Abstract
High volume resuscitation and damage control surgical methods, while responsible for significantly decreasing morbidity and mortality from traumatic injuries, are associated with pathophysiological derangements that lead to subsequent end organ edema and dysfunction. Alterations in hydrostatic and oncotic pressures frequently result in intestinal edema and subsequent dysfunction. The purpose of this review is to examine the principles involved in the development of intestinal edema, current and historical models for the study of edema, effects of edema on intestinal function (particularly ileus), molecular mediators governing edema-induced dysfunction, potential role of mechanotransduction, and therapeutic effects of hypertonic saline. We review the current state of the science as it relates to resuscitation induced intestinal edema and resultant dysfunction.
Keywords: Resuscitation, Edema, Intestine, Review
I. Background
Survival from trauma has increased considerably over the last several decades secondary to multiple factors, including early and high volume resuscitation with crystalloid and blood products, advances in operative and non-operative management, and the widespread adoption and practice of damage control surgery, including abdominal packing. High volume resuscitation and abdominal packing alter hydrostatic and oncotic pressure differentials, contributing to the formation of intestinal edema. This may occur even in the absence of intra-abdominal injury. [1-7] One of the major focuses of our research is the pathophysiology associated with resuscitation-induced intestinal edema and associated interventions. In this review, we detail the pre-clinical work that has contributed to our understanding of the mechanisms leading to intestinal edema and subsequent dysfunction as well as the evidence and potential mechanisms governing possible therapeutic intervention.
II. Principles Governing Edema Development
Several key principles play a role in the development and propagation of intestinal edema including alterations in microvascular permeability, venous and lymphatic pressure and flow, and in serum protein concentration. Key to the mechanism behind edema formation is perturbed microvascular fluid exchange as determined by hydrostatic and colloid oncotic pressures described by the Starling equation (Equation 1). Alterations in microvascular and tissue hydrostatic and colloid oncotic pressures result in accumulation of fluid in the interstitium. Factors that govern these pressure variables are a central theme to the pathogenesis of intestinal edema.
Equation 1: Starling equation [8]
Jv – fluid filtration rate; Kf – filtration coefficient; Pc – microvascular (capillary) hydrostatic pressure; Pi – interstitial hydrostatic pressure; πc – microvascular colloid osmotic pressure; πi – interstitial colloid osmotic pressure; σ – reflection coefficient to protein
In the multiply injured trauma patient, high volume crystalloid resuscitation and abdominal packing serve to alter these variables (i.e., hydrostatic and osmotic pressures), leading to extravasation of fluid into tissues and subsequent tissue edema, including in the nervous system (brain), respiratory system (lung) and digestive system (gut). [9, 10] High volume crystalloid resuscitation decreases plasma oncotic pressures (πc) and abdominal (specifically peri-hepatic) packing, as a component of damage control resuscitation, increases mesenteric venous pressures leading to increased capillary hydrostatic pressures (Pc). Decreased plasma oncotic pressure and increased capillary pressure both favor movement of fluid from the vasculature into the interstitium.
Increases in capillary permeability (σ), secondary to shock/resuscitation (ischemia/reperfusion mediated injury), also contribute to net fluid flux into the interstitium. [8, 11, 12] As the lymphatics that drain the interstitium flow into the venous system, elevated central venous pressure during resuscitation impedes movement of fluid out of the interstitium. [13] The overall result of these alterations is the development of intestinal edema. [14]
With a transcapillary pressure gradient of 12 mmHg, seen with situations of hemodilution (i.e., high volume resuscitation), “filtration secretion” occurs. [15] With the accumulation in interstitial fluid (i.e., edema), there is a subsequent increase in the pressure of mucosal fluid. This leads to an efflux of protein (i.e., albumin) and fluid into the lumen via enlarged mucosa inter-epithelial channels. When studied, the composition of the fluid is similar to lymph; this indicates that the increased intraluminal fluid is likely escape of interstitial fluid. [16]This represents what is seen clinically, i.e., intestinal edema is accompanied by large, fluid filled loops of bowel.
III. Models of Intestinal Edema
A. Ischemia/Reperfusion
Much work has been done on ischemia/reperfusion and the subsequent effect on intestinal tissue water and resultant intestinal dysfunction. It is well accepted that ischemia/reperfusion can lead to the development of intestinal edema, and much of the published literature reflects the mechanisms and potential therapeutic interventions in ischemia/reperfusion injury. [3] This is especially true in cases of hemorrhagic and septic shock, which are components of the early and late course of a significant number of victims of traumatic injuries. [17] Ischemia/reperfusion injury causes edema by various mechanisms, including increased microvascular and mucosal permeability and necrosis, free radical formation and neutrophil activation and infiltration, and release of pro-inflammatory molecules and cytokines. [18, 19] Ischemia/reperfusion and inflammation increase capillary permeability to proteins (σ) and result in a net fluid flux out of the capillary into the interstitium. There are well described animal models examining the role of ischemia/reperfusion injury and effect on intestinal edema, many of which rely on occlusion of the superior mesenteric artery for a period of time (usually 30 minutes to one hour) followed by a variable period of reperfusion . [20, 21]
B. Hypoproteinemia/Hemodilution
Hypoproteinemia, frequently resulting from high volume crystalloid resuscitation has been shown, in pre-clinical and clinical studies, to contribute to the development of intestinal edema. Decreased colloid osmotic pressure associated with crystalloid infusion and decreased serum protein levels leads to increased intestinal edema in patients undergoing gastrointestinal surgery, secondary to an increased net flow of water into tissues. [22]
A large body of work regarding tissue edema was initially published in the 1940’s by Ravdin and colleagues. Among his many observations was the fact that reduction in oncotic pressure secondary to decreased protein levels (“nutritional edema”) is a key factor in the initiation/propagation of tissue edema; this is exacerbated by large volumes of crystalloid solution. The models utilized were predominantly large animal models (i.e., dog) and relied on induced decreases in plasma protein concentration, either through plasmapheresis, dietary restriction, or a combination of both. A correlation was made between hypoproteinemia and decreased gastrointestinal motility, manifested by delayed gastric emptying and small bowel transit. [23-26] These long term studies (i.e., over several weeks) provide much insight into the pathophysiology of edema formation, especially in relation to the role of serum protein concentration. Acute reductions in serum protein have been demonstrated to result in ileus. [27] Large volume crystalloid resuscitation frequently leads to hypoprotenemia, even in patients without pre-existing conditions with normal serum albumin levels. [28]
Recent animal studies have demonstrated an association between high volume resuscitation and post-surgical complications. High volume crystalloid resuscitation negatively impacts gastrointestinal anastomoses, both functionally and structurally as manifested by bursting pressure and tissue hydroxyproline content. [29] Multiple studies in surgical patients have demonstrated that decreased peri-operative administration of fluids leads to improved outcome, including decreased morbidity, hospital length of stay, wound complications, cardiopulmonary complications, and faster return to normal gastrointestinal function. [30-32] This may be secondary to reduced fluid-related tissue edema formation.
