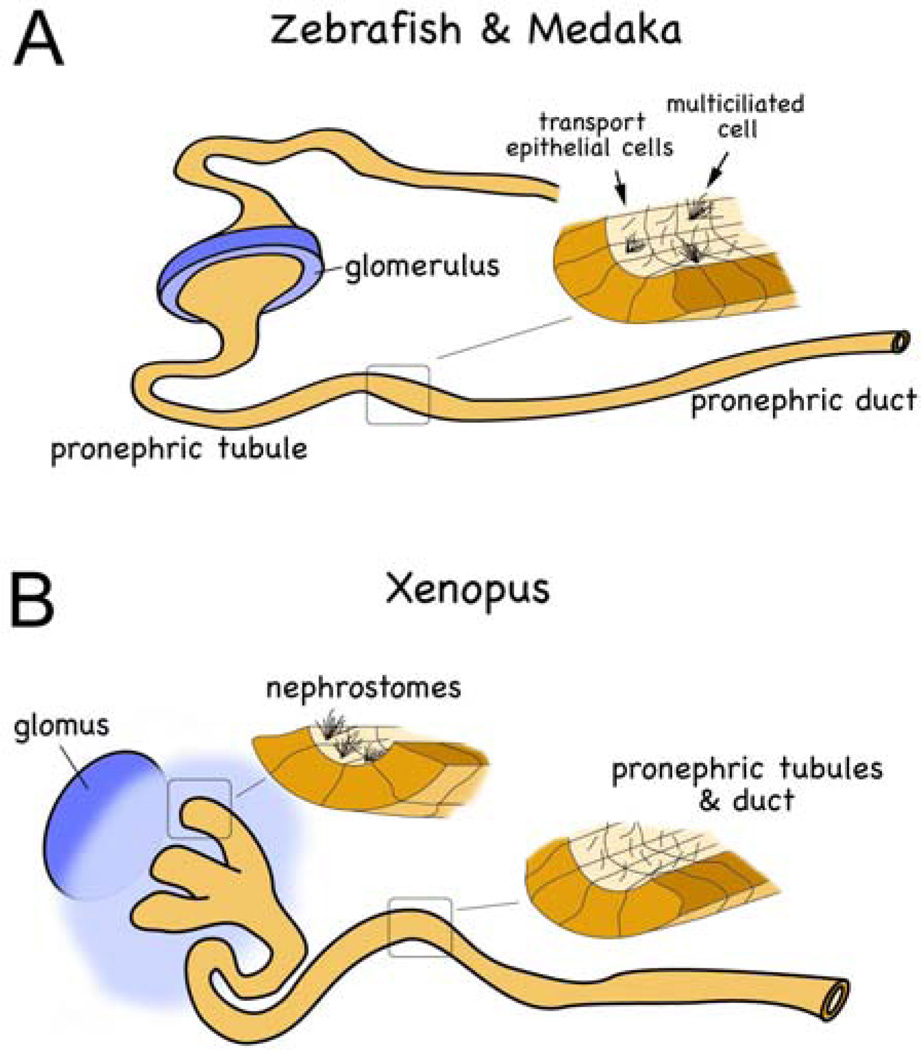
The kidney is a complex organ which filters blood, maintains fluid and ion balance and disposes of metabolic waste. In vertebrates, kidney development is characterized by the successive emergence of three kidney forms, the pronephros, the mesonephros and the metanephros. The metanephros is the functional kidney in higher vertebrates. In amphibians and fish the mesonephros is the adult kidney form. However, the pronephros has to be functional during the earliest stages of embryogenesis to maintain the water homeostasis of the aquatic larvae (Figure 2). While the three kidney types differ in complexity, the functional unit, the nephron is similar for each of them. Moreover, the pronephric kidney contains most of the cell types found in metanephric kidney. In the glomerulus, podocytes, mesangial cells and the endothelia cells are necessary for proper blood filtration and - as shown in zebrafish - podocytes form foot processes of the slit-diaphragm (70). Similarly, highly polarized epithelial cells characterize the pronephric tubules and ducts and they display a proximal-distal organization comparable to that found in the nephrons of the metanephric kidney (71, 72, 73). Finally, at the molecular level, gene regulatory cascades are evolutionarily conserved and many genes function similarly in the pronephros and the metanephros. Thus, over the years the pronephric kidney has been successfully used as a simple model for kidney developmental (74, 75, 76).
Figure 2.
Schematic diagram of the zebrafish/medaka (A) and the Xenopus (B) pronephros. Note that multiciliated cells are present in a salt-and-pepper fashion in teleosts, while they are restricted to the nephrostomes in amphibians.
Zebrafish has provided significant evidences supporting a role for cilia in kidney development and in the pathogenesis of human kidney diseases. The first ENU mutagenesis (77, 78, 79) and insertional mutagenesis screens (80, 81) identified 24 recessive mutations that form pronephric cyst by 2–2.5 dpf. These mutations fall into five distinct phenotypic classes. The first group contains 17 mutations that resulted in cyst formation and body axis curvature. These included the mutant double bubble (dbb) that is characterized by a loss of cilia structure (77, Tomoko Obara and Iain Drummond unpublished data). The second group (pao pao tang (pap) and cyster (cst)) displayed only pronephric cyst formation and no other phenotypes (77). The third mutant group exhibited pronephric cyst formation, body axis curvature and eye defects. They contained fleer (flr) and elipsa (eli) and both have been shown to induce cell death in the photoreceptor cell layer at 3–5 dpf (77, 82). The pronephros flr mutant displayed distended capillaries loop formation and cyst formation, but its molecular identity is still unknown (77). The fourth group of mutants was originally noted for defects in left-right asymmetry, but also displayed kidney cyst and body axis curvature defects (78, 79). However, the molecular nature of the two mutations in this class, locke (lok) and unresolved, are still unknown. Finally, the fifth type of pronephric mutation is characterized by pronephric kidney cyst and undeveloped liver and pancreas (81). So far, only one gene is in this group, variant Hnf1 (vHnf1/HNF1beta). It encodes a homeobox gene, which is known to associate with glomerulocystic kidney disease and diabetes (83, 84). In addition to the initial screens, the group of Nancy Hopkins has identified additional mutants that cause pronephric cyst using retroviral insertional mutagenesis (14). They identified 12 different genes two of which, vhnf1 and pkd2, are linked to human cystic kidney. Three others turned out to be orthologues of genes, which have been shown in Chlamydomonas to encode proteins involved in IFT and cilia formation. Unfortunately, the function and mechanism of many of these mutants still remain unknown. In the future, it will be interesting to see how these relate to ciliogenesis or whether they will further advance the molecular mechanism of cilia-mediated signaling.
