Abstract
Type 1 diabetes mellitus (T1DM) is a T cell-mediated autoimmune disease resulting in islet β cell destruction, hypoinsulinemia, and severely altered glucose homeostasis. T1DM has classically been attributed to the pathogenic actions of auto-reactive effector T cells (Teffs) on the β cell. Recent literature now suggests that a failure of a second T cell subtype, known as regulatory T cells (Tregs), plays a critical role in the development of T1DM. During immune homeostasis, Tregs counterbalance the actions of autoreactive Teff cells, thereby participating in peripheral tolerance. An imbalance in the activity between Teff and Tregs may be crucial in the breakdown of peripheral tolerance, leading to the development of T1DM. In this review, we summarize our current understanding of Treg function in health and in T1DM, and examine the effect of experimental therapies for T1DM on Treg cell number and function in both mice and humans.
Keywords: autoimmunity, effector T cells, regulatory T cells, Teffs, Tregs, Type 1 diabetes mellitus
Introduction—Pathogenesis of type 1 diabetes and the role of T lymphocytes
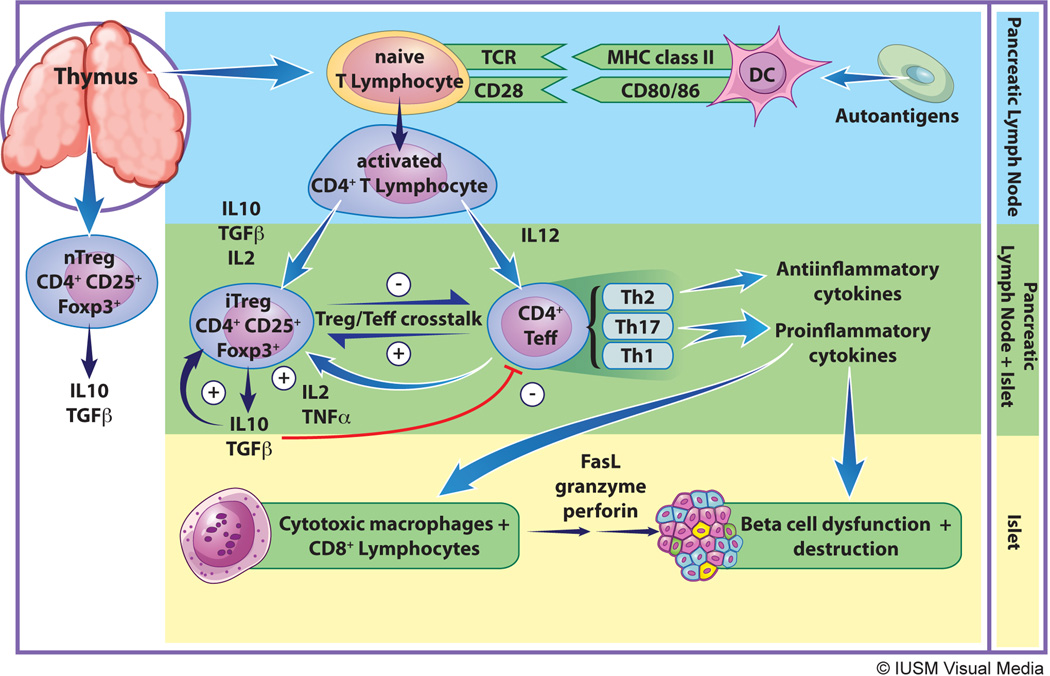
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized largely by T cell-mediated destruction of insulin-producing pancreatic β cells. Individuals become symptomatically hyperglycemic when a critical amount of β cell mass has been lost and the residual β cell mass is unable to match insulin demand. A phenotype similar to human T1DM is seen in the non-obese diabetic (NOD) mouse, and much of our understanding of the pathogenesis of T1DM arises largely from studies of this animal model [1]. The pathogenesis of T1DM is thought to begin when low-level β cell death results in the exposure of β cell antigens, which are then engulfed, processed and presented on the cell surface of antigen presenting cells (APCs) in the context of MHC class II molecules (Fig. 1). It is unclear whether the initial release of β cell autoantigens is prompted by endogenous β cell defects and/or an exogenous trigger, such as a viral infection. In response to antigen presentation and costimulation by APCs, CD4+ T lymphocytes in the local pancreatic lymph nodes proliferate and differentiate into auto-reactive CD4+ effector T cells (Teffs). Teff expansion and function is facilitated further by the immune cell-derived complement (C3a and C5a), which is activated locally during this T cell/APC interaction. Within the pancreatic islets, these activated Teffs release a host of cytokines including IFN-γ and IL-2, resulting in recruitment of cytotoxic macrophages and CD8+ T lymphocytes. Cytotoxic inflammatory cells ultimately infiltrate and destroy the islet cells in a process called “insulitis.” β cell death ensues, partly as a result of direct perforin/granzyme-mediated toxicity by CD8+ T cells, and partly as a result of the release of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-1β) by macrophages. Chemokines released by injured β-cells promote further mononuclear cell recruitment, and the release of additional auto-antigens allows for expansion and propagation of the autoreactive Teff response [2] (see Fig. 1).
