Abstract
Developmental lead (Pb) exposure impairs various cognitive processes and behaviors in both humans and animals. In particular, specific deficits in spatial learning and memory have been described in Pb-exposed rats. It is also known that rearing environment (i.e., non-enriched vs. enriched) can have significant influences on cognitive performance and that rearing environment and sex may modify the influence of Pb exposure on learning and memory processes. It is also known that behavioral testing can alter hippocampal gene expression and interactive effects of environment. Little is known however about the molecular correlates of developmental Pb-exposure on expression of key sets of cognition-relevant genes in the hippocampus and how sex and environmental rearing condition may modify these effects. The present study examined expression profiles of neurobiologically-relevant genes (i.e., neurotrophic factors, NMDA receptors, metabotropic glutamate receptors, synaptic function/plasticity, and transcription/gene regulation ) in behaviorally naïve rats with perinatal exposure (i.e., gestation through weaning) to different levels of Pb (250, 750 and 1500ppm Pb acetate) in males and females raised in a non-enriched environment (standard housing without toys) or an enriched environment (large cage containing toys changed twice weekly). Unlike previous studies identifying gene changes following behavioral testing, which alters expression analysis, we identified both sex and environmental related changes in hippocampal genes following Pb exposure alone. The gene expression changes described may be associated with learning and memory and may pre-determine how cognitive profiles develop following Pb exposure.
Keywords: Lead, Sex, Environment, Gene Expression, Hippocampus
Introduction
The developing brain is a particularly vulnerable target for lead (Pb) with developmental Pb exposure resulting in cognitive deficits (for example, in attention, memory, learning, executive functioning) as well as behavioral and emotional deficits that persist into adulthood (Cory-Slechta, 1995; Kuhlmann et al., 1997; Mushak et al., 1989; Winneke et al., 1996). Although the effects of Pb on the developing brain have been studied for decades, there are still gaps in our understanding of how this ubiquitous environmental toxicant influences brain development and function. In particular, the ways in which Pb interacts with environmental factors and sex to influence nervous system development and function are areas in which our knowledge is scant.
In children, socioeconomic status (SES) has attracted some attention as a potentially important influence on vulnerability and response to childhood Pb poisoning. Exposure to neurotoxicants, including Pb, is often greater among low SES children (Baghurst et al., 1999; Brody et al., 1994). Although a child’s SES may be a risk factor that affects the likelihood of exposure to Pb, it may also significantly influence the outcome from Pb exposure (Bellinger, 2008; Cory-Slechta et al., 2008). There is the possibility that the social/behavioral concomitants of low SES, many of which are associated with high levels of stress, may enhance the neurotoxicity of Pb (Rutter, 1983). Rutter [1983] hypothesized that economically disadvantaged children, because of a neuropsychological status rendered fragile by environmental influences, might be more vulnerable to the neurotoxic effects of Pb. In support of this hypothesis, Winneke and Kraemer (Winneke and Kramer, 1997) reported that SES interacted with the effects of Pb on visual-motor integration and reaction time, with performance deficits greater in low SES Pb-exposed children compared to their more economically fortunate counterparts.
Thus, different environmental milieus in early life may have powerful effects on the response of the brain to a toxic insult. In animals, being raised in a non-enriched environment may exacerbate the neurotoxicity of Pb whereas being raised in an enriched environment could have potential mitigating effects. Previous results from our laboratory (Schneider et al., 2001) and others (Guilarte et al., 2003) suggest this may be the case. However, both the amount of Pb exposure and sex may potentially influence the outcome of such studies and neither factor was examined in the prior work mentioned above. Additionally, previous studies included very limited examinations of effects on gene expression in the brain (specifically the hippocampus) and examined these changes in animals that had been involved in behavioral protocols. This may introduce potential interpretation problems as exposure to behavioral tests may in and of itself influence gene expression in the hippocampus (Falkenberg, 1992). Therefore, while numerous studies have attempted to model the cognitive effects of developmental Pb exposure, with specific focus on hippocampal-associated learning and memory, the interpretation of gene expression outcomes in such studies has been limited by the experimental design. Thus, the current study was performed to further examine the influences of Pb exposure, sex and rearing environment on expression profiles of a number of neurobiologically relevant genes (i.e., genes for neurotrophic factors, NMDA receptors, metabotropic glutamate receptors, synaptic function/plasticity genes, and genes related to transcription regulation) in the hippocampus of behaviorally naïve rats exposed to Pb during gestation and lactation. The hippocampus was chosen for study since it is a brain region known to be sensitive to the effects of developmental Pb exposure and one that has been well studied for several decades in relation to effects of Pb on its structure and function.
2. Materials and Methods
2.1 Animals and treatments
Long Evans dams (Harlan Laboratories) were fed Pb-containing food (RMH 1000 chow with or without added Pb acetate: 0 ppm, 250 ppm, 750ppm or 1500 ppm) for ten days prior to breeding and remained on the same diet through weaning. Litters were culled to equal numbers of pups to standardize litter size, with an aim of having eight pups per litter. Equal numbers of males and females were maintained wherever possible and were exposed to Pb from gestation through lactation (i.e., to postnatal day 21). At weaning one male and one female were taken from each litter and combined with animals from other litters to form environment cohorts, with each litter an experimental n of 1, per sex. Each experimental group consisted of 6 animals, requiring 48 males and 48 females for the entire study. Rats were either housed 3 to a cage (47.6 × 25.9 cm) with no added stimuli (non-enriched) or 6 to a cage (61 × 43.5 cm) containing a variety of toys, climbing and nesting materials, and tunnels that were changed twice per week (enriched group). All cages contained a 50:50 mix of Alpha-Dri:Beta-Cob bedding material (Shepherd Specialty Papers), to a standard depth of 1cm. Other than the differences already described, animals were handled in exactly the same manner and were exposed to a 12h:12h light:dark cycle for the duration of the study. All animals were euthanized at day 55. Animals from different groups were euthanized at the same time of day by decapitation and hippocampi were rapidly removed, flash frozen on dry ice and stored at −80 °C until processed. The use of animals was in compliance with NIH Guidelines for the Care and Use of Laboratory Animals and the study was approved by the institutional animal care and use committee at Thomas Jefferson University.
2.2. Blood and Brain Lead Measurements
Blood was collected at the time of euthanasia and analyzed for Pb levels by either graphite furnace atomic absorption with Zeeman background correction (ESA Labs, MA) for blood Pb level. Additionally, blood samples were obtained from dams prior to Pb exposure and at parturition and weaning. Blood Pb levels were also obtained from different groups of pups at day 21 (weaning).