In zebrafish, the cilia in the pronephric kidney, the spinal cord and KV, the equivalent of the mouse embryonic node, are motile and of the 9+2 structure as characterized by electron microscopy (16). To examine the motility of these pronephric cilia, 2.5 dpf embryos were treated with butanedione monoxime to stop the heartbeat and circulation thereby eliminating the glomerular filtration pressure and the ultrafiltrate flow. High-speed video-microscopy revealed active cilia beating and not passive reflection (16). Moreover, the rotational cilia movement generated a corkscrew-like wave pattern in the lumen of the duct directed toward the cloaca. To understand the biological relevance of these cilia movements in vivo, the structure of cilia was manipulated using MOs against the IFT proteins polaris/IFT88/osm-5 and hippi/IFT57/che-13. In addition, the mutant fish strain oval (ovltz288b), which carries a point mutation (L260X) in the zebrafish homolog of polaris/IFT88/osm-5 which leads a truncated protein was also evaluated. All the morphants (i.e. embryos injected with MOs) and ovlfz288b, developed pronephric cyst, hydrocephalus, curved body axis and pericardial edema in 72 hpf (hours post-fertilization). As expected, the cilia were severely shortened or absent, however, the 9+2 microtubule doublet ultrastructure was relatively normal. Moving cilia were rarely detected and the remaining motile cilia displayed a faster, uncoordinated, and flickering movement. To determine whether disturbed cilia motility impacted the fluid output from the pronephric cloaca, dye excretion was examined by using Tetramethylrhodamine-conjugated dextran (70 kDa) injected into the cardinal vein of living 3.5 dpf embryos. In wild-type embryos the dye is filtered at the glomerulus and excreted via pronephros cloaca and the fluorescent urine output can be observed after 3–8 min post-injection. In the morphants and ovltz288b, no dye excretion could be detected suggesting that cilia function is required to maintain normal rates of fluid flow in the pronephros. To demonstrate that impaired fluid flow can lead to cyst formation, the investigators mechanically obstructed the pronephric ducts near the cloaca and verified rapid pronephric cyst formation within 30 min (16).
One interesting observation is that cilia in zebrafish and 9+0 mouse node cilia are motile but, cilia in metanephric kidney (9+0 type) are not motile and are thought to sense fluid flow instead of propelling fluid (85). However, in humans, 9+2 cilia have been detected during fetal kidney development (86) and in adult human kidneys with pathological symptoms (87, 88, 89). More specifically, 9+2 motile cilia seem to be important for kidney development to maintain proper lumen diameter and kidney function. As development proceeds cilia motility seems to be lost and the cilia take on a new sensory function in the mature mammalian kidney. The fact that the cilia movements and not only a sensory function is important for the development of the pronephros has been directly shown in zebrafish by disrupting dynein heavy chain 9 (dhc9). The outer doublet microtubules and associated dynein arms are critical for the initiation and propagation of ciliary bending. In order to disrupt cilia beat rate and pattern, a MO targeted to the orthologue gene (dhc9) to human dynein heavy chain 9 (DYH9) at the splice donor site of the P1 domain, the primary ATP binding site that is essential for dynein motor was injected in zebrafish. The dhc9 morphant phenotype included reduction in beat frequency and slower cilia movement, but without changes in cilia structure that caused the phenotypes associated with IFT loss of function (16).
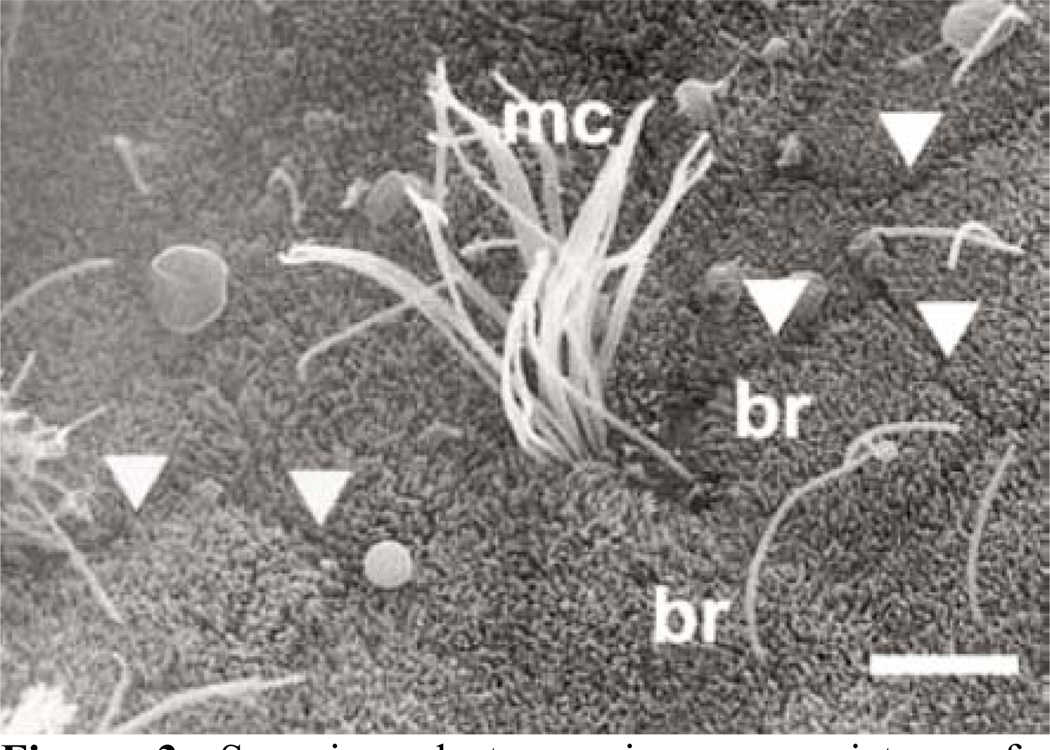
One important realization in understanding cilia function is that zebrafish embryos have two types of ciliated cells in the early distal segment of the pronephros: single ciliated cells that morphologically resemble transporting epithelial cells and multiciliated cells (72, 73) (Figure 2A). The two cell types are also found in the pronephros of medaka embryos (39, Tomoko Obara unpublished data) (Figure 3) and in the amphibian pronephros (90, 91, 92). In zebrafish and medaka, the two cell types are distributed as an alternative “salt and pepper” pattern in the early distal segment of pronephros. In amphibians the multi-ciliated cells are restricted to the nephrostomes (91, 92). This difference may be explained by anatomical differences of the pronephros. The fish pronephros is a semi-integrated nephron and ultrafiltrate passes directly across the glomerular filtration barrier into the pronephric tubules and is propelled within the tubules (70). In Xenopus, the ultrafiltrate moves into the coelomic cavity prior to entering the pronephric tubules using a specialized structure, the nephrostomes. In Xenopus the nephrostome contains the multi-ciliated cells as shown both by immunofluorescence for acetylated alpha-tubulin as well as molecular markers characteristic for multi-ciliated cells (91 and Oliver Wessely unpublished data) (Figure 2B).