Figure. Pathogenesis of type 1 diabetes.
The figure shows the general events from antigen presentation at the pancreatic lymph node (top) to immune cell-mediated destruction of beta cells (bottom), focusing on key events and cell types involved in the processes. Details are provided in the text. MHC class II – major histocompatibility complex class II, DC – dendritic cell, TCR – T cell receptor, IL10 – interleukin-10, TGFβ – transforming growth factor-beta, IL2 – interleukin-2, TNFα – tumor necrosis factor-alpha, iTreg – induced regulatory T cell, nTreg – natural regulatory T cell, Teff – effector T cells, FasL – Fas ligand, Th2 – T helper lymphocyte 2, Th17 – T helper lymphocyte 17, Th1 – T helper lymphocyte 1.
At the earliest stages of T1DM, however, a significant residual mass of β cells exist (perhaps 10–20%) that still produces insulin, but is ineffective at the time of clinical diagnosis owing to hyperglycemia or acidosis [3–5]. This significant residual β cell mass presents a potential therapeutic opportunity to reverse T1DM and is the premise behind a number of immunomodulatory approaches to halt further autoimmune destruction. As such, research into therapeutic interventions in T1DM has long focused on decreasing or blocking Teff activity during the early period following T1DM onset. There is mounting evidence, however, that a second subset of T lymphocytes, known as regulatory T cells (Tregs), is either quantitatively or qualitatively defective during T1DM pathogenesis. Thus, an underlying propensity to develop T1DM may occur when there is an imbalance in the number or function of Treg vs. Teff cells [6–9]. In accordance with this perspective, there has been emergence of therapies that increase the activity of Tregs and restore the balance between the Treg and Teff response. In this review, we will focus on how current T1DM therapies tested in both animal models and humans might be achieving their efficacy via the manipulation of the Treg response.
The Regulatory T cell (Treg) in T1DM
In the 1970s and 1980s, the existence of a population of T cells that could suppress immunity was inferred from studies of neonatally thymectomized mice, which developed multi-organ autoimmunity [10]. The notion of a population of “suppressor cells” capable of inhibiting Teffs fell out of favor in the late 1980s due to concerns that the results were neither reproducible nor generalizable. Despite these questions, the concept of T cells with inhibitory capability was renewed with a seminal study by Sakaguchi in which mice receiving an adoptive transfer of T cells devoid of CD4+CD25+ cells developed multi-organ system autoimmunity [11]. It has been appreciated that this population of T cells, now known as regulatory T cells (Tregs), can keep autoreactive T cells “in check”, thereby preventing undesirable immune responses, such as autoimmune diabetes [12]. As such, Tregs may be able to target T cell responses and perhaps antigen-specific responses without broadly immunosuppressive effects.
1. Physiologic Treg activity
There are two subpopulations of Tregs, “natural” Tregs (nTregs) and “induced” Tregs (iTregs), both of which participate in the maintenance of peripheral tolerance. nTregs, such as CD3+CD4+CD25+Foxp3+ cells, originate in the thymus during ontogeny, whereas iTregs derive from T cells that are activated by antigen in the periphery. In the organ-specific autoimmunity of T1DM, iTregs likely play a more important role than nTregs owing to their high affinity self-antigen recognition and efficient infiltration of areas with active inflammation [13].
All Tregs constitutively express CD25, the high-affinity receptor for IL-2α, and are defined by their preferential expression of the forkhead winged helix transcription factor Foxp3 [14–16]. The absence of Foxp3, as in the fatal diseases of scurfy in mice and IPEX syndrome in humans, results in multi-system autoimmunity, highlighting the importance of Tregs in self-tolerance. Interestingly, the most common endocrinopathy encountered in IPEX is T1DM, suggesting that Tregs may be central to the suppression of circulating β cell- specific T cells, and such pre-effector T cells may be in relative abundance compared to other self-reactive T cells [17–19].