C. Hydrostatic Factors
One of the initial animal models of edema (nutritional edema) relied on manipulation of oncotic pressures by induced hypoproteinemia (plasmapheresis), as discussed by Ravdin and colleagues. In this model, delayed gastric and intestinal transit was noted. [24, 26] In development of our model, we focused on the manipulation of hydrostatic and oncotic pressure differentials. High volume resuscitation results in hemodilution and alterations in oncotic pressures. Venous hypertension, frequently a consequence of abdominal packing, leads to alterations in hydrostatic pressures. [14] We have developed an edema model that relies on alterations in hydrostatic and oncotic pressures. This has allowed us to study intestinal edema in a setting without the influence of significant ischemic or inflammatory factors.
Adult male Sprague Dawley rats undergo an upper midline laparotomy. Mesenteric venous hypertension is induced by tying a 4-0 silk suture over PE-10 sized tubing around the superior mesenteric vein. The tubing is then removed, creating a reproducible non-occlusive stenosis that causes a sustained elevation in mesenteric venous pressure. An external jugular venous catheter is utilized for administration of high volume crystalloids. [33]
This model leads to a sustained elevation in mesenteric venous pressure (20 ± 3 mmHg from a baseline of 11 ± 1 mmHg) and development of significant intestinal edema (as evidenced by increased wet to dry ratios). Intestinal edema is demonstrated (by wet to dry ratios) as early as 30 minutes following surgery and lasts for at least 12 hours after surgery. We also detect no evidence of ischemia (as evidenced by no difference in portal venous lactate), neutrophil recruitment (as evidenced by myeloperoxidase activity), or reperfusion induced mucosal injury (as evidenced by Chiu scoring) in this model. [33]
There are several advantages of this model. First, edema develops in a reproducible manner with respect to time of development and amount of edema. Second, the effects of edema can be studied without confounding elements due to ischemia/reperfusion or inflammatory related injury. This model has formed the basis for our work examining the mechanisms behind non-ischemic/inflammatory-related, resuscitation-induced intestinal edema-mediated dysfunction, which we review in detail.
IV. Edema as a Mediator of Intestinal Dysfunction
In addition to changes in permeability, a major functional consequence of edema is ileus, or delay in intestinal transit. Ileus frequently is a consequence of systemic illness, such as sepsis, and is well documented in the post-operative environment. [34] Fluid resuscitation has been shown to induce intestinal edema in a hemorrhage/resuscitation model. [35, 36] In an animal model, intestinal edema induced by high volume crystalloid resuscitation and mesenteric venous hypertension decreases intestinal transit, measured at 6 and 12 hours post surgery. [33] Edema-induced ileus is of significant clinical significance; it results in delayed initiation of enteral feeds, increased hospital morbidity, and increased hospital length of stay and healthcare costs.
Ileus is mediated by two major factors; mechanical dysfunction secondary to coupling of the muscle cell to surrounding tissue and decreases in intestinal contractility (dysfunction of the intestinal smooth muscle cell). Additionally, ileus may serve to contribute to edema formation. Recent work has begun to define the signal transduction pathways initiated by the onset of interstitial edema. Ultimately, transcription factors/gene products and mediators converge on myosin light chain20 (MLC) phosphorylation to regulate intestinal contractility and transit.
A. Ileus as a Contributor to Edema Formation
While edema functionally leads to ileus, some evidence also suggests that ileus may serve to contribute to, or propagate, edema formation. Lymphatic outflow serves to decrease intestinal edema by providing a conduit for the escape of accumulated interstitial fluid. [37, 38] Unthank and Bohlen demonstrated that lymphatic vessels within the bowel wall are valve-less. [39] This implies that flow of lymph from the intestine depends on peristalsis for forward flow. Lack of peristalsis (secondary to ileus) leading to lack of lymphatic outflow may serve to increase the severity of edema, creating a propagating cycle of dysfunction.
B. Intestinal Contractility
Decreased intestinal contractility is a well-documented consequence of intestinal injury, observed in models of ischemia/reperfusion, hemorrhagic shock, and colitis. [40-43] Signaling pathways governing smooth muscle contractility primarily converge on the MLC phosphorylation/de-phosphorylation apparatus. (Figure 1) The decrease in MLC phosphorylation and subsequent decrease in contractility may be mediated by upstream signaling events that converge on MLC mediated smooth muscle contractile apparatus. These include the stress signaling pathways signal transduction and activator of transcription (STAT)-3 and NF-kappa B, which may lead to increased nitric oxide (NO) production. [44][Uray, Critical Care Medicine, In Press] Increased concentrations of nitric oxide (NO) may at least partially explain the effect of edema on intestinal smooth muscle contractility. Activation of these pathways may also be important in other situations resulting in ileus, such as post-operative and endotoxin-induced ileus. [45-51]
Figure 1.
Regulation of MLC phosphorylation in edema-induced intestinal dysfunction. Intestinal contractility is regulated by phosphorylation of the MLC, which in turn is controlled by the activity of kinases and phosphatases. Preliminary data demonstrate that the control of MLC in edema is likely through the effects on the activity of the phosphatase. (MLC, myosin light chain; MLCK, MLC kinase; MYPT1, myosin phosphatase targeting subunit 1 of MLC phosphatase; CPI17, PKC-potentiated inhibitory protein; ZIPK, zipper interacting protein kinase; ILK, Integrin linked kinase; PAK, P21 activated kinase; ROK, Rho Kinase; PKC, protein kinase C)
1. STAT-3
The STAT family of transcription factors is important in activating intracellular signaling pathways and is activated (primarily by phosphorylation by Janus kinases (JAK) and mitogen activated protein (MAP) kinases) in response to stress, cytokines, and various growth factors. [52] Although little is known about the role of STAT-3 activation on intestinal smooth muscle function, there are a few studies that suggest that STAT-3 may play a role in intestinal contractile function. For example, STAT-3 activation has been demonstrated in the intestinal muscularis in response to surgical manipulation and ischemia-reperfusion injury. [41, 45] Both surgical manipulation and ischemia-reperfusion injury have also been shown to decrease intestinal contractile activity. The increase in STAT-3 in these models is believed to be mediated by cytokine production (such as IL-6).
Edema induces increased STAT-3 signaling in the intestinal muscularis compared to non-edematous intestine including increased STAT-3 phosphorylation, increased nuclear translocation and increased STAT-3 binding to its DNA consensus site. [44] STAT3 inhibition attenuates edema-induced intestinal dysfunction, including edema-induced intestinal contractile dysfunction and decreased intestinal smooth muscle MLC phosphorylation. [44] We conclude from these data that STAT-3 activation is involved in edema-induced intestinal contractile dysfunction.