Figure 3.
Scanning electron microscopy picture of a pronephric tubule section of medaka. The cell borders are indicated by arrowheads; mc, multi-ciliated cell; br, brush border. Reproduced with permission from Y. Wakamatsu (39) and Nature publishing group.
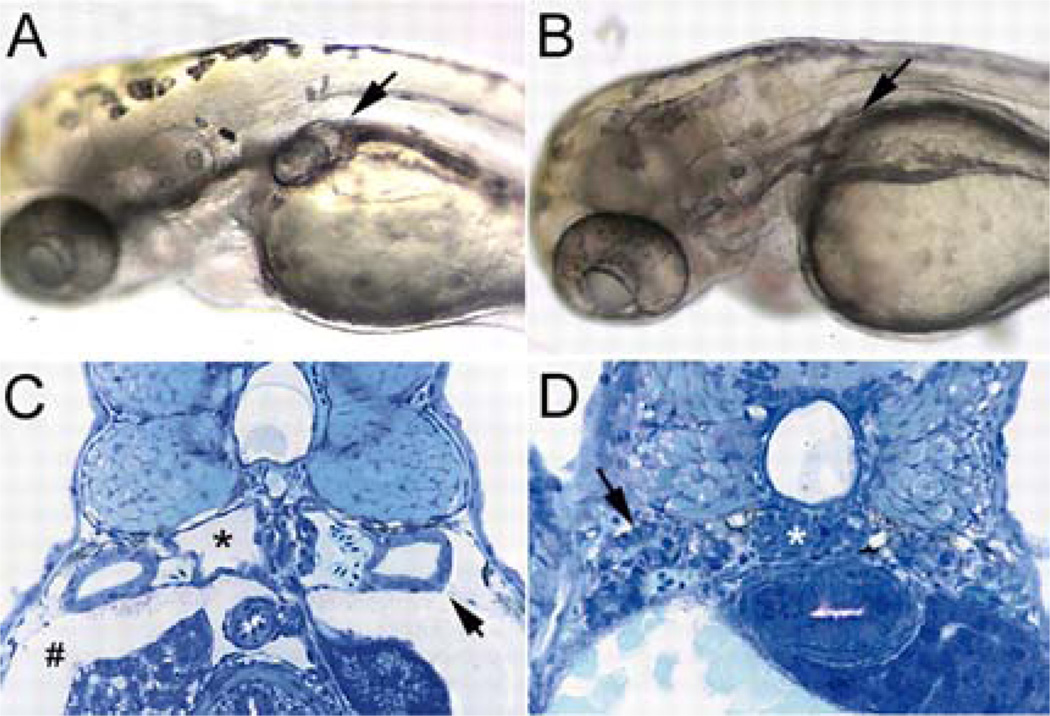
Because Notch signaling has been shown to regulate pattern formation and cell fate determination through cell-cell interactions, the “salt and pepper” pattern of the single- and multi-ciliated pronephros cell types suggested that lateral inhibition by Notch signaling was responsible for the formation of the multi-ciliated cells. Notch is a transmembrane receptor that interacts with transmembrane ligands (Delta and Serrate/Jagged) expressed on neighboring cells (93, 94, 95, 96). Upon ligand binding Notch undergoes regulated intramembrane cleavage, releasing the Notch intracellular domain (NICD), which moves into the nucleus and activates the CSL (CBF-1, Supressor of Hairless (Rbpsuh), Lag-1) transcriptional complex. This initiates a transcriptional cascade involving the expression of Hes and HRT/HER/Hey transcriptional repressors and thus changes the cell fate of neighboring cells (93, 94, 95, 96). Notch signaling has an important role during kidney development. In the mouse metanephric kidney Jagged1/Notch-2 signaling has been implicated in the development of glomerular vasculature and in segmentation of the nephron (93, 94, 95, 96). Similarly, in Xenopus, Notch signaling regulates proximal-distal development of the pronephric kidney (96). The recent study by the Drummond’s and Jiang’s group showed that another Notch ligand, jagged2, is required to regulate the formation of multi-ciliated cells (72, 73). jagged2 is expressed in the multi-ciliated cells and jagged2 knockdown by MOs resulted in the formation of ectopic multi-ciliated cells via up-regulating a key transcriptional regulator of the ciliogenesis, rfx2. These studies indicate that neighboring cells regulates the specification of multi-ciliated cells via lateral inhibition involving the interaction between Jagged2 and Notch-3. Another interesting observation from this study was that the pronephric defect was rescued when the jagged2 MO was injected into dbb homozygotes zebrafish, while similar rescue experiments in other zebrafish mutant strains affecting cilia structure, cilia motility and/or pronephric cyst formation were negative (Figure 4). This suggested that the not-yet-identified dbb gene is a crucial mediator of Jagged2/Notch-3 signaling cascade and further supports the power of genetic screens to identify novel aspects in ciliogenesis (72).