Naïve T lymphocytes and T lymphocyte subsets (such as Th1, Th2, Th17, and Tregs), possess a great deal of plasticity in their differentiation, largely dependent on the local microenvironment. The differentiation of T cells into CD4+ iTregs is favored by a microenvironment rich in transforming growth factor (TGF-β) [20, 21 {Luo, 2007 #64] (Figure 1). Active secretion of the potently anti-inflammatory cytokines IL-10 and TGF-β contributes to both the immune suppressive functions and the expansion of iTregs [22–25]. iTregs suppress Teff activity through multiple mechanisms, many of which are still poorly defined and require more study. However, it is known that Tregs can specifically suppress Teff cells via the secretion of the afore-mentioned inhibitory cytokines (TGF-β, IL-10) [26–28], and can directly target Teff for cytolysis via the release of perforin and granzymes [29, 30].
To maintain immune homeostasis, Tregs interplay with Teff cells to achieve a balance between the pro-inflammatory and anti-inflammatory responses. Neither T lymphocyte subset is capable of acting in isolation. In fact, Teff cell activity is required for maintenance of Treg cell function and proliferation. Locally, Teff and Tregs interface through the IL-2/IL-2R pathway, allowing for direct feedback between the two cell types [31–33]. Teffs and Tregs that have the same TCR compete for the same (auto) antigen, and both become activated and proliferate in response to antigen. Tregs possess a higher affinity for self-antigen and are less reliant on costimulation than Teffs, which may be a mechanism that enhances Treg generation in non-inflammatory states, thus promoting self-tolerance [9, 34–36]. Conversely, in pro-inflammatory states, Teffs proliferate more rapidly than Tregs in response to antigen, promoting a Teff/Treg balance favoring pathogenesis. Factors thought to promote inflammatory states and enhance Teff number and function (i.e. TNF and IL2), may paradoxically enhance Treg number and function at sites of inflammation, perhaps mitigating active pathogenic responses or participating in the contraction and resolution of an immune response [37, 38]. Globally, it is clear that Teffs and Tregs are responsive to similar agents (such as antigens and cytokines), but endogenous factors specific to the cell subtype and the local immune microenvironment influence the critical balance of these cells, determining an outcome of either tolerance or pathogenesis. For a therapy to be effective at preventing or reversing human T1DM, Tregs will need to be expanded or spared, while β cell-specific Teffs are deactivated or eliminated.
2. The natural history of Tregs in T1D
The non-obese diabetic (NOD) mouse is arguably the best animal model for T1DM developed to date. Many therapies that have been tested in humans have first shown efficacy in this model [39–43], and NOD mice remain the proving ground for new targets in T1DM. NOD mice develop spontaneous and progressive T-cell infiltration into the islets of Langerhans, resulting in insulitis [1]. The lag time (many weeks) between the initiation of insulitis and the development of overt diabetes in the NOD mouse is suggestive of a progressive breakdown in peripheral regulatory mechanisms, namely Treg cell function. It remains controversial, however, if there is a quantitative vs. qualitative defect in Tregs that predisposes to development of T1DM (reviewed in [44]). In some studies, Treg cell populations were shown to remain constant, if not increased, in affected lymphoid tissues prior to diabetes onset [38, 45]. Yet it is not uncommon in other T cell mediated conditions (i.e. organ rejection) to have an influx of Tregs at sites of inflammation. Nevertheless, the persistent development of T1DM in these studies suggests that the Treg response is insufficient to control the marked Teff cell up-regulation. In part, this insufficient Treg response may be related to a decrease in Treg cell potency with age [38, 45, 46]. NOD mice with a genetically engineered disruption of co-stimulatory molecules (such as in CD40, CD28, and B7-1/B7-2) develop diabetes more quickly and at higher rates than wild-type NOD mice, suggesting that these molecules are critical for Treg cell generation and/or maintenance [47–49]. CD28−/− mice develop profound lymphoproliferative diseases, presumed secondary to a paucity of Treg cell numbers [50, 51]. Interestingly, CD154−/− NOD mice have normal levels of functional Tregs despite CD154 being one of the requisite costimulatory molecules for Teffs [8]. Knock-out models resulting in a reduction in Treg number or function do not clearly demonstrate how or where Tregs are adversely affected. For example, are Tregs affected during thymic generation, homeostatic peripheral maintenance, or during expansion to a specific antigen in pro-inflammation states?