2.3 Tissue handling, RNA manipulation, and quantitative RT-PCR
Approximately 60μg of total RNA was extracted from each hippocampal sample for subsequent qPCR analysis. Total RNA was isolated using Qiagen miRNeasy kits (which extracts both total and miRNA) according to the manufacturers suggested protocol. Samples were homogenized in RNase/DNase/Protease free micro-pestle and mortars (Kimble-Kontes Inc.) and mRNA was extracted using an automated Qiagen QiaCube, followed by QA/QC on a GE Nanovue spectrophotometer, followed by RNA integrity number (RIN) analysis on an Agilent Bioanalyzer. Samples were only processed further if they had a 260/280 reading of 1.9-2.1 on the spectrophotmoter and a RIN of greater than 8.5. mRNA was reverse transcribed using the Omniscript RT kit according to the manufacturer’s instructions. Real-Time PCR was then performed using a LightCycler 480 (Roche Diagnostics, Ltd) with gene specific optimized primers (Idaho Biotech, UT and SAB Biosciences) (Table 1) and LC480 SYBR Green I Master Mix (Roche Diagnostics, Ltd). A typical reaction took approximately 50 minutes to complete and included a 5 minute denaturation step at 95°C, followed by 35 cycles of 95°C for 5s (melting), 55°C for 5s (annealing) and 72°C for 5s (extension). To confirm specificity of amplification, the products were subjected to a melting curve analysis at the end of the final extension period. Standard curve amplification was performed using known amplicon dilutions ranging from 10−6–10−1 attomoles. The standard curve for each primer set was stored as a reference curve for use in future experiments and compared to standards in each run and against the unknown samples.
Table 1.
Primer Sequences and annealing temperatures for all primers.
| Primer Name | Forward Primer Sequence | Reverse Primer Sequence | Annealing Temp (°C) |
|---|---|---|---|
| bFGF | CGTCAAACTACAGCTCCA | CAGTGCCACATACCAAC | 55 |
| NT3 | GCCATTGACATTCGGG | GCTCGGACGTAGTTT | 55 |
| NGF | AGCCACGGACATCAAG | CGTCTGTTGTCAACGC | 55 |
| BDNF | GCGTGTGTGACAGTATTAG | GGCATTGCGAGTTCCA | 55 |
| NR1 | ACACAATTACGAGAGCG | CCGAACCCATGTCTTATC | 55 |
| NR2A | GGATTAACCGACAGCAC | CCCGATAACTAAGCGTTG | 55 |
| NR2B | AAGCCCGACTAATTCCA | AAGGGTTTTGCGTGAC | 55 |
| NR3A | CTACATACACAGCGAACT | TGGCACGTTGTACCTT | 55 |
| NR3B | GGGAAACCGAACAACAC | CCATACCTTGAAATGCCG | 57 |
| mGluR3 | TCCTACGACAGCGTGA | AACCAGGGGTTACGAT | 55 |
| mGluR4 | TTTGTGCGCTACAACG | CGGTAAATGCGGTTGG | 57 |
| mGluR5 | CAGTGAACCGTGTGAGA | GGAGTGTCCCGATAAATG | 55 |
| CaMKIIα | ACCCGATTCACGGAAG | GGAGTCGGACGATATTGG | 55 |
| CaMKIIβ | TTTGGATTTGCGGGAAC | GTAACGGTGTCCCACT | 55 |
| RIM1 | CAACCACCACTGAACA | AGATTCCACACGTCGG | 55 |
| Spinophilin | AGGTACGCAAGATCAAAC | CCTCTCTAGTGTCGCC | 55 |
| MeCP2 | GGGCAAACATGAACCAC | CGCGTCCAACCTTCAG | 55 |
| Zfp111 | CACGATGTGATGCTGG | AGTAGTGCTGGAATGGT | 55 |
2.3 Data Analysis
Analysis of variance (ANOVA) was used to model differences in gene expression levels with respect to dose groups (Control, 250 ppm, 750 ppm and 1500ppm). Separate models were fit for each gene. Differences in dose groups were considered significant if the adjusted p-value was <0.05. Separately, p-values from all possible pairwise comparisons of dose groups (regardless of the significance of the overall F-test from the ANOVA) are reported only for genes with significant group differences with differences considered significant when the adjusted p-value was <0.05 using a Bonferroni multiple comparison test.
3.0 Results
3.1 Animal Characteristics
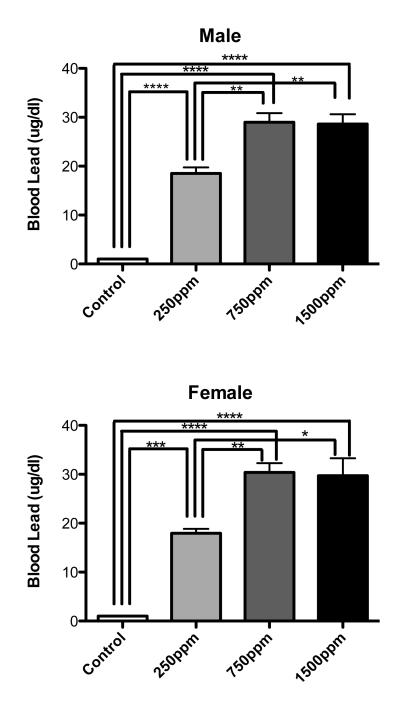
There were no significant differences in body weight between Pb-exposed animals and controls within a sex and across environments at the end of the study (Table 2). The mean blood Pb levels for control animals was <1.0 μg/dl. Mean blood Pb levels for all Pb-exposed groups at the conclusion of the study were not significantly different from control (i.e., all animals had blood Pb levels at or below detectable limits i.e., <1.0 μg/dl). Blood Pb levels of littermates at the end of the Pb exposure period showed concentration dependent lead levels up to 750ppm then increased no further with 1500ppm exposure (Figure 1). Blood Pb levels were not significantly different between males and females at any dose of Pb (Figure 1).
Table 2.