The mechanism by which STAT-3 is activated in intestinal edema is unclear. As mentioned previously, in both ischemia/reperfusion and surgical manipulation models of intestinal dysfunction of the gut, up-regulation of IL-6 in the muscularis and mucosal layers induced STAT-3 activation. [41, 45] Although IL-6 was up-regulated due to surgical stress in our intestinal edema model, we were unable to detect differences in IL-6 levels in the intestinal muscle with edema development compared to non-edematous tissue. [44] Furthermore, AG490, a JAK-2 inhibitor, did not completely block STAT-3 activation in the edematous intestinal smooth muscle suggesting that STAT-3 may be activated through a non-canonical pathway.
Mechanotransduction may be responsible for STAT-3 activation in our model either directly or indirectly (i.e., through a mechanosensitive channel such as the sodium hydrogen exchanger (NHE)). In cardiomyocytes, stretch has been shown to increase STAT-3 expression. [53-57] Edema alters the mechanical properties of intestinal tissue, representing a potential mechanism.
2. NF-kappa B
NF-kappa B is a transcription factor present in a variety of cells that regulates expression of such factors as cytokines, growth factors, inducible nitric oxide synthase (iNOS), and adhesion molecules. It has been implicated widely in inflammatory processes, and it may play a role in intestinal contractility. [58] In human colonic smooth muscle cells, NF-kappa B activation by TNFα was shown to suppress cell contractility via induction of ICAM-1. [59] In an animal model of Crohn’s disease, NF-kappa B was shown to decrease colonic circular smooth muscle contractility. [60]
We initially became interested in NF-kappa B for several reasons including the results of a microarray analysis performed comparing genetic expression in non-edematous and edematous intestinal smooth muscle. Analysis of the microarray data for common function-specific regulatory elements using two different software analyses revealed the importance of NF-kappa B signaling in edema-induced intestinal dysfunction. (DIRE and oPOSSUM) [61, 62] [Uray, Critical Care Medicine, In Press] Subsequently, we demonstrated increased NF-kappa B signaling in edematous tissue compared to non-edematous tissue. Inhibition of NF-kappa B attenuates edema-induced intestinal contractile dysfunction including decreased contractile activity and decreased intestinal smooth muscle MLC phosphorylation. These data suggest that NF-kappa B is involved in the signaling pathway leading to decreased intestinal contractile activity.
NF-kappa B may mediate some of its downstream effects on intestinal contractility by transcription of iNOS. iNOS protein levels are increased in edematous intestine; additionally inhibition of iNOS results in improved intestinal transit. [36]
The mechanism of NF-kappa B activation in intestinal edema is unclear; however, mechanical changes in intestinal tissue may play a role. Edema causes more than a 6-fold increase in intestinal smooth muscle tissue mechanical stress. [48] Stretch has been shown to activate NF-kappa B in other cell types. Mechanical stretch of rat ventricular myocytes stimulated BNP gene transcription in an NF-kappa B dependent manner. [55] Biomechanical stress was shown to stimulate IL-6 production via NF-kappa B in vascular smooth muscle cells. [63] In bladder smooth muscle cells, mechanical stretch activated NF-kappa B through increased actin polymerization. [53] In our model, we also observed altered actin polymerization that may play a role in edema-induced activation of NF-kappa B. [48, 49, 51]
3. NO
iNOS/NO is a known mediator of smooth muscle relaxation (correlating with ileus in the small intestine) in models of ischemia/reperfusion and post-surgical ileus (bowel manipulation models of ileus). [64-67] Additionally, iNOS has been implicated in barrier dysfunction in burn induced intestinal dysfunction. [68] Although constitutive levels of NO are important, overproduction can lead to pathological effects. [68] iNOS has been shown to mediate changes in intestinal contractility; inhibition of iNOS has been shown to attenuate decreased intestinal contractility in animal models, including in colitis and decreased ileal contractility induced by peritonitis. [43, 69]
iNOS is a constitutively active enzyme that increases production of NO by the conversion of arginine to citrulline and NO. NO binds to soluble guanylyl cyclase (sGC), converting GTP to cGMP. Increased levels of cGMP may mediate decreased phosphorylation of MLC by increasing the activity of MLC phosphatase (MLCP). Additionally, it may lead to hyperpolarization of the cell membrane, leading to an inhibition of calcium release and subsequent decreased contractility. Other potential mechanisms include inhibition of certain neurotransmitters, including acetylcholine and substance P. [70, 71]
NF-kappa B activates transcription of iNOS. We know that NF-kappa B is increased in edematous intestinal smooth muscle. We have demonstrated that iNOS expression is increased in intestinal edema after initiation of edema. [72] iNOS protein levels also are increased with edema, and inhibition of iNOS with a selective inhibitor (L-NIL) results in marked improvement in intestinal transit. Increases in iNOS have been observed in other models resulting in ileus, including endotoxin induced ileus, models of mechanical ileus and ischemia/reperfusion injury. [53, 55] Whether iNOS mediates dysfunction through the classic soluble guanylyl cyclase/cGMP pathway or via a non-canonical pathway is a subject of investigation in our group.
4. Myosin Light Chain Phosphorylation
We have observed that decreases in MLC phosphorylation in our intestinal edema model correlate well with changes in both intestinal contractile activity and transit. In general, pharmacologic treatments that attenuated edema-induced contractile activity also prevented edema-induced decreases in intestinal smooth muscle MLC phosphorylation. This includes NF-kappa B inhibition with pyrrolidinedithiocarbamate (PDTC) and STAT-3 inhibition with AG490. [44][Uray, Critical Care Medicine, In Press] While STAT-3 and NF-kappa B signaling have been implicated in edema-induced decreases in MLC phosphorylation, the mechanism is unclear. However, preliminary data show that MLC phosphorylation is altered through regulation of MLCP. [73]
MLC phosphorylation is an obligatory step in smooth muscle contraction and is highly regulated. The phosphorylation status of MLC depends on the balance between phosphorylation by MLC kinase (MLCK) and dephosphorylation by MLCP. MLCK is dependent on Ca2+-calmodulin; thus, smooth muscle contraction is responsive to intracellular Ca2+. However, both MLC phosphorylation and smooth muscle contractile activity can be modulated via Ca2+-independent pathways. These Ca2+-independent pathways affect contractile activity through direct phosphorylation of MLC or through regulation of MLCP activity. MLC can be directly phosphorylated by a number of kinases other than MLCK including Rho kinase (ROK), integrin-linked kinase (ILK), and zipper interacting protein kinase (ZIPK). MLCP activity is regulated via phosphorylation of the regulatory subunit of the enzyme, MYPT1, or by phosphorylation of the endogenous inhibitor of MLCP, protein kinase C (PKC)-potentiated inhibitory protein, CPI17. [74] The regulation of MLC phosphorylation is demonstrated in Figure 1.