Th17 cells are known to play a role in the pathogenesis of several autoimmune diseases, but a pathogenic role (if any) for Th17 cells in T1DM remains poorly understood. It appears that Th17 cells may act indirectly via their differentiation into Teffs [52, 53]. Th17 cells are also capable of differentiating into Tregs in a milieu rich in TGF-β and TNF [52–54]. Th17 cell function, as measured by IL-17 levels, is known to increase as T1DM progresses [55]. In fact, anti-IL-17 antibody delays the onset of T1DM in NOD mice, concordant with upregulation of Treg populations [56], indicating that Th17 cells play an important role in the Treg/Teff balance.
For at least two reasons, studies of Tregs in humans are more limited than in NOD mice. First, there is yet no marker that appears to be sufficiently reliable for identifying CD4+ Tregs in humans. CD25 is not a specific marker for activated Tregs, as activated Teff cells also transiently express CD25 [57], thereby making it difficult to discern Treg from Teff cell populations. Second, whereas in NOD mice it is straightforward to identify Treg populations relevant to T1DM by analysis of pancreatic lymph nodes or even islets directly, in humans this is not possible, and the interpretation of Treg populations collected in the peripheral blood is likely not analogous to the Treg cell number and function in the inflamed pancreas. Antigen-specific Treg cell assays would be valuable in discerning T1DM-specific Treg cell activity and in monitoring response to therapy, but there is currently no assay with adequate reproducibility [58]. Fortunately, the methylation profile of the Foxp3 gene is specific to activated Tregs in humans, as activated Tregs show complete demethylation of the Foxp3 promoter region compared to the partial methylation seen in activated non-Treg cells [59–61].
Despite the limitations inherent to Treg detection in humans, there have been several attempts to study Treg cell frequency and function in people with T1DM [62]. Individuals with T1DM appear to have normal Treg frequencies, but decreased functional responses as demonstrated by reduced levels of iTreg cell-secreted IL-10 [63]. Additionally, children with T1DM have a transient loss of Treg suppressive function (measured by incubation of CD4+CD25+ cells with anti-CD3 or anti-CD3/CD28) during the first 3–6 months from diagnosis with subsequent recovery to that of non-diabetic controls by nine months [64]. It remains largely unknown, however, what is occurring to the Treg/Teff balance in the years or months preceding diagnosis of T1DM in humans.
3. Defects in Treg cell function in T1DM
Based on the aforementioned studies in humans and NOD mice, there is an emerging consensus that Tregs exhibit reduced functionality (perhaps absolute, relative, or both) with respect to Teff cell suppression in T1DM. As described earlier, reduced Treg function results in an imbalance in the Treg/Teff response and a failure to maintain self-tolerance; this favors the pro-inflammatory Teff response and the resultant Th1-mediated β-cell destruction. A reason for impaired Treg function in T1DM may lie in alterations in the crosstalk between Teffs and Tregs (Figure 1). Defective or decreased IL-2/IL-2R signaling may be one of the main culprits for the altered Treg/Teff cell balance. For example, NOD mice have reduced pancreatic IL-2 levels, conferring reduced stimulation of Tregs [65]. This decline in Treg cell function likely feeds the proliferation of auto-reactive Teff cells. Treg function and number may result from defective signaling from dendritic cells (DCs), but it is unclear if this affects the maintenance of basal homeostatic Tregs or antigen-specific activation of Tregs during a pro-inflammatory response [66]. As with mice, humans may be more prone to development of T1DM because of defective or immature DC signaling. Indeed, individuals with T1DM have decreased circulating DCs compared to healthy controls [67]. Recently, immature gut mucosal DCs in individuals with T1DM were found responsible for reduced differentiation of gastrointestinal CD4+CD25+Foxp3+ Tregs [68]. This defective de novo generation of iTregs by gut mucosa may adversely affect the Treg/Teff cell balance, leading to a failure of self-tolerance.
Similar to CD4+ Tregs, CD8+ Treg induction may be limited by impaired DC presentation. The induction of potent CD8+ Tregs requires activation by the heat shock protein Hsp60sp, which is bound to the MHC class II HLA-E. Many individuals with T1DM have CD8+ T cells defective in their ability to recognize HLA-E/Hsp60sp [69], offering a potential mechanism for T1DM pathogenesis. Interestingly, this CD8+ defect can be corrected by administration in vitro of autologous immature DCs loaded with Hsp60sp peptide [69].