Body weights at study conclusion.
| Body Weight (g) |
||||
|---|---|---|---|---|
| 0 ppm | 250 ppm | 750 ppm | 1500 ppm | |
| Male Impoverished | 254.5 ± 5.6 | 251.8 ± 8.2 | 239.5 ± 7.4 | 235.0 ± 5.7 |
| Male Enriched | 189.0 ± 4.7 | 191.5 ± 6.9 | 190.5 ± 4.6 | 187.8 ± 3.6 |
| Female Impoverished | 187.2 ± 3.0 | 187.3 ± 7.4 | 193.5 ± 6.4 | 182.2 ± 5.3 |
| Female Enriched | 189.0 ± 4.7 | 191.5 ± 6.9 | 190.5 ± 4.5 | 187.8 ± 3.6 |
There were no significant differences in body weights within gender regardless of lead exposure levels or rearing environmental.
Figure 1.
Blood lead levels (BLL) in animals on the last day of lead exposure. Both male and female postnatal day 21 animals showed significantly elevated BLL when compared to age matched controls not exposed to lead. There were no significant differences between males and females in BLL at any level of lead exposure. Interestingly, BLL’s did not increase in 1500ppm exposed animals when compared to 750ppm exposed animals. Data are mean blood lead level ± S.E.M. All statistically significant differences between exposures are designated on the graphs using connecting lines. * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001
3.2 Gene Expression
The following analyses were performed: (1) Within Sex: all Pb exposure levels with each sex/environment combination, i.e., Female Enriched Control vs. Female Enriched 250ppm or, Male Non-enriched 250ppm vs. Male Non-enriched 750ppm); (2) Between Sex, each Pb exposure/environment combination (i.e., Enriched 250ppm Female vs. Enriched 250ppm Male); and (3) Between Environments, each Pb exposure/sex combination (i.e., Female Enriched 250ppm vs. Female Non-enriched 250ppm).
3.3 Neurotrophic Factor Gene Expression
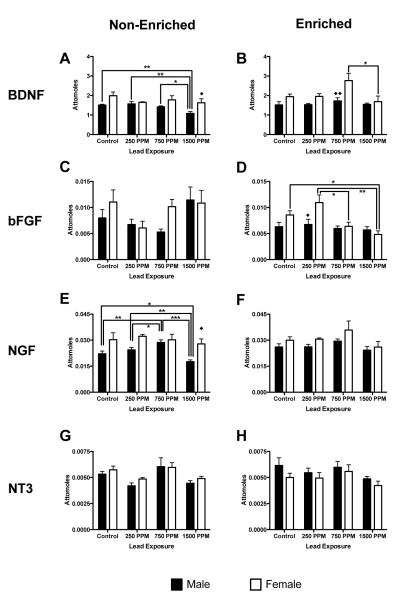
Gene expression for brain-derived neurotrophic factor (Bdnf), basic fibroblast growth factor (Fgf2), nerve growth factor (Ngf) and neurotrophin-3 (Nt-3) were examined due to their presence in and importance to the function of the hippocampus. Bdnf mRNA: In males, Bdnf mRNA expression was unchanged by Pb exposure in non-enriched animals in all but 1500ppm exposed group, which had significantly reduced BDNF mRNA expression, compared to control, 250ppm and 750ppm exposure groups (p<0.01, p<0.01 and p<0.05 respectively; Figure 2A). There was no effect of Pb exposure on Bdnf mRNA expression in any enriched males. There was an overall significant effect of environment on Bdnf mRNA expression in males (F(1,40) = 6.06, p = 0.0182):1500ppm-exposed non-enriched males had lower Bdnf mRNA expression than enriched males (p<0.05, Figures 2A and 2B), with no significant differences seen at any other exposure level across environments. An overall significant effect of both Pb exposure and environment on Bdnf mRNA expression was observed in females (F(3,40) = 2.91, p = 0.0463 and F(1,40) = 4.47, p = 0.0407, respectively), with an increase in expression in 750ppm-exposed enriched animals compared to enriched 1500ppm females (p<0.05; Figure 2B). Comparison of Bdnf mRNA expression across sexes by environment and Pb exposure level was significant at two dose/environment combinations; enriched 750ppm females expressed Bdnf mRNA at higher levels than males (p<0.01, Figure 2B), while non-enriched 1500ppm females expressed Bdnf mRNA at a higher level than non-enriched male 1500ppm-exposed animals (p=0.05, Figure 2A).
Figure 2.
Effects of perinatal lead-exposure on neurotrophic factor gene expression profiles in the hippocampus of male and female rats housed in either a non-enriched or enriched environment. Quantitative PCR analysis of mRNA expression levels of brain derived neurotrophic factor (Bdnf; A, B), basic fibroblast growth factor (Fgf2; C, D), nerve growth factor (Ngf; E, F) and neurotrophic factor-3 (Nt-3; G, H) in the hippocampus of control and lead-exposed animals. Sex, environment and dose specific patterns of response to lead were observed for Bdnf, Ffg2 and Ngf gene expression. Data are mean number of attomoles of mRNA ± S.E.M. * p < 0.05, ** p < 0.01, and *** p < 0.001 within sex analysis. ◆ p<0.05, and ◆◆ p < 0.01 across sex analysis
Fgf2 mRNA
Fgf2 mRNA expression was not significantly changed from control levels at any Pb exposure in either enriched or non-enriched males (Figures 2C and 2D). There were no overall significant differences in Fgf2 mRNA expression within males across environment, although 1500ppm non-enriched animals had a significantly higher expression level than 1500ppm enriched male animals (p<0.01, Figure 2C and 2D). In non-enriched females, there were no statistically significant changes in Fgf2 mRNA expression with any level of Pb exposure, compared to controls. In enriched females significantly lower Fgf2 mRNA expression was observed in 1500ppm Pb-exposed animals compared to controls (p<0.05, Figure 2D). There was also significantly greater expression of Fgf2 mRNA in enriched 250ppm Pb-exposed females compared to both enriched 750ppm and 1500ppm-exposed animals (p<0.05 and p<0.01 respectively, Figure 2D). However, non-enriched 250ppm Pb exposed animals were found to have decreased levels of mRNA expression (Figure 2C), while enriched 250ppm Pb-exposed animals had increased Fgf2 mRNA levels relative to controls. There were no significant differences in Fgf2 mRNA expression within females across environments, however, there was an effect of sex and Pb-exposure level (F(1,40) = 5.70, p=0.028 and F(3,40) = 6.16, p=0.015, respectively, Figures 2C and 2D), with differences between enriched males and females at the 250ppm exposure level (p<0.05).