Figure 4.
Enhanced ciliogenesis and double bubble-mutant rescue by inhibition of Jagged/Notch signaling. (A) dbb-mutant homozygote at 2.5 dpf showing bilateral cyst formation in the proximal pronephros (arrow). (B) dbb-mutant homozygote injected with jagged 2 exon 20 MO at the one-cell stage, showing the absence of cyst formation (arrow; mutant rescue). (C) Histological section of dbb-homozygote pronephros showing a cystic pronephric tubule (*), dilated pronephric tubules (arrow) and edema (#). (D) Histological section of a dbb homozygote injected with jagged 2 exon 20 MO at the one-cell stage, showing complete absence of cystic pathology and edema (arrow, wild-type-appearing pronephric tubules; *, normal glomerular structure). Reproduced with permission from I. A. Drummond (79) and the Company of Biologists Ltd.
In addition to Delta-Notch signaling non-canonical Wnt signaling seems to be important for ciliogenesis. Park et al. (97) examined two Xenopus homologues of the Drosophila planar cell polarity (PCP) pathway, fuzzy (fy) and inturned (in). While loss-of-function studies clearly showed a role for both proteins in non-canonical Wnt signaling, they also revealed additional phenotypes resembling defects in vertebrate hedgehog signaling, which is dependent on the presence of primary cilia. They went on to show that ciliogenesis was defective in the Xenopus embryos lacking in or fy. Interestingly, this was caused by a defect in the microtubule-mediated assembly of the apical actin meshwork present in ciliated cells. Along the same line, the group of J. C. Izpisúa-Belmonte (98) recently identified in a screen for genes involved in zebrafish left-right patterning a putative phosphorylation-dependent cytoskeletal regulatory molecule named duboraya (dub). Knockdown of dub in zebrafish embryos resulted in randomization of the left-right asymmetry patterning due to defects in cilia formation. Molecular analysis showed that dub was regulated by phosphorylation downstream of Frizzled-2. Thus, dub seems to be an essential component of non-canonical Wnt signaling, and similar to in and fy regulates ciliogenesis by organizing the actin cytoskeleton in epithelial cells.
5.2. A reverse genetic approach towards ciliogenesis
Dysfunctional primary cilia have been implicated in disease pathogenesis and zebrafish has been a popular animal model to better understand the function and structural role of genes discovered through the human mutation studies (22). One particularly fruitful example is Nephronothisis (NPHP), which is caused by many different genetic defects, many of which have been characterized by the group of F. Hildebrandt. So far, positional cloning has identified six genes (NPHP1 through 6), all of which have been demonstrated to be expressed in primary cilia or in centrosomes of renal epithelial cells. This connection of NPHP to cilia function has led to the concept of “ciliopathies” as a term describing human diseases caused by dysfunctional cilia (22).
One gene, which was extensively characterized in Xenopus and zebrafish as model systems, is Inversin (inv), the gene mutated in NPHP2 (99, 100, 101). Biochemical studies in cells showed that Inversin, Nephrocystin (the product of the gene mutated in NPHP1) and beta-tubulin form a protein complex that localizes to primary cilia of renal tubular cells. As one would expect, knockdown of inv in zebrafish resulted in a classical cilia-related phenotype, i.e. PKD-like renal cysts and randomization of heart looping. However, these data also supported the observation that the multiprotein complex with other NPHP proteins in the cilia is important for Inversin activity. Deletion of only the putative nephrocystin-binding domain (the C-terminal IQ2 domain) by targeting the splice donor site corresponding to the human exon 14 with a MO resulted in cyst formation similar to the knockdown of the entire protein.
Interestingly, the PKD-like phenotype of zebrafish embryos lacking Inversin protein could be completely rescued by co-injection of mRNA encoding mouse Inversin as well as zebrafish diversin a molecule structurally related to inversin. Diversin has been shown to inhibit canonical Wnt/beta-Catenin signaling and controlling gastrulation movements in zebrafish embryos (99, 100) suggesting a role for Wnt signaling. These findings were substantiated in subsequent studies using Xenopus and zebrafish embryos. In Xenopus, ectopic expression of Inversin inhibited canonical Wnt/beta-Catenin pathway by targeting cytoplasmic Dishevelled protein for ubiquitin-mediated degradation; concomitantly, loss-of-function studies using MOs demonstrated that Inversin was required for the convergent-extension movements of the mesoderm, a classical paradigm for non-canonical Wnt signaling. Similar data were obtained in zebrafish. The transcriptional regulator bozozok (boz) is a direct target of Wnt/beta–Catenin signaling and is expressed at the 60% epiboly stage in the dorsal yolk syncytial layer. In zebrafish embryos lacking inversin, boz expression was extended to the lateral, ventral and animal yolk syncytial layer, indicating that Inversin is required to restrict Wnt/beta-Catenin-mediated gene transcription. Together these data suggested that Inversin acts as a molecular switch between canonical and noncanonical Wnt signaling. When extrapolated to kidney development these findings suggest that beta-Catenin/TCF-dependent gene transcription has to be curtailed to allow normal tubular differentiation. Even though the role of Wnt/beta-Catenin signaling in PKD is still unclear, these findings have a strong conceptual impact on the understanding of Polycystic Kidney Disease (17, 23, 24).
Many other genes associated with ciliogenesis have been examined in zebrafish. These include NPHP CEP290, which causes NPHP6 (102). Cep290 encodes a centrosomal protein involved in chromosome segregation and cell cycle regulation via its interaction with the transcription factor ATF4. Knockdown of cep290 in zebrafish recapitulates at least some aspects of its human phenotype (Joubert syndrome) and causes renal, retinal and cerebellar phenotypes, which was not caused by structural or motility defects of cilia.