C. Mechanical Changes as a Contributor to Ileus
As important as dysfunction in the smooth muscle cell is to the pathogenesis of edema-induced ileus, it is important to remember that muscle cells function in the framework of the intestinal tissue matrix. Thus, alterations in tissue properties may serve to alter the behavior of smooth muscle cells. Mechanical changes induced by edema are probably partially responsible for ileus. There are alterations in cytoskeletal architecture and mechanical properties induced by edema, including changes in calponin and vimentin (intermediate filament) levels and F:G actin ratio and alteration of interstitial and lymphatic pressures. [49] In addition, the mechanical properties of edematous intestine are altered, as manifested by decreased stress and residual stiffness. [49, 51] Certain therapeutic modalities, including hypertonic saline, reverse intestinal dysfunction induced by edema, including ileus, possibly by allowing for more efficient force transmission through intestinal tissue because of the prevention of cytoskeletal changes induced by edema. [48-51]
D. Barrier Dysfunction/Effects on Intestinal Permeability
Intestinal edema, both in the presence or absence of venous hypertension, decreases tissue resistance and increases mucosal permeability. [33] This finding has been seen in other disease processes in which intestinal edema is a component of the manifestation in both clinical and preclinical studies. Patients with chronic heart failure have been shown to have increased intestinal permeability. [75] The increase in permeability is secondary to multiple factors, including alterations in tight junction proteins (notably occludin and claudin), apoptosis of epithelial cells, oxidant stress (by activating certain isoforms of protein kinase C, causing lipid peroxidation in epithelial cells, altering mitochondrial function, and inhibiting ion exchange), nitric oxide (by decreasing epithelial cell viability and/or altering the cytoskeleton), and pro-inflammatory cytokines (IL-1, IL-4, IL-6, tumor necrosis factor-alpha, and interferon-gamma) (possibly, in part by increasing production of nitric oxide). [76-78] Dysfunctions in intestinal barrier integrity and function are thought to be involved in the pathogenesis of infection and subsequent multi-organ failure. [79] Barrier dysfunction may also contribute to edema secondary to translocation of serum proteins from the intravascular space, notably albumin. This is especially true in the post-operative period and in cases of sepsis, which may complicate the treatment of traumatically injured patients. [80]
V. Mechanotransduction
A. Basic Principles
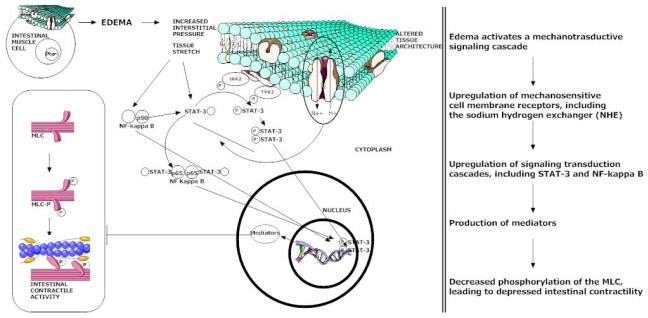
Cell and tissue stretch may be an important initiating event of signal cascades known to mediate intestinal dysfunction. In cardiomyocytes, stretch has been shown to activate sodium hydrogen exchange (NHE) receptors. [81] Additionally, stretch has been demonstrated to activate the STAT-3 and NF-kappa B pathway, which we have demonstrated to be involved in edema-induced intestinal dysfunction. [53, 55, 56, 82] [Uray, Critical Care Medicine, In Press]
Our interest in a mechanical explanation for activation of signaling cascades known to result in resuscitation induced intestinal edema is borne out of the observation that edema alters the mechanical properties of intestinal tissue. Intestinal edema is associated with cytoskeletal changes, including changes in intermediate filaments (calponin and vimentin) and in F:G actin ratio. There are alterations in tissue properties, including a decrease in stiffness and residual stress. Therapeutic modalities associated with improvement in edema-induced dysfunction, particularly hypertonic saline, are associated with return of intermediate filaments, F:G actin ratio, interstitial pressures, tissue stiffness and residual stress towards control levels, which may account for at least part of its therapeutic effect. [48, 49, 51]
The transcription factors NF-kappa B and STAT-3 are frequently activated by cytokines and other byproducts of inflammatory processes. In our model, there are no significant inflammatory stimuli present, leading us to explore non-canonical pathways of NF-kappa B and STAT-3 activation. The evidence for significant, early mechanical changes in intestinal edema indicates that the signals for activation of these cascades in intestinal edema are early and may be mechanical in nature. As others have demonstrated the role of stretch in other organ system related dysfunction, we have pursued development of this potential mechanism in intestinal edema-induced dysfunction.
B. Sodium Hydrogen Exchanger
Secondary to the early, substantial changes in the mechanical properties of edematous intestinal tissues, we hypothesized that stretch coupled ion exchangers were involved in the pathophysiology of edema-induced intestinal dysfunction. The NHE is a cytoskeletal linked ion exchange protein that has been shown to be activated by stretch. Given its properties as a mechanosensor and the fact that mechanical changes occur early in the course of intestinal edema, we have been interested in NHE as a potential initiator of dysfunctional signaling cascades in edema. NHEs are intimately involved in the regulation of intracellular pH and volume. It is also involved in intestinal absorption. There are nine identified isoforms. [83] The NHE is expressed throughout the intestine, with isoforms 2 and 3 being present primarily on the intestinal epithelial cell brush border. NHE isoform 1 is expressed in both mucosa and muscle [56, 81, 84, 85]
The NHE has been implicated in various intestinal pathologies, including colitis and hemorrhage/resuscitation models. [86, 87] It has been associated with vascular endothelial cell dysfunction in hemorrhagic shock and consequent hypoperfusion. [87] Administration of the sodium hydrogen exchange inhibitor amiloride has been demonstrated to ameliorate increased intestinal permeability in hemorrhagic shock and improves lung injury when combined with hypertonic saline resuscitation. [88, 89] Additionally, NHE inhibition in a colitis model has been shown to ameliorate disease activity and decrease IL-8 activation. [86] NHE inhibition has been shown to improve cardiac contractility in ischemia reperfusion injury. [90]
NHE may also play a role in edema-induced intestinal dysfunction. NHE inhibition ameliorates myocardial edema after cardioplegic arrest and cardiopulmonary bypass. [91] We have demonstrated edema induces increases in NHE 1-3 expression. [92] Hypertonic saline, which improves ileus induced by intestinal edema, prevents increased NHE expression. Inhibition of NHE with 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) results in improved contractile activity and intestinal transit, increased MLC phosphorylation, and decreased STAT-3 phosphorylation. [92] The potential mechanism of NHE in edema-induced intestinal dysfunction is demonstrated in Figure 2.
Figure 2.
Role of NHE in edema-induced intestinal dysfunction. Inhibition of NHE by EIPA results in decreased STAT-3 activation, amelioration of the decrease in MLC phosphorylation and improvements in intestinal contractility and transit. It also suggests that STAT-3 activation in edema is regulated by NHE, as has been demonstrated in other studies, particularly those involving cardiomyocytes.
C. Mechanical Stretch
Longitudinal stretch of intestinal tissues activates signaling pathways involved in edema. In preliminary data, when the intestine is stretched to correlate to the approximate stress seen in our in-vivo model of intestinal edema, there is activation of STAT-3, NF-kappa B and decreased phosphorylation of the MLC. Additionally, there is increased expression of NHE. [Shah, Journal of the American College of Surgeons, In Press] (Figure 3) Preliminary data also indicates that stretch of human intestinal smooth muscle cells activates STAT-3 and NF-kappa B. [73] This is the first body of work published in the literature examining the effect of mechanical stretch in intestinal tissues and indicates a potential role for mechanotransduction in edema-induced intestinal dysfunction. This also creates the possibility for an in-vitro model to study intestinal edema.