Ngf mRNA
In non-enriched males, Ngf mRNA expression was significantly altered in 750ppm (increased) and 1500ppm (decreased) Pb-exposed animals, compared to controls (p<0.01 and p<0.05 respectively, Figure 2E). There were also significant differences between 250ppm and both 750ppm (increased) and 1500ppm (decreased) Pb exposures (p<0.05 and p<0.01 respectively, Figure 2E), as well as between 750ppm and 1500ppm Pb-exposed animals (p<0.001, Figure 2E). There was no effect of Pb exposure on Ngf mRNA expression in enriched males. An overall significant effect of environment upon expression of Ngf mRNA (F(1,40) = 8.81, p=0.005,) was observed, with expression levels in enriched 1500ppm Pb-exposed males higher than in non-enriched males (p<0.05). Expression of Ngf mRNA was not changed at any Pb exposure level in either enriched or non-enriched females. (Figures 2E and 2F). The only significant difference between the sexes was found in non-enriched animals at the highest exposure level, with1500ppm Pb-exposed females having a higher level of Ngf mRNA expression than comparable males (p<0.05, Figure 2E).
Nt-3 mRNA
There were no significant effects on Nt-3 gene expression at any Pb exposure, regardless of sex or environment (Figures 2G and 2H).
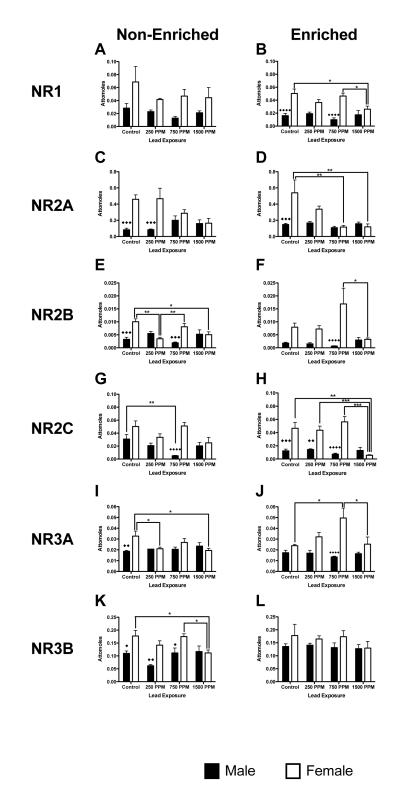
NMDA Receptor Subtype Gene Expression
Gene expression for NMDA receptor subtypes Nr1a, Nr2a, Nr2b, Nr2c, Nr3a and Nr3b was examined due to their potential sensitivity to Pb exposure and their functional role in the hippocampus. Nr1a mRNA: There were no significant changes in Nr1a mRNA expression within environment for non-enriched or enriched males at any level of Pb exposure (Figures 3A and 3B). In enriched females, Nr1a mRNA expression was significantly reduced in 1500ppm Pb-exposed animals when compared to both controls and 750ppm Pb–exposed females (p<0.05 for both comparisons, Figure 3A). There were no significant effects of Pb exposure on Nr1a mRNA expression in non-enriched females, although the trend was for decreased expression relative to controls (Figure 3B). In enriched animals there was a significant interaction between sex and lead exposure level (F(3,40) = 4.08, p=0.0128) with both control and 750ppm exposures having significantly lower expression levels in males than females (p<0.0001 for both comparisons). There was no overall effect of environment on Nr1a mRNA expression in either males or females.
Figure 3.
Effects of perinatal lead-exposure on NMDA receptor subtype gene expression profiles in the hippocampus of male and female rats housed in either a non-enriched or enriched environment. Quantitative PCR analysis of mRNA expression levels for five NMDA receptor subtypes: Nr1 (A, B), Nr2a (C, D), Nr2b (E, F), Nr3a (G, H) and Nr3b (I, J) in the hippocampus of control and lead-exposed animals. Significant sex, environment and dose specific effects were observed. Data are mean number of attomoles of mRNA ± S.E.M. * p<0.05, ** p < 0.01, and *** p<0.001 within sex analysis. ◆ p<0.05, ◆◆ p < 0.01, ◆◆◆ p<0.001 and ◆◆◆◆ p<0.0001 across sex analysis.
Nr2a mRNA
In males, there were no statistically significant effects of Pb exposure or environment on Nr2a mRNA expression levels. In females, there was an overall effect of Pb with increasing Pb levels decreasing Nr2a mRNA expression (F(3,40) = 8.46 p=0.0002). Nr2a mRNA levels were significantly affected by Pb exposure only in enriched animals in which decreased Nr2a mRNA expression was observed in 750ppm and 1500ppm Pb-exposed animals (p<0.01 for both comparisons, Figure 3D). Comparison of sex by environment and Pb exposure for Nr2a mRNA expression found significant differences in expression between males and females in control animals as well as in 250ppm Pb-exposed non-enriched animals (Figures 3C and 3D).
Nr2b mRNA
In males, there was an overall effect of both Pb exposure and environment on Nr2b mRNA expression (F(3,40) = 4.39, p<0.0092 and F(1,40) = p<0.0005 respectively), with lower expression in enriched animals compared to non enriched animals at all exposure levels with the exception of 1500ppm (Figure 3E and 3F). In females, there was an overall effect of Pb (F(3,40) = 4.95, p<0.0051). In non-enriched females, expression levels were lower in 250ppm and 1500ppm exposed animals compared to controls and 750ppm-exposed animals (p<0.01 and p<0.05 respectively, Figure 3E). In the enriched females, there was a significant difference in expression only between 1500ppm and 750ppm Pb-exposed animals (p<0.05, Figure 3F). Comparison of sex by environment and Pb exposure was significant in both non-enriched and enriched animals (F(1,40)=13.43,, p=0.0007 and F(1,40) =18.79, p<0.0001, respectively). Males had lower expression of Nr2b mRNA then females in the non-enriched condition (control and 750ppm Pb-exposure, p<0.001 and p<0.001 respectively, Figure 3E) and in enriched animals at 750ppm Pb-exposure (p<0.0001, Figure 3F).
Nr2c mRNA
There was an overall effect of both environment and Pb on Nr2c expression in males (F(1,40) =7.02, p=0.0115 and F(3,40) =5.48, p=0.0030, respectively). In non-enriched males, mRNA expression was significantly reduced in 750ppm-exposed animals compared to controls (p<0.01, Figure 3G). In females there was a significant effect of Pb exposure (F(3,40) = 11.71, p<0.0001), but no effect of environment. In non-enriched females there was no significant effect of Pb exposure, however, Nr2c mRNA expression levels in enriched females exposed to 1500ppm Pb were significantly decreased compared to control (p<0.01, Figure 3H) and the 250ppm and 750ppm exposure groups (p<0.001 for both comparisons, Figure 3H). Comparison of sex within environment and by Pb was significant for both sex and Pb-exposure in non-enriched animals (F(1,40) = 23.9, p<0.001 and F(3,40) = 3.17, p=0.0346 respectively) and enriched animals (F(1,40) = 49.39, p<0.0001 and F(3,40) = 7.87, p<0.0003 respectively), with females showing higher expression of Nr2c mRNA in both non-enriched and enriched environments at all but the highest Pb exposure level (Figures 3G and H).