Tregs as a therapeutic target in T1DM
Given that T1DM is a T cell mediated process, strategies to increase Treg cell number and/or function have been viewed as potential therapeutic approaches. In this respect, there is an ongoing clinical trial of intravenous infusions of autologous Tregs expanded ex vivo in individuals newly diagnosed with T1DM (currently enrolling, NTC01210664). Similarly, human cord blood stem cells, which are rich in Tregs and also may be capable of favoring differentiation of naïve T lymphocytes to Tregs, are an active area of research in diabetes [70, 71]. Given the expense and technical difficulty in infusing antigen-specific Tregs or harvesting cord blood stem cells, however, therapies that target endogenous Treg cell function are clearly desirable. The discussion below will focus on approaches to increase endogenous Treg cell production and/or function (summarized in Table).
Table.
Therapeutic approaches to increase endogenous regulatory T cell populations and function as demonstrated by murine studies and clinical trials
| Reference | |||
|---|---|---|---|
| Murine Studies | Human Studies | ||
| B lymphocyte blockade | |||
| Anti-CD20 mAb | [151] | ||
| Enhanced Treg differentiation | |||
| Foxp3 demethylation | [152] | ||
| Improved Treg activation | |||
| Dendritic cell presentation | |||
| G-CSF | [79, 80] | NCT01106157 NCT00662519 | |
| Antigen-loaded DC | [76, 78] | NCT00445913 | |
| Flt3 | [153–155] | ||
| Rapamycin | [129, 130] | [131–133] NCT00525889 | |
| GAD-alum | [43, 135–137] | [138–140] | |
| Complement inhibition | [83] | ||
| Co-stimulatory molecules | |||
| CD28 and/or CD154 antagonism | [9] | ||
| Increased Treg proliferation/expansion | |||
| DPP-IV inhibitors | [103–105, 111] | NCT01155284 | |
| IL-10 agonists | [94–96] | ||
| IL-17 antibody | [56] | ||
| Improved Teff-Treg interaction | |||
| IL-2 agonists | [38, 65] | NCT00525889 | |
| Anti-CD3 mAb | [151] | ||
| Selective targeting of highly diabetogenic Teffs while sparing Tregs | |||
| LFA3Ig (putative) | NCT00965458 | ||
1. Modification of dendritic cell function to induce Tregs
Treg cell maintenance during immune quiescence is linked to the direct interaction with DCs [72, 73], the only major APC subset involved in activating the T lymphocyte response to self-antigen [74, 75]. In the NOD mouse, Tregs expanded by antigen-loaded DC offered protection and restored euglycemia in overt diabetes [76]. Additionally, iTregs can be induced when naïve T cells are treated with β-islet antigen pulsed DC in a TGF-β-rich environment [77]. These iTregs have proven protective in preventing islet graft rejection, suggesting tolerogenic DCs may have a therapeutic role in the destructive autoimmunity of T1DM [76, 78]. G-CSF, a hematopoietic growth factor of the myeloid lineage, has also been shown to increase tolerogenic DCs and Tregs in the peripheral blood of healthy human subjects. In NOD mice, G-CSF caused recruitment of immature tolerogenic DCs, which subsequently led to recruitment of CD4+ Tregs [79, 80].
The complement system, which links the innate and adaptive immune response, is another potential therapeutic target in modifying DC function. Cognate T cell/DC signaling results in activation of the complement system, particularly C3a and C5a fragments, which is implicated in Th1-mediated autoimmune disease pathogenesis [81, 82]. Activated complement fragments amplify the pro-inflammatory Th17 and Th1 response [82]. Conversely, inhibition of complement results in Treg expansion and function [83–85]. Interestingly, complement C3-deficient mice remain insulitis- and diabetes-free in response to STZ but do have an expanded TGF-β-dependent Treg population [83]. Complement appears requisite for diabetes development [86], perhaps by shifting the Treg/Teff balance towards pro-inflammatory Teffs.
2. IL-10 therapy
Tregs secrete high levels of IL-10, a potent anti-inflammatory cytokine that promotes Treg differentiation and function. IL-10 allows antigen-activated Tregs to suppress in a non-antigen-specific manner, permitting broader suppressive activity [22]. Clinically, IL-10-producing Tregs naturally regulate tolerance in the setting of bone marrow and solid organ transplantation, as the incidence of graft-versus-host disease is inversely related to endogenous IL-10 production [87–90]. In fact, it is this “non-specific” immune suppressive activity that may be of concern in clinical translation as alterations to the Teff/Treg balance may adversely affect critical and desirable protective T cell immune responses.