Figure 3.
Mechanotrasduction as a mechanistic explanation for edema-induced intestinal dyscfunction. Intestinal edema results in profound changes in the characteristics of intestinal tissue, including increased interstitial pressure. Stretch has been shown to modulate activation of various signaling pathways in cardiomyocytes. Intestinal stretch (in the absence of edema) that mimics similar magnitude forces as measured in edematous intestine results in increased expression of NHE 1-3, increased NF-kappa B activation, increased phosphorylation of STAT-3 and decreased MLC phosphorylation. This indicates that stretch may be the stimulus for activation of dysfunctional signaling pathways in intestinal edema.
VI. Therapeutic Modalities
A. Hypertonic Saline
Hypertonic saline has been studied and has shown therapeutic benefit in animal models of intestinal ischemia reperfusion. It has been shown to improve intestinal transit, decrease mucosal injury and tissue myeloperoxidase levels, and decrease iNOS protein expression. It has also been shown to reduce injury to lung and liver, presumably secondary to its gut protective effects. [93-95] In hemorrhagic shock, administration of hypertonic saline improves intestinal perfusion, reduces small intestinal mucosal apoptosis, and improves barrier function. [96-99] We have studied hypertonic saline in resuscitation induced intestinal edema, both in an attempt to better understand the pathophysiology behind edema-induced intestinal dysfunction and as a therapeutic intervention.
Administration of hypertonic saline decreases tissue edema, and reduction of tissue edema may be secondary to redistribution of tissue water to the peritoneal, intraluminal, and vascular spaces. This may be due, in part, to upregulation or prevention of a decrease in expression of water transport proteins, specifically the aquaporin 4 receptor. [Radhakrishnan, Critical Care Medicine, In Press] The decrease in intestinal edema is accompanied by a concurrent improvement in intestinal transit. [50]
Edema is associated with alterations in tissue architecture, and hypertonic saline reverses these alterations towards control levels. Hypertonic saline reverses the decrease in tissue stiffness, stress, and strain and prevents the increase in interstitial pressure due to edema. [48, 50] It prevents the decrease in stress fiber formation induced by edema (as measured by calponin and vimentin) and prevents thealterations in F:G actin ratio induced by edema. Hypertonic saline preserves tissue architecture and possibly allows for more efficient transmission of force through intestinal tissue.[49] Additionally, hypertonic saline prevents the increase in central venous pressure and lymphatic flow seen with intestinal edema. [48-50] Hypertonic saline may modulate some of the early mechanotransductive signals responsible for mediating subsequent intestinal dysfunction, including preventing an increase in NHE expression.
VI. Conclusion and Future Directions
Edema is a well accepted component in the pathophysiology of many disease processes involving the intestine, including that of ischemia/reperfusion, hemorrhagic shock, and inflammatory mediated processes. While many of these play a role in traumatic injury and resuscitation (notably hemorrhagic shock and ischemia/reperfusion), edema traditionally has not been viewed as an initiator or amplifier of signal transduction cascades that leads to end organ dysfunction. We have developed a body of literature separating edema from other confounding factors, notably ischemia/reperfusion, and examined it as an isolated disease process. Through our work, we have isolated edema as an initiator and propagator of pathways that lead to end organ dysfunction.
These studies have led to a better understanding of factors involved in the development of intestinal edema, specifically alterations in tissue architecture and alterations in interstitial pressures. The signal transduction cascades initiated with edema, including STAT-3, NF-kappa B, and possibly iNOS, mediate decreased MLC phosphorylation, subsequently decreasing intestinal contractility resulting in ileus.
Mathematical modeling may serve as a useful tool in predicting edema development in various organ systems. Factors, such as microvascular filtration, lymphatic resistance, osmotic and interstitial pressures, and interstitial compliance, can be utilized to mathematically predict edema development. This has applied to a variety of models with validation with experimental data. [100-109] Allowing one to predict development of edema as well as factors contributing to edema may allow for initiation of various protective strategies or alternative resuscitation strategies to prevent/ameliorate tissue water accumulation.
Our current work focuses on more specifically delineating the mechanotransductive pathways responsible for activation of signaling cascades known to mediate dysfunction. This includes expanding on the role of mechanosensors, including the NHE family of ion channels. One focus involves examining specific cytoskeletal elements, specifically water transport proteins, that may serve a therapeutic effect by decreasing tissue edema and hence the increase in interstitial pressure. Additionally, we are interested in understanding the mechanism by which activation of specific transcription factors (i.e., STAT-3 and NF-kappa B) lead to decreased MLC phosphorylation.
Acknowledgments
Sources of support: NIH Grants KO1 DK 070758, RO1 HL 36115, and P50 GM 38529
Footnotes
Conflict Statement There are no known conflicts between the authors and the information presented in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madigan MC, Kemp CD, Johnson JC, et al. Secondary abdominal compartment syndrome after severe extremity injury: are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008;64:280–285. doi: 10.1097/TA.0b013e3181622bb6. [DOI] [PubMed] [Google Scholar]
- 2.Rodas EB, Malhotra AK, Chhitwal R, et al. Hyperacute abdominal compartment syndrome: an unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am Surg. 2005;71:977–981. doi: 10.1177/000313480507101113. [DOI] [PubMed] [Google Scholar]
- 3.Balogh Z, McKinley BA, Cocanour CS, et al. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–543. doi: 10.1016/s0002-9610(02)01050-4. discussion 543-534. [DOI] [PubMed] [Google Scholar]
- 4.Fleming A, Bishop M, Shoemaker W, et al. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg. 1992;127:1175–1179. doi: 10.1001/archsurg.1992.01420100033006. discussion 1179-1181. [DOI] [PubMed] [Google Scholar]