As expected mutations in intraflagellar transport proteins are the cause for ciliopathies. Ift80 is mutated in Jeune asphyxiating thoracic dystrophy (103). Its orthologue in zebrafish (zfitf80) is expressed in the craniofacial region, otocyst and pronephric duct and the knockdown results in a classical “loss-of-cilia” phenotype, i.e. body axis curvature, kidney cysts, cardiac edema, abnormal anterior neurocranial development, with midline fusion of trabeculae (103). Similarly, another IFT protein, Ift88/polaris/Tg737, was originally described as the causative mutation of a mouse model of ARPKD (104). Ift88 is a protein present in intraflagellar transport particles and is important for assembly and maintenance of the outer segment of the cilia structure (9, 10). In zebrafish, the ovl mutation has been shown to be located in ift88 (13). Cilia of ovl mutant fish cilia initially form, but are not maintained resulting in kidney cysts, and the degeneration of photoreceptor cells, auditory hair cells and olfactory sensory neurons, all of which have been shown to be ciliated (13, 14). Similar, but weaker phenotypes could also be observed in zebrafish embryos lacking other IFT proteins (Ift52, Ift57 and Iftl40) (13).
A third such ciliopathy is the Bardet-Biedl syndrome (BBS), a pleiotropic disorder with primary features that include age-related retinal dystrophy, obesity, Polydactyly, renal dysplasia, reproductive tract abnormalities and cognitive impairment (20, 21, 105). It is genetically heterogeneous and so far mutations in eight genes have been identified (BBS1–8). All of the genes are expressed exclusively in ciliated organs. Knockdown of zebrafish bbs2, bbs4, bbs5, bbs6, bbs7 and bbs8 resulted in left-right patterning defect due to disruption of ciliogenesis in KV (106). This study was the first to demonstrate that the diverse group of BBS genes share a common role in intracellular trafficking required for ciliogenesis. Interestingly, these kinds of studies also defined a subset of BBS genes, BBS6, BBS10 and BBS12, as a vertebrate-specific branch of chaperonin-like proteins (106, 107, 108, 109).
Several studies in Drosophila, C. elegans and mouse raised the possibility that BBS genes have a role in the PCP pathway (20, 21, 105). In zebrafish, this pathway has been studied extensively, since several mutant fish strains with defects in PCP genes have been identified (e.g. trilobite, a mutation of vangl2; silberblick - a mutation of wnt11; pipetail - a mutation of wnt5and knypek) (109). Interestingly, using the interaction between BBS genes in trilobite mutants enhanced the already abnormal convergent-extension movements observed in trilobite mutant fish during gastrulation. Together with the observation that Vangl2 protein is localized around the base of the cilium and perinuclear region, as well as punctuate staining along the length of ciliary axonome, these data suggest that BBS genes are involved in the PCP signaling process. This further strengthens the hypotheses of other studies (20, 21, 105, 109) suggesting a connection between cilia and PCP signaling.
In contrast to many animal models of PKD, which are caused by defects in ciliogenesis (19, 110), human ADPKD is caused by mutations in two genes involved in cilia-regulated signaling. Mutations in PKD1 account for 85% of the ADPKD cases, but the function of the PKD1 gene product PC1 is still poorly understood. The current working model is that the primary cilia in the kidney function as a mechanosensor for luminal fluid flow and PC1 is an essential component of regulating the release of intracellular calcium (68, 69, 110, 111, 112). Cultured renal epithelia from PC1 null mice contain cilia but fail to respond to flow-mediated bending of their cilia with an elevation of intracellular calcium (68, 69). In addition, the C-terminal part of the cytoplasmic tail of PC1 has been implicated in regulating multiple signal transduction pathways such as Wnt signaling (114), G-protein signaling (115) and the activation of transcription factors such as AP-1 and STAT1 (116, 117, 118, 119). In zebrafish PC1 has not yet been extensively studied. However, zebrafish has already been instrumental in better understanding the function of PC1. Recent studies have shown that luminal flow results in proteolytic cleavage of PC1, releasing its cytoplasmic tail, which then translocates to the nucleus and stimulates STAT6-dependent transcription (120). Interestingly, when the intracellular cytoplasmic tail of PC1 was ectopically expressed in zebrafish embryos, they develop renal cysts. These data suggest that the function of PC1 in kidney development is conserved and that zebrafish will also be an informative model to better understand the molecular mechanism of PC1 activity. PC1 and PC2 function in medaka has also been explored as a model of cystogenesis (Tomoko Obara unpublished data).
The other gene mutated in ADPKD, pkd2, has been studied in zebrafish by several groups (15, 121, 122, 123). In addition to knock down approaches using MOs, the laboratory of N. Hopkins (14) has identified the zebrafish orthologue of pkd2 by insertional mutagenesis and Schottenfeld et al. (123) have characterized curly up (cup), another allele of pkd2, isolated from a large-scale ENU mutagenesis screen.
Immunofluorescence analysis of PC2 revealed protein expression in the pronephros, in muscle cells and in a variety of sensory cells that are associated with mechanotransduction, including the ear, the lateral line organ, and the olfactory placodes (122). As expected from studies in mouse and cell lines, in zebrafish PC2 could be detected at the subcellular level in motile cilia and in intracellular membranes of the pronephric kidney. Interestingly, the intracellular localization of PC2 was not uniform in the kidney, but segmental specific; in the proximal nephron segment, PC2 was localized to basolateral membranes, whereas in the caudal pronephric segment, PC2 was concentrated in subapical cytoplasmic vesicles (122).
Two main roles for PC2 have so far been described in zebrafish, one in kidney development and one in the establishment of left-right body axis. The later phenotype resembles human heterotaxia and includes changes in asymmetric gene expression in the lateral plate and in the dorsal diencephalon of the brain (15, 122, 123). Moreover, by targeting the MOs specifically to the dorsal forerunner cells and KV, Yost et al. (15) could demonstrate that the expression of PC2 in these cells is necessary for normal left-right patterning. In contrast to mouse, zebrafish PC2 does not activate signaling via Nodal homologue southpaw, but instead regulates the propagation of Nodal signals and restrict the expression of southpaw to the left lateral plate mesoderm (123). In the pronephros, loss-of-PC2 resulted in cyst formation, which - unlike zebrafish IFT mutants - was not associated with cilia defects. Instead, it resulted in reduced kidney fluid output, expansion of caudal apical cell membranes, and occlusion of the caudal pronephric nephron segment (122). Interestingly, while both PC2 mutant fish strains (15, 122, 123) displayed left-right asymmetry defects, neither strain developed pronephric cyst or defects in kidney patterning. Only zebrafish embryos injected with MO directed against PC2 developed pronephric cyst and defects in left–right asymmetry. While puzzling, the reason why the MO injections have a stronger phenotype than the genetic mutation is still not yet known and will be cause for future studies.