Nr3a mRNA
In males, there was a significant effect of environment with all enriched animals having lower Nr3a mRNA expression than non-enriched animals (F(1,40) = 11.90, p=0.0013, Figures 3I and 3J). In females, there was both an effect of environment and Pb exposure on Nr3a mRNA expression (F(1,40) = 5.01, p=0.0308 and F(3,40) = 3.79, p=0.0175, Figures 3I and 3J). In non-enriched females, Nr3a expression was decreased, compared to controls, at the lowest and highest levels of Pb exposure (p<0.05 for both comparisons, Figure 3I). Conversely, in enriched females, Pb-exposure increased Nr3a mRNA expression in 750ppm Pb-exposed animals, compared to control and 1500 ppm exposures (p<0.05 for both comparisons, Figure 3J). In non-enriched condition, significant differences were seen between control males and females (p<0.01, Figure 3I), while in enriched animals, only 750ppm Pb-exposed males and females were significantly different from each other (p<0.00001, Figure 3J).
Nr3b mRNA
In males there was no statistically significant effect of Pb exposure under either environmental condition. There was however an overall significant difference within males across environment (F(1,40) = 10.66, p=0.0022; enriched 250ppm Pb-exposed males had significantly higher Nr3b mRNA expression than non-enriched 250ppm Pb-exposed males (p<0.01, Figures 3K and 3L). Females showed no overall effect of either environment or Pb exposure on Nr3b expression. In non-enriched females, exposure to 1500ppm Pb produced significant reductions in Nr3b mRNA expression compared to both control and 750ppm Pb-exposed animals (p<0.05 in both cases, Figure 3K). Comparison across sex by Pb exposure and environment showed male/female differences only in non-enriched animals at all but the highest level of Pb exposure (Figure 3K).
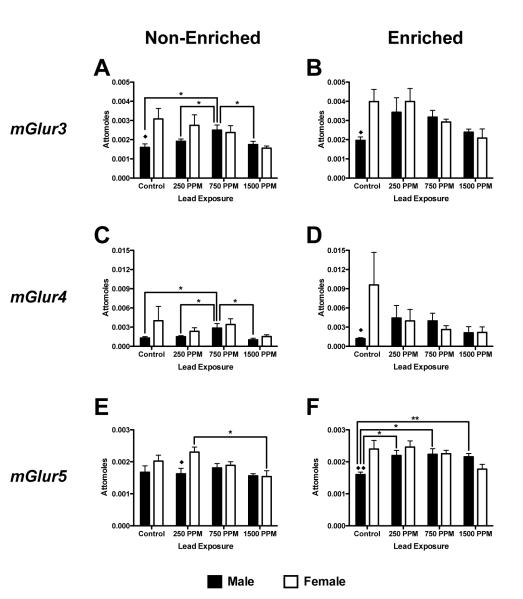
Metabotropic Glutamate Receptor (mGluR) Subtype Gene Expression
As mGluRs are associated with control of intracellular calcium release and are know to function in synaptic plasticity and development in the hippocampus (Corti et al., 2002), mGluR mRNA expression was assessed.
mGluR3 mRNA
In males there was both an effect of environment and Pb exposure on mGluR3 mRNA expression (F(1,40) = 11,41, p=0.0016 and F(3,40) = 4.42, p=0.0089). Non-enriched males exposed to 750ppm lead had significantly increased mGlur3 mRNA expression compared to controls and other Pb exposure groups (p<0.05 for all comparisons, Figure 4A). There were no significant differences between exposure groups in enriched males. Additionally, enriched 250ppm Pb-exposed males had significantly higher mGluR3 expression than non-enriched males (p<0.05, Figures 4A and 4B). Neither non-enriched nor enriched females showed a significant effect of Pb exposure on mGluR3 expression. Comparisons across sex showed a significant difference between the sexes only between control animals, with females having higher levels of expression in both non-enriched (p<0.05, Figure 4A) and enriched animals (p<0.05, Figure 4B).
Figure 4.
Effects of perinatal lead-exposure on metabotropic glutamate receptor subtype gene expression profiles in the hippocampus of male and female rats housed in either a non-enriched or enriched environment. Quantitative PCR analysis of mRNA expression levels for metabotropic glutamate receptors mGluR3 (A, B), mGluR4 (C, D) and mGluR5 (E, F) in the hippocampus of control and lead-exposed animals. Significant sex, environment and dose effects were observed. Data are mean number of attomoles of mRNA ± S.E.M. * p<0.05 and ** p<0.01 within sex analysis. ◆ p<0.05 and ◆◆ p < 0.01 across sex analysis.
mGlur4 mRNA
Non-enriched males exposed to 750ppm lead had significantly increased expression of mGluR4 mRNA compared to controls and other exposure groups (p<0.05 for all comparisons, Figure 4C) but there was no significant differences within males across environments or across Pb exposure levels. Neither non-enriched nor enriched females showed a significant effect on mGluR4 expression at any level of Pb exposure (Figures 4C and 4D). There was no significant difference within females across environments or across Pb exposure levels or between males and females.
mGluR5 mRNA
In males, there was no overall effect of Pb, but there was an overall effect of environment on mGluR5 mRNA expression (F(1,40) = 13.85, p<0.0006, Figures 4 E and 4F). In non-enriched males there was no significant effect of Pb-exposure on mGluR5 mRNA expression levels (Figure 4E) but enriched males showed increased mGlur5 expression at all exposure levels, compared to controls (p<0.05, p<0.05 and p<0.01 respectively, Figure 4F). In females, there were overall significant effects of both environment and Pb on mGluR5 expression (F(1,40) = 5.15, p=0.0287 and F(3,40) = 6.25, p=0.0014 respectively; Figures 4E and 4F). In non-enriched females, 250ppm Pb-exposed animals had higher mGlur5 expression compared to 1500ppm –exposed animals (p<0.01, Figure 4E). There were no significant effects of Pb exposure in the enriched females (Figure 4F). Sex-related differences in expression were observed in non-enriched 250ppm –exposed animals (p<0.05, Figure 4E) and in enriched controls (p<0.01, Figure 4F).