- 5.Bishop MH, Shoemaker WC, Appel PL, et al. Relationship between supranormal circulatory values, time delays, and outcome in severely traumatized patients. Crit Care Med. 1993;21:56–63. doi: 10.1097/00003246-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Ivy ME, Atweh NA, Palmer J, et al. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387–391. doi: 10.1097/00005373-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 7.O’Mara MS, Slater H, Goldfarb IW, et al. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58:1011–1018. doi: 10.1097/01.ta.0000162732.39083.15. [DOI] [PubMed] [Google Scholar]
- 8.Staub NC, Taylor AE. Edema. Raven Press; New York: 1984. [Google Scholar]
- 9.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc. 2005;2:206–213. doi: 10.1513/pats.200501-009AC. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Moore AN, Clifton GL, et al. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein PD, Doerfler ME. Systemic complications of fluid resuscitation. Crit Care Clin. 1992;8:439–448. [PubMed] [Google Scholar]
- 12.Davis MJ, Gore RW. Capillary pressures in rat intestinal muscle and mucosal villi during venous pressure elevation. Am J Physiol. 1985;249:H174–187. doi: 10.1152/ajpheart.1985.249.1.H174. [DOI] [PubMed] [Google Scholar]
- 13.Laine GA, Allen SJ, Katz J, et al. Outflow pressure reduces lymph flow rate from various tissues. Microvasc Res. 1987;33:135–142. doi: 10.1016/0026-2862(87)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Stewart RH, Laine GA. Flow in lymphatic networks: interaction between hepatic and intestinal lymph vessels. Microcirculation. 2001;8:221–227. doi: 10.1038/sj/mn/7800081. [DOI] [PubMed] [Google Scholar]
- 15.Granger DN, Mortillaro NA, Taylor AE. Interactions of intestinal lymph flow and secretion. Am J Physiol. 1977;232:E13–18. doi: 10.1152/ajpendo.1977.232.1.E13. [DOI] [PubMed] [Google Scholar]
- 16.Granger DN, Barrowman JA. Microcirculation of the alimentary tract I. Physiology of transcapillary fluid and solute exchange. Gastroenterology. 1983;84:846–868. [PubMed] [Google Scholar]
- 17.Teke Z, Sacar M, Yenisey C, et al. Activated protein C attenuates intestinal mucosal injury after mesenteric ischemia/reperfusion. J Surg Res. 2008;149:219–230. doi: 10.1016/j.jss.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Moore RM, Muir WW, Granger DN. Mechanisms of gastrointestinal ischemia-reperfusion injury and potential therapeutic interventions: a review and its implications in the horse. J Vet Intern Med. 1995;9:115–132. doi: 10.1111/j.1939-1676.1995.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 19.Teke Z, Kabay B, Ozden A, et al. Effects of tempol, a membrane-permeable radical scavenger, on local and remote organ injuries caused by intestinal ischemia/reperfusion in rats. J Surg Res. 2008;149:259–271. doi: 10.1016/j.jss.2007.12.791. [DOI] [PubMed] [Google Scholar]
- 20.Kozar RA, Holcomb JB, Hassoun HT, et al. Superior mesenteric artery occlusion models shock-induced gut ischemia-reperfusion. J Surg Res. 2004;116:145–150. doi: 10.1016/s0022-4804(03)00301-9. [DOI] [PubMed] [Google Scholar]
- 21.Mallick IH, Yang W, Winslet MC, et al. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 22.Prien T, Backhaus N, Pelster F, et al. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990;2:317–323. doi: 10.1016/0952-8180(90)90077-g. [DOI] [PubMed] [Google Scholar]
- 23.Ravdin IS. Hypoproteinemia and Its Relation to Surgical Problems. Ann Surg. 1940;112:576–583. doi: 10.1097/00000658-194010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barden RP, Thompson WD, Ravdin IS, Frank IL. The influence of the serum protein on the motility of the small intestine. Surg Gynecol Obstet. 1938;66:819–821. [Google Scholar]
- 25.Barden RP, Ravdin IS, Frazier WD. Hypoproteinemia as a factor in the retardation of gastric emptying after operations of the Billroth I or II types. Am J Roentgenol. 1937;38:196–202. [Google Scholar]
- 26.Mecray PM, Barden RP, Ravdin IS. Nutritional edema: its effect on the gastric emptying time before and after gastric operations. Nutrition. 1990;6:278–289. 1937. discussion 290. [PubMed] [Google Scholar]
- 27.Moss GS. Plasma albumin and postoperative ileus. Surg Forum. 1967;18:333–336. [Google Scholar]
- 28.Lobo DN, Stanga Z, Simpson JA, et al. Dilution and redistribution effects of rapid 2-litre infusions of 0.9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: a double-blind crossover study. Clin Sci (Lond) 2001;101:173–179. [PubMed] [Google Scholar]
- 29.Marjanovic G, Villain C, Juettner E, et al. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg. 2009;249:181–185. doi: 10.1097/SLA.0b013e31818b73dc. [DOI] [PubMed] [Google Scholar]
- 30.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 31.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Moore-Olufemi SD, Xue H, Attuwaybi BO, et al. Resuscitation-induced gut edema and intestinal dysfunction. J Trauma. 2005;58:264–270. doi: 10.1097/01.ta.0000133571.64393.d2. [DOI] [PubMed] [Google Scholar]
- 34.Bauer AJ, Schwarz NT, Moore BA, et al. Ileus in critical illness: mechanisms and management. Curr Opin Crit Care. 2002;8:152–157. doi: 10.1097/00075198-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Moon PF, Hollyfield-Gilbert MA, Myers TL, et al. Effects of isotonic crystalloid resuscitation on fluid compartments in hemorrhaged rats. Shock. 1994;2:355–361. doi: 10.1097/00024382-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Moore-Olufemi SD, Xue H, Allen SJ, et al. Inhibition of intestinal transit by resuscitation-induced gut edema is reversed by L-NIL. J Surg Res. 2005;129:1–5. doi: 10.1016/j.jss.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 37.Drake RE, Teague RA, Gabel JC. Lymphatic drainage reduces intestinal edema and fluid loss. Lymphology. 1998;31:68–73. [PubMed] [Google Scholar]
- 38.Balogh Z, McKinley BA, Cox CS, Jr, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock. 2003;20:483–492. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 39.Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol. 1988;254:G389–398. doi: 10.1152/ajpgi.1988.254.3.G389. [DOI] [PubMed] [Google Scholar]
- 40.Sayan H, Ozacmak VH, Altaner S, et al. Protective effects of L-arginine on rat terminal ileum subjected to ischemia/reperfusion. J Pediatr Gastroenterol Nutr. 2008;46:29–35. doi: 10.1097/01.mpg.0000304450.54057.96. [DOI] [PubMed] [Google Scholar]
- 41.Hierholzer C, Kalff JC, Chakraborty A, et al. Impaired gut contractility following hemorrhagic shock is accompaied by IL-6 and G-CSF production and neutrophil infiltration. Dig Dis Sci. 2001;46:230–241. doi: 10.1023/a:1005524021552. [DOI] [PubMed] [Google Scholar]
- 42.Kalff JC, Hierholzer C, Tsukada K, et al. Hemorrhagic shock results in intestinal muscularis intercellular adhesion molecule (ICAM-1) expression, neutrophil infiltration, and smooth muscle dysfunction. Arch Orthop Trauma Surg. 1999;119:89–93. doi: 10.1007/s004020050363. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg S, Holst M, Hellstrom PM. Expression of iNOS mRNA associated with suppression of colonic contraction in rat colitis. Acta Physiol (Oxf) 2006;187:489–494. doi: 10.1111/j.1748-1716.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 44.Uray KS, Laine GA, Xue H, et al. Edema-induced intestinal dysfunction is mediated by STAT3 activation. Shock. 2007;28:239–244. doi: 10.1097/shk.0b013e318033eaae. [DOI] [PubMed] [Google Scholar]