6. PERSPECTIVES
The emergence of cilia as a signaling unit has had a broad impact on developmental biology and to understanding of various diseases. Lower vertebrates such as zebrafish, medaka and Xenopus have helped to better understand not only the process of ciliogenesis, but also the signaling events downstream of the cilia. In this respect, the roles of cilia in the formation of the left-right axis and in the development of the pronephros are two best-studied scenarios. Since cilia, however, can be found on most cell types, it is likely that cilia-regulated signaling is important for many more events during organogenesis.
One of the important future aspects studying cilia is to understand the mechanism of fluid dynamics. In case of the pronephros, cilia regulate the organization of the tubular epithelial structures, in the node they establish an asymmetric signaling center and in the spinal cord they sense a gradient of hedgehog signaling. The complexity of these scenarios clearly illustrates that cilia need to be studied in vivo in their cellular context. Analyzing them as isolated cells in cell culture will only allow small aspects of their potential. Thus, lower vertebrates, zebrafish, medaka and Xenopus, offer unique advantages. The ease and speed of molecular manipulation using for example injections of MOs to eliminate a gene allows testing new scenarios within weeks. In addition, mutagenesis screens still prove to be very fertile grounds to discover new aspects in cilia signaling. The realization, that the zebrafish mutant double bubble (dbb) is involved in the development of ciliated cells in the pronephros demonstrates that some of the non-identified mutant genes may unravel completely unexpected aspects of ciliogenesis. Furthermore, with the technological advances more directed genetic screens are possible to address specific problems in cilia-mediated signaling.
ACKNOWLEDGEMENT
O. W. and T. O. contributed equally to this article. We apologize that due to space limitations we were prevented from citing all of the literature relevant to this review. This work has been supported in part by grants from the National Institutes of Health to T. O. (5R21DK069604-02, 1R01DK078209-01) and O. W. (5R21DK070671-03) and Polycystic Kidney Disease Foundation to T. O. (69a2r) and O. W. (103a2r). We also would like to thank Jeff Schelling, Albert Ong, Uyen Tran and Genevieve Okenka for useful comments on the manuscript.
Abbreviations
- IFT
intraflagella transport
- ENU
N-ethyl-N-nitrosourea
- dpf
days post-fertilization
- hpf
hours post-fertilization
- MOs
morpholino antisense oligos days post-fertilization
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- PC2
Polycystin-2
- KV
Kupffer’s vesicle
- DFCs
dorsal forerunner cells
- GRP
gastrocoel roof plate
- PC1
Polycystin-1
- ARPKD
autosomal recessive PKD
- BBS
Bardet-Biedl syndrome
- NPHP
nephronophthisis
- dbb
double bubble
- pap
pao pao tang
- cst
cyster
- flr
fleer
- eli
elipsa
- lok
locke
- vHnfl/HNFlbeta
varient Hnfl
- ovltz288b
oval
- dhc9
dynein heavy chain 9
- DYH9
human dynein heavy chain 9
- NICD
Notch intracellular domain
- CSL
CBF-1, Supressor of Hairless (Rbpsuh), Lag-1)
- PCP
planar cell polarity
- fy
fuzzy
- in
inturned
- dub
duboraya
- inv
Inversin
- boz
bozozok
- ovl
oval
- TRP
transient receptor potential
- cup
curley up
REFERENCES
- 1.Wheatley DN. Primary cilia in normal and pathological tissues. Pathobiology. 1995;63:222–238. doi: 10.1159/000163955. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 3.Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 4.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 7.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 8.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development. 2001;128:1493–1505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- 11.Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK, Identification of CHE-13. a novel intraflagellar transport protein required for cilia formation. Exp Cell Res. 2003;284:251–263. doi: 10.1016/s0014-4827(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 12.Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J Cell Sci. 2006;119:4088–4100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- 13.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Dev Biol. 2005;287:274–288. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 15.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 17.Germino GG. Linking cilia to Wnts. Nat Genet. 2005;37:455–457. doi: 10.1038/ng0505-455. [DOI] [PubMed] [Google Scholar]
- 18.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 19.Harris PC, Torres VE. Understanding pathogenic mechanisms in polycystic kidney disease provides clues for therapy. Curr Opin Nephrol Hyperten. 2006;15:456–463. doi: 10.1097/01.mnh.0000232888.65895.e7. [DOI] [PubMed] [Google Scholar]
- 20.Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildebrandt F, Zhou W. Nephronophthisis-associatd ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 23.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 24.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 25.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 26.Aida T. On the inheritance of Color in a Fresh-Water Fish, APLOCHEILUS LAPTIS Temmick and Schlegel, with Special Reference to Sex-Linked Inheritance. Genetics. 1921;6:554–573. doi: 10.1093/genetics/6.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto T. Medaka (Killifish)-Biology and Strains. Tokyo: Keigaku Publishing Co; 1975. [Google Scholar]
- 28.Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- 29.Method in Cell Biology, The zebrafish: genetics and genomics volume 60. Oxford: Academic Press; [Google Scholar]
- 30.Wittbrodt J, Shima A, Schartl M. Medaka-a model organism from the Far East. Nat Rev Genet. 2002;3:53–64. doi: 10.1038/nrg704. [DOI] [PubMed] [Google Scholar]
- 31.Grunwald DJ, Streisinger G. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res. 1992;59:103–116. doi: 10.1017/s0016672300030317. [DOI] [PubMed] [Google Scholar]
- 32.Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13:2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara M, Naruse K, Sasaki S, Nakatani Y, Qu W, Ahsan B, Yamada T, Nagayasu Y, Doi K, Kasai Y, Jindo T, Kobayashi D, Shimada A, Toyoda A, Kuroki Y, Fujiyama A, Sasaki T, Shimizu A, Asakawa S, Shimizu N, Hashimoto S, Yang J, Lee Y, Matsushima K, Sugano S, Sakaizumi M, Narita T, Ohishi K, Haga S, Ohta F, Nomoto H, Nogata K, Morishita T, Endo T, Shin-I T, Takeda H, Morishita S, Kohara Y. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714–719. doi: 10.1038/nature05846. [DOI] [PubMed] [Google Scholar]