Genes Related to Synaptic Function/Plasticity
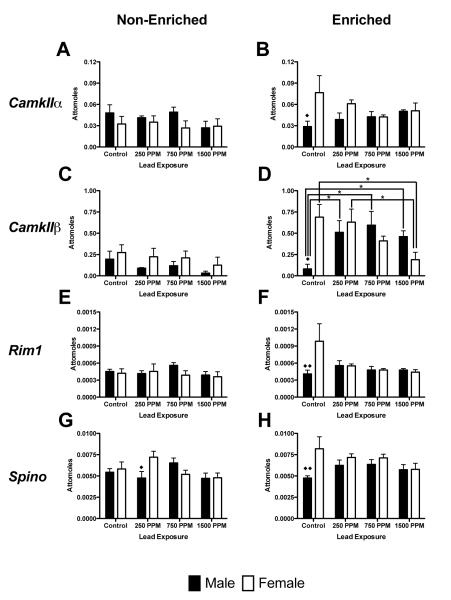
Several genes that play important roles in synaptic function and plasticity in the hippocampus were examined, including the α and β forms of the Ca2+/calmodulin-dependent protein kinases (CamKIIα and CamKIIβ), and genes involved in regulating synaptic membrane exocytosis (Rim1 and Spinophilin (Spino). In non-enriched animals, there was no significant effect of Pb exposure on any of the genes assessed. With the exception of CamKIIβ, there was no significant effect of Pb exposure on any of the genes assessed in enriched animals. Enriched males showed increased CamKIIβ expression at all Pb exposure levels (p<0.05 vs. control for all comparisons, Figure 5D). In enriched females, there was a significant decrease in CamKIIβ expression in 1500ppm Pb–exposed animals compared to control and 250ppm Pb– exposed animals. There was a significant effect of environment in males (F(1,40) = 22.72, p<0.0001), with the enriched animals in all Pb-exposure groups showing higher CamKIIβ expression compared to non-enriched animals (p<0.01 for all comparisons, Figures 5C and 5D). Similarly, there was a significant effect of environment in females (F(1,40) = 13.08, p<0.0008); enriched control and 250ppm Pb-exposured animals had higher expression of CamKIIβ compared to non-enriched animals (p<0.05 for all comparisons, Figures 5C and 5D). Comparisons across sexes showed significant differences between control animals in the enriched environment in CamKIIα, CamKIIβ, Rim1, and Spino expression, with females having higher levels of expression than males in each instance. In non-enriched animals, males and females differed only in Spino expression in the 250ppm exposure group (Figure 5G).
Figure 5.
Effects of perinatal lead-exposure on genes related to synaptic function and plasticity in the hippocampus of male and female rats housed in either a non-enriched or enriched environment. Quantitative PCR analysis of mRNA expression levels for Ca2+/calmodulin-dependent protein kinase II alpha (CamkIIα; A, B), Ca2+/calmodulin-dependent protein kinase II beta (CamkIIβ; C, D), regulating synaptic membrane exocytosis 1 (Rim1; G ,H) and spinophilin (Spino; I, J) in the hippocampus of control and lead-exposed animals. Significant effects were only seen in CamkIIβ expression. Data are mean number of attomoles of mRNA ± S.E.M. * p<0.05 within sex analysis. ◆ p<0.05 and ◆◆ p < 0.01 across sex analysis.
Genes Related to Transcription and Gene Regulation
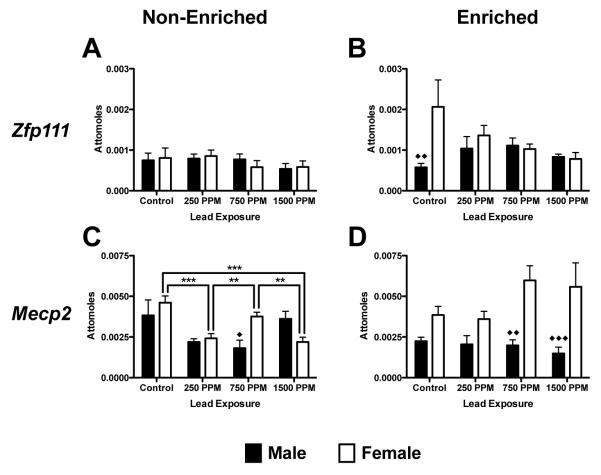
Zfp111 mRNA
Zfp111, a gene involved in negative regulation of transcription (Figure 6E and 6F) was not significantly changed in either males or females by Pb-exposure, environment or sex (Figure 6A and 6B).
Figure 6.
Effects of perinatal lead-exposure on mRNA expression of genes related to transcription and regulation in the hippocampus of male and female rats housed in either a non-enriched or enriched environment. Quantitative PCR analysis of mRNA expression levels for zinc finger protein 111 (Zfp111; A, B) and Methyl CpG binding protein 2 (Mecp2; C, D) and in the hippocampus of lead exposed animals. A number of structure and dose specific effects were observed. Data are mean number of attomoles of mRNA ± S.E.M. * p<0.05, ** p < 0.01, and *** p<0.001 within sex analysis. ◆ p<0.05, ◆◆ p < 0.01 and ◆◆◆ p<0.001 across sex analysis.
Mecp2 mRNA
There were no significant changes in Mecp2 mRNA expression associated with Pb exposure in enriched or non-enriched males. Overall there was an effect of environment on Mecp2 mRNA expression in males (F(1,40) = 6.75, p=0.0131), however, only at the 1500ppm level of exposure (p<0.05, Figures 6C and 6D), with non-enriched animals showing a higher expression level. In non-enriched females there was lower expression of Mecp2 mRNA expression in 250ppm and 1500 ppm Pb-exposed animals compared to controls (p<0.001) and to 750 ppm-exposed animals (p<0.01) (Figure 6C). No significant effects of Pb exposure were observed in enriched females (Figure 6D). There was an overall effect of environment in females (F(1,40) = 9.31, p=0.0040); enriched 1500ppm Pb-exposed females had higher expression of Mecp2 mRNA than non-enriched animals (p<0.01, Figures 6C and 6D). Comparison of influences of sex by environment and Pb-exposure showed male/female differences in Mecp2 mRNA expression in 750ppm and 1500ppm-exposed enriched animals and in Zfp111 expression in control animals.