- 45.Wehner S, Schwarz NT, Hundsdoerfer R, et al. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436–446. doi: 10.1016/j.surg.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Kalff JC, Turler A, Schwarz NT, et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz NT, Engel B, Eskandari MK, et al. Lipopolysaccharide preconditioning and cross-tolerance: the induction of protective mechanisms for rat intestinal ileus. Gastroenterology. 2002;123:586–598. doi: 10.1053/gast.2002.34777. [DOI] [PubMed] [Google Scholar]
- 48.Cox CS, Jr., Radhakrishnan R, Villarrubia L, et al. Hypertonic saline modulation of intestinal tissue stress and fluid balance. Shock. 2008;29:598–602. doi: 10.1097/SHK.0b013e318157eba7. [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan RS, Radhakrishnan HR, Xue H, et al. Hypertonic saline reverses stiffness in a Sprague-Dawley rat model of acute intestinal edema, leading to improved intestinal function. Crit Care Med. 2007;35:538–543. doi: 10.1097/01.CCM.0000254330.39804.9C. [DOI] [PubMed] [Google Scholar]
- 50.Radhakrishnan RS, Xue H, Moore-Olufemi SD, et al. Hypertonic saline resuscitation prevents hydrostatically induced intestinal edema and ileus. Crit Care Med. 2006;34:1713–1718. doi: 10.1097/01.CCM.0000218811.39686.3D. [DOI] [PubMed] [Google Scholar]
- 51.Radhakrishnan RS, Xue H, Weisbrodt N, et al. Resuscitation-induced intestinal edema decreases the stiffness and residual stress of the intestine. Shock. 2005;24:165–170. doi: 10.1097/01.shk.0000168873.45283.4c. [DOI] [PubMed] [Google Scholar]
- 52.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23:177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 53.Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608–20622. doi: 10.1074/jbc.M600214200. [DOI] [PubMed] [Google Scholar]
- 54.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- 55.Liang F, Gardner DG. Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism. J Clin Invest. 1999;104:1603–1612. doi: 10.1172/JCI7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan J, Fukuda K, Saito M, et al. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–1136. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 57.Wang TL, Yang YH, Chang H, et al. Angiotensin II signals mechanical stretch-induced cardiac matrix metalloproteinase expression via JAK-STAT pathway. J Mol Cell Cardiol. 2004;37:785–794. doi: 10.1016/j.yjmcc.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology. 2003;124:1369–1380. doi: 10.1016/s0016-5085(03)00263-4. [DOI] [PubMed] [Google Scholar]
- 59.Pazdrak K, Shi XZ, Sarna SK. TNFalpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology. 2004;127:1096–1109. doi: 10.1053/j.gastro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita K, Sato K, Hori M, et al. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn’s colitis model. Am J Physiol Gastrointest Liver Physiol. 2003;285:G483–493. doi: 10.1152/ajpgi.00038.2003. [DOI] [PubMed] [Google Scholar]
- 61.Gotea V, Ovcharenko I. DiRE: identifying distant regulatory elements of co-expressed genes. Nucleic Acids Res. 2008;36:W133–139. doi: 10.1093/nar/gkn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho Sui SJ, Mortimer JR, Arenillas DJ, et al. oPOSSUM: identification of over-represented transcription factor binding sites in co-expressed genes. Nucleic Acids Res. 2005;33:3154–3164. doi: 10.1093/nar/gki624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zampetaki A, Zhang Z, Hu Y, et al. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-kappaB signaling pathways. Am J Physiol Heart Circ Physiol. 2005;288:H2946–2954. doi: 10.1152/ajpheart.00919.2004. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz NT, Kalff JC, Turler A, et al. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004;126:159–169. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 65.Turler A, Moore BA, Pezzone MA, et al. Colonic postoperative inflammatory ileus in the rat. Ann Surg. 2002;236:56–66. doi: 10.1097/00000658-200207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hassoun HT, Weisbrodt NW, Mercer DW, et al. Inducible nitric oxide synthase mediates gut ischemia/reperfusion-induced ileus only after severe insults. J Surg Res. 2001;97:150–154. doi: 10.1006/jsre.2001.6140. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura J, van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- 68.Chen LW, Wang JS, Hwang B, et al. Reversal of the effect of albumin on gut barrier function in burn by the inhibition of inducible isoform of nitric oxide synthase. Arch Surg. 2003;138:1219–1225. doi: 10.1001/archsurg.138.11.1219. [DOI] [PubMed] [Google Scholar]
- 69.Koyluoglu G, Kaya T, Bagcivan I, et al. Effect of L-NAME on decreased ileal muscle contractility induced by peritonitis in rats. J Pediatr Surg. 2002;37:901–905. doi: 10.1053/jpsu.2002.32907. [DOI] [PubMed] [Google Scholar]
- 70.Zyromski NJ, Duenes JA, Kendrick ML, et al. Mechanism mediating nitric oxide-induced inhibition in human jejunal longitudinal smooth muscle. Surgery. 2001;130:489–496. doi: 10.1067/msy.2001.116414. [DOI] [PubMed] [Google Scholar]
- 71.Grasa L, Rebollar E, Arruebo MP, et al. The role of NO in the contractility of rabbit small intestine in vitro: effect of K+ channels. J Physiol Pharmacol. 2005;56:407–419. [PubMed] [Google Scholar]
- 72.Shah S, Xue H, Uray K, Laine G, Cox C. iNOS inhibition increases intestinal contractile frequency in resuscitation induced intestinal edema. Shock. 2009;31:41. [Google Scholar]
- 73.Uray KS, Hummel JA, Shah S, et al. The effects of increasing cyclical stretch on human intestinal smooth muscle cell signaling. Taos, Keystone Symposia, Mechanotransduction in Physiology and Disease. 2009 [Google Scholar]
- 74.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 75.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shao L, Huang Q, He M, et al. Changes of occludin expression in intestinal mucosa after burn in rats. Burns. 2005;31:838–844. doi: 10.1016/j.burns.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 80.Lobo DN. Fluid, electrolytes and nutrition: physiological and clinical aspects. Proc Nutr Soc. 2004;63:453–466. doi: 10.1079/pns2004376. [DOI] [PubMed] [Google Scholar]
- 81.Cingolani HE, Perez NG, Pieske B, et al. Stretch-elicited Na+/H+ exchanger activation: the autocrine/paracrine loop and its mechanical counterpart. Cardiovasc Res. 2003;57:953–960. doi: 10.1016/s0008-6363(02)00768-x. [DOI] [PubMed] [Google Scholar]