IL-10-secreting islet antigen-specific T cell responses, markers of Treg function, are present but reduced in individuals with T1DM compared to healthy, antibody-negative, controls [63, 91–93]. This deficit in Treg function is amplified by an increased Teff function, as reflected by increased antigen-specific IFN-γ secretion [63, 91, 92]. Higher frequencies of IL-10-secreting Tregs at diagnosis were associated with improved glycemic control at 3 months of disease [93].
As a therapeutic angle, IL-10 has been found to reduce diabetes development in the murine model through up-regulation of Tregs [94, 95]. IL-10 has also been found capable of inducing tolerogenic DCs. IL-10-conditioned dendritic cells are capable of delaying and preventing diabetes onset in NOD and HLA transgenic animal models, a protective effect also transferrable via adoptive transfer and associated with increased CD4+CD25+Foxp3+ Treg cell populations [96]. Interestingly, transgenic expression of IL-10 in the islet β cells of NOD mice actually caused acceleration of diabetes, suggesting over-expression may play a role in β cell stress [95, 97]. As such, there again appears to be a critical balance between promoting Treg number and function and increasing β cell antigenicity and destruction by Teffs. It is possible that unbridled Treg activity as a therapeutic intervention would likely be harmful; therefore, striking a delicate balance between Teff and Treg function remains the goal.
3. Approaches to increasing TGF-β
As discussed previously, TGF-β is critical in promoting Treg differentiation, expansion, and function. Therefore, therapies that increase TGF-β, especially early in the T cell response, may be beneficial in altering Treg/Teff cell interplay and mitigating β cell destruction. Supporting the valuable role for increased endogenous TGF-β is the finding of reduced TGF-β levels preceding diabetes development in NOD mice and in individuals with a positive family history [98, 99]. NOD mice genetically engineered to constitutively express islet-specific TGF-β from birth have reduced diabetes incidence [100]. TGF-β appears to alter APC signaling [101] and induce apoptosis of infiltrating lymphocytes [100]. Unfortunately, constitutive expression of TGF-β was associated with the undesirable outcome of extensive pancreatic fibrosis [100].
Dipeptidyl-peptidase IV (DPP-IV) inhibitors promote Treg development through their positive effects on endogenous TGF-β1. DPP-IV is a ubiquitous serine-type protease that degrades the incretin hormones of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). DPP-IV inhibitors prevent this degradation, thereby prolonging endogenous incretin activity. As reviewed by Drucker, incretin hormones also have direct β cell effects including increased insulin synthesis and secretion, as well as increased β cell mass by enhanced proliferation and suppressed apoptosis [102]. The ability of DPP-IV inhibitors to directly preserve β cell mass and function have made them an attractive therapy in T1DM. In fact, GLP-1 stimulation delays diabetes development in NOD mice [103, 104] with the effect magnified when the GLP-1 receptor agonist is combined with the immunosuppressant therapies, such as anti-CD3 monoclonal antibody [105].
Apart from these direct effects on the β cell, however, DPP-IV inhibitors are also immunomodulatory, a property perhaps more relevant to T1DM pathogenesis. Their immunomodulatory effects occur through CD26, a lymphocytic cell surface peptidase with intrinsic DPP-IV activity [106]. CD26 contributes to T cell development, maturation, and migration, as well as cytokine production and T cell-dependent antibody production (reviewed in [107]). As such, CD26/DPP-IV inhibition results in T cell and cytokine suppression and TGF-β up-regulation [108–110]. It is unclear if the enhanced TGF-β levels are a cause or effect of Teff cell suppression. Regardless, DPP-IV inhibition results in reversal of diabetes in NOD mice, albeit non-sustained with treatment cessation [111]. Diabetes remission in this study was associated with increased TGF-β levels and CD4+CD25+Foxp3+ T cells, especially in the inflamed pancreas [111]. Interestingly, re-occurrence of disease was paralleled by reductions in Treg populations and TGF-β1 levels. Currently, a clinical trial is underway using a DPP-IV inhibitor (sitagliptin) combined with a proton-pump inhibitor (lansoprazole) in children and adults with newly diagnosed T1DM (NCT01155284).