- 35.Tomita H. Mutant genes in the medaka-Medaka (Killifish) Biology and Strains. Tokyo: Keigaku Publishing Company; 1975. [Google Scholar]
- 36.Shima A, Shimada A, Development of a possible nonmammalian test system for radiation-induced germ-cell mutagenesis using a fish. the Japanese medaka (Oryzias latipes) Proc Natl Acad Sci U S A. 1991;88:2545–2549. doi: 10.1073/pnas.88.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loosli F, Koster RW, Carl M, Kuhnlein R, Henrich T, Mucke M, Krone A, Wittbrodt J. A genetic screen for mutations affecting embryonic development in medaka fish (Oryzias latipes) Mech Dev. 2000;97:133–139. doi: 10.1016/s0925-4773(00)00406-8. [DOI] [PubMed] [Google Scholar]
- 38.Wakamatsu Y, Pristyazhnyuk S, Kinoshita M, Tanaka M, Ozato K. The see-through medaka: a fish model that is transparent throughout life. Proc Natl Acad Sci U S A. 2001;98:10046–10050. doi: 10.1073/pnas.181204298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mochizuki E, Fukuta K, Tada T, Harada T, Watanabe N, Matsuo S, Hashimoto H, Ozato K, Wakamatsu Y. Fish mesonephric model of polycystic kidney disease in medaka (Oryzias latipes) pc mutant. Kidney Int. 2005;68:23–34. doi: 10.1111/j.1523-1755.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 40.Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- 41.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet. 2006;7:283–293. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- 43.Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 45.Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389:963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development. 1999;126:5495–5504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Essner JJ, Vogan KJ, Wagner MK, Tabin CJ, Yost HJ, Brueckner M. Conserved function for embryonic nodal cilia. Nature. 2002;418:37–38. doi: 10.1038/418037a. [DOI] [PubMed] [Google Scholar]
- 48.Von Kupffer C. Untersuchungen fiber die Entwicklung des Harn-und Geschlechtssystems. Arch, mikr Anat. 1866;2:473–389. [Google Scholar]
- 49.Von Kupffer C. Beobachtungen fiber die Entwicklung der Knochenfische. Arch. f. Mikr. Anat. 1868;4:209–272. [Google Scholar]
- 50.Cooper MS, D’Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184–198. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]
- 51.Melby AE, Warga RM, Kimmel CB. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development. 1996;122:2225–2237. doi: 10.1242/dev.122.7.2225. [DOI] [PubMed] [Google Scholar]
- 52.Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- 53.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 54.Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- 55.Kikuchi Y, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu X, Huang J, Dong Y, Choi J, Langenbacher A, Chen JN. Na,K-ATPase alpha2 and Ncx4a regulate zebrafish left-right patterning. Development. 2007;134:1921–1930. doi: 10.1242/dev.02851. [DOI] [PubMed] [Google Scholar]
- 57.Shook DR, Majer C, Keller R. Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev Biol. 2002;248:220–239. doi: 10.1006/dbio.2002.0718. [DOI] [PubMed] [Google Scholar]
- 58.Shook DR, Majer C, Keller R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol. 2004;270:163–185. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation. 2007;75:133–146. doi: 10.1111/j.1432-0436.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 60.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 61.Morgan D, Goodship J, Essner JJ, Vogan KJ, Turnpenny L, Yost HJ, Tabin CJ, Strachan T. The left-right determinant inversin has highly conserved ankyrin repeat and IQ domains and interacts with calmodulin. Hum Genet. 2002;110:377–384. doi: 10.1007/s00439-002-0696-4. [DOI] [PubMed] [Google Scholar]
- 62.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 63.The International Polycystic Kidney Disease Consortium: Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 64.Burn TC, Connors TD, Dackowski WR, Petry LR, Van Raay TJ, Millholland JM, Venet M, Miller G, Hakim RM, Landes GM, Klinger KW, Qian F, Onuchic LF, Watnick T, Germino GG, Doggett NA. Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. The American PKD1 Consortium (APKD1 Consortium) Hum Mol Genet. 1995;4:575–582. doi: 10.1093/hmg/4.4.575. [DOI] [PubMed] [Google Scholar]
- 65.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2 a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 66.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 67.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 68.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 69.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 70.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA, Organization of the pronephric filtration apparatus in zebrafish requires Nephrin. Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carroll T, Wallingford J, Seufert D, Vize PD. Molecular regulation of pronephric development. Curr Top Dev Biol. 1999;44:67–100. doi: 10.1016/s0070-2153(08)60467-6. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 73.Ma M, Jiang YJ. Jagged2a–notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007;3:133–145. doi: 10.1371/journal.pgen.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drummond I. Making a zebrafish kidney: a tale of two tubes. Trends Cell Biol. 2003;13:357–365. doi: 10.1016/s0962-8924(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 75.Hostetter CL, Sullivan-Brown JL, Burdine RD. Zebrafish pronephros: a model for understanding cystic kidney disease. Dev Dyn. 2003;228:514–522. doi: 10.1002/dvdy.10371. [DOI] [PubMed] [Google Scholar]
- 76.Hentschel DM, Bonventre JV. Novel non-rodent models of kidney disease. Curr Mol Med. 2005;5:537–546. doi: 10.2174/1566524054553469. [DOI] [PubMed] [Google Scholar]
- 77.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 78.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nusslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- 79.Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- 80.Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, Lin SY, Nissen RM, Hopkins N. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–140. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- 81.Sun Z, Hopkins N. vhnfl, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001;15:3217–3229. doi: 10.1101/gad946701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malicki J, Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL, Stainier DY, Abdelilah S, Zwartkruis F, Rangini Z, Driever W. Mutations affecting development of the zebrafish retina. Development. 1996;123:263–273. doi: 10.1242/dev.123.1.263. [DOI] [PubMed] [Google Scholar]