- 82.Halachmi S, Aitken KJ, Szybowska M, et al. Role of signal transducer and activator of transcription 3 (STAT3) in stretch injury to bladder smooth muscle cells. Cell Tissue Res. 2006;326:149–158. doi: 10.1007/s00441-006-0204-6. [DOI] [PubMed] [Google Scholar]
- 83.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 84.Chang E, O’Donnell ME, Barakat AI. Shear stress and 17beta-estradiol modulate cerebral microvascular endothelial Na-K-Cl cotransporter and Na/H exchanger protein levels. Am J Physiol Cell Physiol. 2008;294:C363–371. doi: 10.1152/ajpcell.00045.2007. [DOI] [PubMed] [Google Scholar]
- 85.Kiela PR, Xu H, Ghishan FK. Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J Physiol Pharmacol. 2006;57(Suppl 7):51–79. [PubMed] [Google Scholar]
- 86.Nemeth ZH, Deitch EA, Szabo C, et al. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G122–132. doi: 10.1152/ajpgi.00015.2002. [DOI] [PubMed] [Google Scholar]
- 87.Zakaria el R, Li N, Matheson PJ, et al. Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation. Surgery. 2007;142:487–496. doi: 10.1016/j.surg.2007.08.007. discussion 496 e481-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujiyoshi N, Feketeova E, Lu Q, et al. Amiloride moderates increased gut permeability and diminishes mesenteric lymph-mediated priming of neutrophils in trauma/hemorrhagic shock. Surgery. 2006;140:810–817. doi: 10.1016/j.surg.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Fujiyoshi N, Deitch EA, Feketeova E, et al. Amiloride combined with small-volume resuscitation with hypertonic saline is superior in ameliorating trauma-hemorrhagic shock-induced lung injury in rats to the administration of either agent alone. Crit Care Med. 2005;33:2592–2598. doi: 10.1097/01.ccm.0000186770.59312.44. [DOI] [PubMed] [Google Scholar]
- 90.Yarbrough WM, Mukherjee R, Escobar GP, et al. Direct inhibition of the sodium/hydrogen exchanger after prolonged regional ischemia improves contractility on reperfusion independent of myocardial viability. J Thorac Cardiovasc Surg. 2003;126:1489–1497. doi: 10.1016/s0022-5223(03)00811-0. [DOI] [PubMed] [Google Scholar]
- 91.Cox CS, Jr., Sauer H, Allen SJ, et al. Sodium/hydrogen-exchanger inhibition during cardioplegic arrest and cardiopulmonary bypass: an experimental study. J Thorac Cardiovasc Surg. 2002;123:959–966. doi: 10.1067/mtc.2002.120715. [DOI] [PubMed] [Google Scholar]
- 92.Uray KS, Cox C. Inhibition of Na/H exchanger attenuates edema-induced intestinal dysfunction. Crit Care Med. 2008;35:A35. [Google Scholar]
- 93.Attuwaybi B, Kozar RA, Gates KS, et al. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma. 2004;56:749–758. doi: 10.1097/01.ta.0000119686.33487.65. discussion 758-749. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez EA, Kozar RA, Suliburk JW, et al. Conventional dose hypertonic saline provides optimal gut protection and limits remote organ injury after gut ischemia reperfusion. J Trauma. 2006;61:66–73. doi: 10.1097/01.ta.0000224190.65542.e2. discussion 73-64. [DOI] [PubMed] [Google Scholar]
- 95.Powers KA, Zurawska J, Szaszi K, et al. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66–74. doi: 10.1016/j.surg.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 96.Zakaria el R, Tsakadze NL, Garrison RN. Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery. 2006;140:579–587. doi: 10.1016/j.surg.2006.05.015. discussion 587-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu YQ, Huang WD, Cai XJ, et al. Hypertonic saline resuscitation reduces apoptosis of intestinal mucosa in a rat model of hemorrhagic shock. J Zhejiang Univ Sci B. 2008;9:879–884. doi: 10.1631/jzus.B0820116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murao Y, Hata M, Ohnishi K, et al. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock. 2003;20:23–28. doi: 10.1097/01.shk.0000078832.57685.6c. [DOI] [PubMed] [Google Scholar]
- 99.Shi HP, Deitch EA, Da Xu Z, et al. Hypertonic saline improves intestinal mucosa barrier function and lung injury after trauma-hemorrhagic shock. Shock. 2002;17:496–501. doi: 10.1097/00024382-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 100.Bert JL, Bowen BD, Reed RK. Microvascular exchange and interstitial volume regulation in the rat: model validation. Am J Physiol. 1988;254:H384–399. doi: 10.1152/ajpheart.1988.254.2.H384. [DOI] [PubMed] [Google Scholar]
- 101.Bert JL, Gyenge CC, Bowen BD, et al. A model of fluid and solute exchange in the human: validation and implications. Acta Physiol Scand. 2000;170:201–209. doi: 10.1046/j.1365-201x.2000.00776.x. [DOI] [PubMed] [Google Scholar]
- 102.Chapple C, Bowen BD, Reed RK, et al. A model of human microvascular exchange: parameter estimation based on normals and nephrotics. Comput Methods Programs Biomed. 1993;41:33–54. doi: 10.1016/0169-2607(93)90064-r. [DOI] [PubMed] [Google Scholar]
- 103.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 104.Gyenge CC, Bowen BD, Reed RK, et al. Transport of fluid and solutes in the body I. Formulation of a mathematical model. Am J Physiol. 1999;277:H1215–1227. doi: 10.1152/ajpheart.1999.277.3.H1215. [DOI] [PubMed] [Google Scholar]
- 105.Gyenge CC, Bowen BD, Reed RK, et al. Transport of fluid and solutes in the body II. Model validation and implications. Am J Physiol. 1999;277:H1228–1240. doi: 10.1152/ajpheart.1999.277.3.H1228. [DOI] [PubMed] [Google Scholar]
- 106.Reed RK, Bowen BD, Bert JL. Microvascular exchange and interstitial volume regulation in the rat: implications of the model. Am J Physiol. 1989;257:H2081–2091. doi: 10.1152/ajpheart.1989.257.6.H2081. [DOI] [PubMed] [Google Scholar]
- 107.Xie SL, Reed RK, Bowen BD, et al. A model of human microvascular exchange. Microvasc Res. 1995;49:141–162. doi: 10.1006/mvre.1995.1012. [DOI] [PubMed] [Google Scholar]
- 108.Dongaonkar RM, Quick CM, Stewart RH, et al. Edemagenic gain and interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R651–659. doi: 10.1152/ajpregu.00354.2007. [DOI] [PubMed] [Google Scholar]
- 109.Dongaonkar RM, Laine GA, Stewart RH, et al. Balance point characterization of interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R6–R16. doi: 10.1152/ajpregu.00097.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah SK, Fogle LN, Aroom KR, et al. Mechanotransduction as a mechanistic explanation for edema induced intestinal dysfunction (abstract) J Am Coll Surg. 2009 (in press) [Google Scholar]
- 111.Uray KS, Wright Z, Kislitsyna K, et al. NF-kappa B activation by edema inhibits intestinal contractile activity. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181ce4aaa. (in press) [DOI] [PubMed] [Google Scholar]
- 112.Radhakrishnan RS, Shah SK, Lance SH, et al. Hypertonic saline alters hydraulic conductivity and upregulates mucosal/submucosal aquaporin 4 in resuscitation induced intestinal edema. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181ab878b. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]