4. Anti-CD3 monoclonal antibody
Anti-CD3 monoclonal antibody (anti-CD3 mAb) has shown promise as a therapeutic approach in T1DM through its ability to directly block antigen-dependent T cell activation [112–114]. A short treatment course with anti-CD3 mAb resulted in diabetes remission in NOD mice [39, 40]. These findings in mice has led to human trials using two modified formations of the hOKT3 monoclonal antibodies, ChAglyCD3 (otelixizumab) and hOKT3γ1(Ala-Ala) (teplizumab). In children with newly diagnosed T1DM, a limited course of hOKT3γ1 attenuated the loss of insulin production in patients (as evidenced by reduced HbA1c and total daily insulin dose), but did not reverse the need for exogenous insulin [42, 115]. Similarly in a trial of ChAglyCD3, β cell function was improved and insulin requirement was lower in drug-treated recipients [116]. Some data suggests that in addition to depleting Teffs, such antiCD3 therapies enhance Treg numbers and perhaps function. Anti-CD3 mAb causes increased expression and function of human and murine CD4+CD25+Foxp3+ Tregs [41], but may have even more profound effects on CD8+ Tregs. hOKT3γ1(Ala-Ala) has been found to increase functional circulating iCD8+ Tregs in individuals with T1DM [117]. The induction of iCD8+ Tregs by hOKT3γ1(Ala-Ala) requires TNF-α, with action mediated by the NF-κΒ cascade [117]. It is possible that TNF-α secreted in the pro-inflammatory milieu of T1DM stimulates these potent CD8+ Tregs.
Recent large Phase 3 industry-supported trials, including DEFEND-2 (using ChAglyCD3 (otelixizumab)) [118] and the Protégé trial (hOKT3γ1(Ala-Ala) (teplizumab)) [119], in newly diagnosed T1DM failed to meet their primary endpoints. Similarly, a Phase 2 trial sponsored by the Immune Tolerance Network again showed that one course of hOKT3γ1(Ala-Ala) (teplizumab) slowed β cell decline [120], but there was no added benefit of a second course one year later despite more adverse events.
5. Manipulation of the IL-2/IL-2R pathway
IL-2, a cytokine secreted by activated Teff cells, binds to the constitutively active trimeric IL-2R (CD25) of Tregs. Through this IL-2/IL-2R interaction, IL-2 acts as an important stimulatory signal for the expansion and function of Tregs [33, 121, 122]. IL-2, and the resultant Treg expansion, is critical for maintenance of self-tolerance, as evidenced by the severe multi-organ autoimmune disease that develops in mice deficient in IL-2 or IL-2R [123, 124]. In both NOD mice and humans, expression of genes that contribute to aberrant IL-2/IL-2R signaling, such as CD25 and PTPN 2, are associated with increased T1DM incidence [57, 65, 125]. In children with T1D, abnormal IL-2R signaling in the CD4+ T lymphocytes was found to contribute to decreased Foxp3 expression and, therefore, a reduction in Tregs [126].
Given these observations, therapies that increase activity of the IL-2/IL-2R pathway, such as IL-2 agonists, may be helpful in preventing T1D. Low-dose IL-2 was found to increase Treg cell populations and largely prevented the development of T1DM in young NOD mice [65]. In overtly diabetic NOD mice, 60% developed long-lasting remission after receiving low-dose IL-2, associated with a concomitant increase in Tregs [38]. Diabetes remission was likely due to expansion of Treg activity, as Treg-deficient CD28−/− NOD mice did not have diabetes remission [37]. Additionally, there was increased expression of Treg-associated molecules (CD25, Foxp3, CTLA-4, and glucocorticoid-induced TNF receptor (GITR)). Notably, low-dose IL-2 treatment minimized the effect of IL-2 on NK and Teff cells, reducing the incidence of cytokine storm seen with higher dose IL-2 agonists [65, 127, 128]. A Phase 1 trial in newly diagnosed T1DM evaluating IL-2 and rapamycin supported by NIAID’s Immune Tolerance network recently completed enrollment, with results pending (NCT00525889).
6. Rapamycin therapy
Rapamycin, a non-calcineurin inhibitor, blocks signaling in response to cytokines and favors differentiation of tolerogenic dendritic cells, perhaps indirectly resulting in increased Treg activity [129]. Rapamycin promotes the differentiation of functional Tregs when added to murine CD4+ lymphocytes in vitro. [130]. Likewise, administration of rapamycin in vivo prevents diabetes onset in NOD mice and allows for expansion of Tregs [131]. Unfortunately, a clinical trial of rapamycin in adults with T1DM did not alter the frequency or proliferation of Tregs [132]. Rapamycin may perform better as an adjunct when used in combination with IL-10 to increase Tregs [133].