- 83.Mache CJ, Preisegger KH, Kopp S, Ratschek M, Ring E. De novo HNF-1 beta gene mutation in familial hypoplastic glomerulocystic kidney disease. Pediatr Nephrol. 2002;17:1021–1026. doi: 10.1007/s00467-002-0975-2. [DOI] [PubMed] [Google Scholar]
- 84.Kolatsi-Joannou M, Bingham C, Ellard S, Bulman MP, Allen LI, Hattersley AT, Woolf AS. Hepatocyte nuclear factor-1 beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12:2175–2180. doi: 10.1681/ASN.V12102175. [DOI] [PubMed] [Google Scholar]
- 85.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 86.Zimmermann HD. Cilia in the fetal kidney of man. Beitr Pathol. 1971;143:227–240. [PubMed] [Google Scholar]
- 87.Duffy JL, Suzuki Y. Ciliated human renal proximal tubular cells. Observations in three cases of hypercalcemia. Am J Pathol. 1968;53:609–616. [PMC free article] [PubMed] [Google Scholar]
- 88.Hassan MO, Subramanyan S. Ciliated renal tubular cells in crescentic glomerulonephritis. Ultrastruct Pathol. 1995;19:201–203. doi: 10.3109/01913129509064222. [DOI] [PubMed] [Google Scholar]
- 89.Katz SM, Morgan JJ. Cilia in the human kidney. Ultrastruct Pathol. 1984;6:285–294. doi: 10.3109/01913128409018587. [DOI] [PubMed] [Google Scholar]
- 90.Mobjerg N, H.Larsen E, Jespersen A. Morphology of the kidney in larvae of Bufo viridis (Amphibia, Anura, Bufonidae) J Morphol. 2000;245:177–195. doi: 10.1002/1097-4687(200009)245:3<177::AID-JMOR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 91.Vize PD, Carroll TJ, Wallingford JB. Induction, Development, and Physiology of the Pronephric Tubules-The Kidney: From Normal Development to Congenital Disease. Amsterdam: Academic Press; 2003. [Google Scholar]
- 92.Tran U, Pickney LM, Ozpolat BD, Wessely O. Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros. Dev Biol. 2007;307:152–164. doi: 10.1016/j.ydbio.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506–512. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- 95.McCright B. Notch signaling in kidney development. Curr Opin Nephrol Hypertens. 2003;12:5–10. doi: 10.1097/00041552-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 96.McLaughlin KA, Rones MS, Mercola M. Notch regulates cell fate in the developing pronephros. Dev Biol. 2000;227:567–580. doi: 10.1006/dbio.2000.9913. [DOI] [PubMed] [Google Scholar]
- 97.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 98.Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- 99.Otto EA, Schemer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2005;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 102.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt FF. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 103.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 104.Schrick JJ, Onuchic LF, Reeders ST, Korenberg J, Chen XN, Moyer JH, Wilkinson JE, Woychik RP. Characterization of the human homologue of the mouse Tg737 candidate polycystic kidney disease gene. Hum Mol Genet. 1995;4:559–567. doi: 10.1093/hmg/4.4.559. [DOI] [PubMed] [Google Scholar]
- 105.Beales PL. Lifting the lid on Pandora's box: the Bardet-Biedl syndrome. Curr Opin Genet Dev. 2005;15:315–323. doi: 10.1016/j.gde.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006;15:667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 107.Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, Jalk N, Vicaire S, Sarda P, Hamel C, Lacombe D, Holder M, Odent S, Holder S, Brooks AS, Elcioglu NH, Silva ED, Rossillion B, Sigaudy S, de Ravel TJ, Lewis RA, Leheup B, Verloes A, Amati-Bonneau P, Megarbane A, Poch O, Bonneau D, Beales PL, Mandel JL, Katsanis N, Dollfus H. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 108.Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, de Ravel TJ, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chappie JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 110.Guay-Woodford LM. Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol. 2006;21:1369–1376. doi: 10.1007/s00467-006-0164-9. [DOI] [PubMed] [Google Scholar]
- 111.Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;279:55455–55464. doi: 10.1074/jbc.M402905200. [DOI] [PubMed] [Google Scholar]
- 112.Hooper KM, Boletta A, Germino GG, Hu Q, Ziegelstein RC, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Renal Physiol. 2005;289:F521–F530. doi: 10.1152/ajprenal.00355.2004. [DOI] [PubMed] [Google Scholar]
- 113.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow- induced Ca2+ signaling. Am J Physiol Renal Physiol. 2006;292:F930–F945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 115.Kim E, Arnould T, Sellin L, Benzing T, Cornelia N, Kocher O, Tsiokas L, Sukhatme VP, Walz G. Interaction between RGS7 and polycystin. Proc Natl Acad Sci U S A. 1999;96:6371–6376. doi: 10.1073/pnas.96.11.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnould T, Kim E, Tsiokas L, Jochimsen F, Gruning W, Chang JD, Walz G. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem. 1998;273:6013–6018. doi: 10.1074/jbc.273.11.6013. [DOI] [PubMed] [Google Scholar]
- 117.Arnould T, Sellin L, Benzing T, Tsiokas L, Cohen HT, Kim E, Walz G. Cellular activation triggered by the autosomal dominant polycystic kidney disease gene product PKD2. Mol Cell Biol. 1999;19:3423–3434. doi: 10.1128/mcb.19.5.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Le NH, van der Wal A, van der Bent P, Lantinga-van Leeuwen IS, Breuning MH, van Dam H, de Heer E, Peters DJ. Increased activity of activator protein-1 transcription factor components ATF2, c-Jun, and c-Fos in human and mouse autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:2724–2731. doi: 10.1681/ASN.2004110913. [DOI] [PubMed] [Google Scholar]
- 119.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(wafl) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 120.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 121.Streets J, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet. 2006;15:1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol. 2006;17:2706–2718. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]