4.0 Discussion
Recently, we reported complex interactions between sex, Pb exposure level and rearing environment on spatial learning and memory in rats (Anderson et al., 2012) and the current work attempted to characterize some of the potential molecular correlates of the behavioral outcomes previously described. Overall, significant differences were observed between the sexes in 11 of the 19 genes analyzed in control animals raised in an enriched environment, compared with only 5 genes significantly differently affected by rearing in the non-enriched environment. In all instances, gene expression levels were higher in females. In Pb-exposed males, we identified only 9 of 19 genes across all Pb exposure groups with expression modified by environment. In Pb-exposed females, we identified only 5 genes modified by environement. Significant differences were also observed across all Pb-exposure groups between the sexes for 7 of 19 genes in animals raised in the enriched environment, compared to 9 genes significantly affected by rearing in the non-enriched environment. Thus, it appears that for the current group of genes interrogated, Pb exposure does not result in a specific or characteristic pattern gene expression changes in the hippocampus that is associable with any single factor (i.e,, Pb exposure level or sex).
The results also suggested that there is minimal effect of environmental enrichment alone on gene expression within a given sex. This is surprising considering significant effects of environmental enrichment on hippocampal learning and memory (Anderson et al., 2012; Pereira et al., 2008; Renner and Rosenzweig, 1986). However, previous gene expression studies have shown that the hippocampus of environmentally enriched male rats differs in expression of only 58 genes (from approximately 3300 genes expressed in the hippocampus) when compared to non-enriched rats (Keyvani et al., 2004). Also, gene expression (Bdnf) in enriched or non-enriched male rats were not different in non-behaviorally tested animals, but was increased in all behaviorally tested animals (Falkenberg et al., 1992a). Thus, while rearing environment may have an effect on behavioral outcomes, it may not significantly alter basal levels of gene expression in the hippocampus. We did not measure the outcome of gene expression changes following environmental enrichment and behavioral testing, but we might expect there to be more significant changes seen in many of the genes selected in behaviorally tested animals.
In Pb-exposed animals, we found complex and not easily defined interactions between Pb-exposure, sex and rearing environment on gene expression in the hippocampus. Although Pb has been reported to affect expression of some neurotrophic factors (Guilarte et al., 2003; Schneider et al., 2001; Schneider et al., 2012), this has not previously been studied in both sexes, at multiple Pb-exposure levels and with animals from different rearing environments. In the present study, we observed significantly higher levels of hippocampal Bdnf mRNA expression in enriched 750ppm Pb-exposed females compared to similarly exposed, non-enriched females, suggesting a potential role for BDNF in the learning and memory benefits previously observed in enriched 750ppm-exposed females (Anderson et al., 2012). Learning and memory in enriched female rats exposed to 750ppm Pb was significantly better than similarly Pb-exposed females raised in a non-enriched environment (Anderson et al., 2012). Why these effects would preferentially occur in the 750ppm exposure group is not presently clear. Additionally, both behavioral and gene expression data suggest that environmental enrichment may partially counteract the effects of a high level Pb exposure (1500ppm) on both Bdnf and Ngf mRNA expression and learning in male rats.
Effects of Pb on the NMDAR complex are well known (Alkondon et al., 1990; Guilarte, 1997; Guilarte and McGlothan, 1998; Guilarte et al., 2003; Nihei and Guilarte, 1999), and have been associated with reduced long-term potentiation (LTP) leading to impaired learning and memory (Nihei et al., 2000). However, virtually all of these studies have examined only males, and only one study assessed any role of environmental enrichment on gene expression. The present analysis of NMDAR subunit mRNA expression in the hippocampus identified significant effects of sex and Pb-exposure level but rarely rearing environment. Although females generally showed greater sensitivity to higher Pb-exposure levels than did males, a profile of limited within sex effects of Pb exposure and little or no effects across environment was seen for all of the NMDARs. Previously, hippocampal Nr1 mRNA expression was reported to be decreased in isolation-reared, 1500ppm Pb-exposed males compared to isolation-reared controls but this was not observed in Pb-exposed males raised in an enriched environment (Guilarte et al., 2003). It is difficult to compare results from that work and the present study since the rearing conditions were significantly different and the prior study examined the brains of animals after behavioral testing, a situation that can dramatically alter gene expression profiles (Falkenberg et al., 1992b).
Expression of mGluR’s may play a role in memory formation, consolidation and recall but not learning (for review see(Riedel et al., 2003)). In the present study, females, in almost all conditions, showed a general decline in mGluR expression with increasing levels of Pb exposure regardless of environment. Fewer changes occurred in males and were seen mostly in enriched animals. It is possible that both Pb exposure and environment influence the processes of memory consolidation and recall through modulation of mGluR’s in the hippocampus and that females are more sensitive to these influences than males.
The genes examined that are associated with synaptic function/plasticity showed very few differences by sex or environment following Pb-exposure, with the exception of CamkIIβ. A decrease in expression of CamKIIβ and CamkIIα mRNA in the hippocampus of females is consistent with impaired learning and memory in these animals, while increased CamKIIβ mRNA, with a similar but non-significant decrease in CamkIIα in the hippocampus of enriched males would be consistent with non-impaired LTP, learning and memory (Silva et al., 1992a; Silva et al., 1992b; Silva et al., 1992c). In enriched Pb-exposed females but not in males, we previously showed that enrichment could not overcome the negative effects of Pb-exposure in non-enriched females, but did overcome the effects of Pb exposure in males (Anderson et al., 2012).
Expression of neither Rim1 nor Spino (Spinophilin) mRNA was found to be sensitive to either lead exposure or environmental enrichment in either of the sexes. This is in contrast to our previous finding that in animals exposed to post-weaning lead there was a significant increase in both Rim1 and Spino mRNA expression in the hippocampus following 1500ppm lead exposure, and a significant reduction in Spino mRNA expression in the hippocampus at low levels of lead exposure (180ppm and 375ppm) (Schneider et al., 2012). The differences between the current findings and those previously reported by us appear to be related to differences in the developmental timing of the Pb exposure (perinatal vs. postnatal).
We previously described increased mRNA expression of Methyl CpG Binding Protein 2 (MECP2) in the hippocampus of male rats following postnatal exposure to 1500ppm Pb (Schneider et al., 2012). Our current results show no significant changes in Mecp2 or Zfp111 mRNA expression in males at any level of Pb exposure in either rearing condition. In females Mecp2 mRNA expression in non-enriched 250 and 1500ppm Pb-exposed animals was reduced compared to control,while expression in enriched females were at (or above) control levels, suggesting that enrichment could potentially counteract detrimental effects of Pb-exposure on Mecp2 expression. The finding that Mecp2 mRNA expression is downregulated following low and high Pb-exposure in non-enriched but not enriched females suggests Pb exposure alone may cause a significant alteration in control of DNA methylation which could drive altered gene expression, possibly leading to perturbations of neuronal development and plasticity.
Several of the genes analyzed here have displayed sex differences in expression, i.e., NMDA receptor subunits were effected more in females than males, while metabatropic glutamate receptors were more effected in males than females following lead and/or environmental enrichment. These effects may in part be due to sex specific expression of neuroactive steroid hormones in the hippocampus modulating gene expression.
The present study has some limitations. Specifically, we have examined only mRNA data and have not analyzed protein expression. Although central dogma suggests a strong correlation between gene and protein expression, past empirical studies in fact suggest only a modest correlation (Nie et al., 2007; Tan et al., 2009). The relationship between genes and proteins can be complicated by post-transcriptional mechanisms affecting mRNA stability and protein degradation, as well as timing differences between gene and protein expression. Therefore, transcriptional, translational and post-translational mechanisms would need to be investigated in order to fully understand the biological impact of Pb and environment on the brain. Further complicating the correlation of mRNA and protein expression in neuronal tissues is that neuronal mRNAs are frequently expressed as proteins in brain regions distant to the site of transcription.. Finally, a potential limitation of the present work is that the entire hippocampus was homogenized for analysis limiting the potential to identify sub-regional differences in gene expression, especially if gene expression patterns are differentially modified by lead exposure in different sub-regions of these structures as has been reported by in situ hybridization for some of the genes analyzed (Guilarte and McGlothan, 2003; Guilarte et al., 2003).
The present study suggests that there are complex, and not easily characterized, interactions between sex, rearing environment and Pb exposure on hippocampal gene expression. In a number of instances, trends in gene expression changes were observed but these did not reach statistical significance and this may be related in part to the use of whole hippocampus for analysis as opposed to sub-regional analysis as some regions may be differentially susceptible to modification following Pb exposure (Guilarte et al., 2003). Further investigation along this line may provide additional information regarding potential interactions between sex, rearing environment, Pb exposure and behavioral outcomes.
Highlights.
Sex alone modifies hippocampal gene expression following lead exposure
Environmental enrichment modifies hippocampal gene expression after lead exposure
Lead exposure may pre-determine cognitive profiles through hippocampal gene expression
Acknowledgements
Funding: This work was supported by the National Institutes of Health grant ES015295.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Letters. 1990;261:124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Anderson DW, Pothakos K, Schneider JS. Sex and rearing condition modify the effects of perinatal lead exposure on learning and memory. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghurst PA, Tong S, Sawyer MG, Burns J, McMichael AJ. Sociodemographic and behavioural determinants of blood lead concentrations in children aged 11-13 years. The Port Pirie Cohort Study. The Medical journal of Australia. 1999;170:63–67. [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008;29:828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW, Paschal DC. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) JAMA. 1994;272:277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annual Review of Pharmacology and Toxicology. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neuroscience Letters. 1992a;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is assosciated with improved spatial memory and enriched environment. Neuroscience Letters. 1992b;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Pb2+ inhibits NMDA receptor function at high and low affinity sites: developmental and regional brain expression. Neurotoxicology. 1997;18:43–51. [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Research. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Research. Molecular Brain Research. 2003;113:37–43. doi: 10.1016/s0169-328x(03)00083-4. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Annals of Neurology. 2003;53:50–56. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- Keyvani K, Sachser N, Witte OW, Paulus W. Gene Expression Profiling in the Intact and Injured Brain Following Environmental Enrichment. Journal of Neuropathology & Experimental Neurology. 2004;63:598–609. doi: 10.1093/jnen/63.6.598. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, McGlothan JL, Guilarte TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neuroscience Letters. 1997;233:101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- Mushak P, Davis JM, Crocetti AF, Grant LD. Prenatal and postnatal effects of low-level lead exposure: integrated summary of a report to the U.S. Congress on childhood lead poisoning. Environmental Research. 1989;50:11–36. doi: 10.1016/s0013-9351(89)80046-5. [DOI] [PubMed] [Google Scholar]
- Nie L, Wu G, Culley DE, Scholten JC, Zhang W. Integrative analysis of transcriptomic and proteomic data: challenges, solutions and applications. Critical Reviews in Biotechnology. 2007;27:63–75. doi: 10.1080/07388550701334212. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99:233–242. doi: 10.1016/s0306-4522(00)00192-5. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Guilarte TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Mol Brain Res. 1999;66:42–49. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Strapasson ACP, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Research. 2008;1218:257–266. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. Social interactions among rats housed in grouped and enriched conditions. Developmental Psychobiology. 1986;19:303–313. doi: 10.1002/dev.420190403. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural Brain Research. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rutter M. Low level lead exposure: sources effects and implications. In: Rutter M, Russel-Jones R, editors. Lead versus health. John Wiley (UK); Chichester: 1983. pp. 333–370. [Google Scholar]
- Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Research. 2001;896:48–55. doi: 10.1016/s0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Mettil W, Anderson DW. Differential Effect of Postnatal Lead Exposure ib Gene Expression in the Hippocampus and Frontal Cortex. Journal of Molecular Neuroscience. 2012;47:76–88. doi: 10.1007/s12031-011-9686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Wang Y, Paylor R, Wehner JM, Stevens CF, Tonegawa S. Alpha calcium/calmodulin kinase II mutant mice: deficient long-term potentiation and impaired spatial learning. Cold Spring Harbor Symposia on Quantitative Biology. 1992c;57:527–539. doi: 10.1101/sqb.1992.057.01.058. [DOI] [PubMed] [Google Scholar]
- Tan CS, Salim A, Ploner A, Lehtio J, Chia KS, Pawitan Y. Correlating gene and protein expression data using Correlated Factor Analysis. BMC Bioinformatics. 2009;10:272. doi: 10.1186/1471-2105-10-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Kramer U. Neurobehavioral aspects of lead neurotoxicity in children. Central European Journal of Public Health. 1997;5:65–69. [PubMed] [Google Scholar]
- Winneke G, Lilienthal H, Kramer U. The neurobehavioural toxicology and teratology of lead. Archives of Toxicology. Supplement. Archiv fur Toxikologie. 1996;(Supplement 18):57–70. doi: 10.1007/978-3-642-61105-6_7. [DOI] [PubMed] [Google Scholar]