7. GAD-alum
Glutamic acid decarboxylase 65 (GAD65) is a major autoantigen in T1DM [134]. In the NOD mouse, introduction of a GAD65 peptide results in immune tolerization and halts further T cell mediated β cell destruction [43]. Inherent to this tolerization, GAD-alum has been found to induce a GAD65-specific Treg response [135–139]. In Phase II clinical trials, alum formulated GAD (GAD-alum), given early in the course of T1DM, resulted in a modest preservation of residual insulin secretion, as reflected by increased fasting C-peptide levels [140]. Nevertheless, recent results from the phase 3 trial of GAD-alum did not show any impact on β cell loss in those with newly diagnosed T1DM. [141].
8. LFA3-Ig/Alefacept
Ideally, a therapeutic agent in T1DM would alter the Teff/Treg balance by targeting Teffs while sparing Tregs. The biologic agent alefacept, FDA-approved for the treatment of plaque psoriasis, now shows promise as an immunomodulatory therapy in T1DM. In psoriasis, alefacept results in prolonged clinical tolerance with remission lasting over 2 years [142, 143]. Alefacept is a fusion protein containing the “head” of human-LFA3 and an IgG “tail” [144, 145]. In humans, LFA3 is found on a variety of antigen presenting cells, and interacts with its cognate ligand CD2 on T cells for adhesion and costimulation. Alefacept appears to both interfere with T cell activation and facilitates NK cell-mediated deletion of T cells [145, 146]. During studies of alefacept in psoriasis, it was recognized that naïve T cells (Tn; i.e., CD3+CD45RO−, CCR7+) were relatively spared, central memory cells (Tcm; i.e., CD3+CD45RO+, CCR7+) were depleted intermediately, and effector memory T cells (Tem; i.e., CD3+CD45RO+CCR7−) were depleted to the greatest extent [147, 148]. Although CD2 is expressed on all T cells, the highest expression is on effector memory T cells and lowest on naïve T cells. Recent data from non-human primates suggest that highly activated and “armed” effector memory T cells involved with active immunopathogenic responses express the highest levels of CD2 [149]. This suggests that T cells with the highest level of expression of CD2 are most susceptible to alefacept. In preliminary studies, CD2 expression on human Tregs is at low levels, nearly equivalent to those of naïve T cells (Rigby, MR personal communication). It is thus plausible that like naïve T cells, Tregs would be relatively spared by alefacept.
Currently, there is an actively enrolling NIAID/Immune Tolerance Network clinical trial (T1DAL, or Type 1 Diabetes with Alefacept) evaluating if alefacept can induce remission in new onset T1DM (NCT00965458). This agent may fit the “model” profile of an immunotherapy in T1DM by targeting the most highly pathogenic diabetes-causing Teffs, while leaving both the naïve protective T cell repertoire and Tregs intact. Thus, alefacept may be a prime example of how to tilt the Teff/Treg balance to favor β cell protection. The development of similar specific therapeutic agents will be facilitated by advances in understanding of the unique surface makers, activation requirements, and unique pathways utilized by both Tregs and Teffs.
Conclusion
To date, no immunomodulatory therapeutic trial has induced long-lasting or significant β cell protection in humans with T1D. Despite a number of clinical trials and decades of research, no clinically beneficial effect is seen or a period of β cell protection is followed by subsequent rate of decline in β cell function equal to that of controls. In no studies had insulin freedom been achieved, yet some studies suggest better glycemic control with early immunomodulatory intervention. The long-term impact for mild successes (i.e. antiCD3) remains debatable. The reasons for this lack of success are unclear, but perhaps therapeutic interventions geared to reduce the Teff cell response also adversely affects Treg function. For example, therapies that block the requisite costimulatory signals may also inhibit Treg differentiation and proliferation. Recently, a cytotoxic T lymphocyte associated molecule-4 (CTLA-4) antagonist, which blocks the CD28:B7 costimulatory interaction, failed to provide long-lasting β-cell protection in recently diagnosed individuals with T1DM [150] This study was performed in spite of preclinical data suggesting that CTLA4Ig therapies affect active pathogenic Teff function little, and affected Treg activation little [9] (Rigby MR, unpublished data}. In addition, the interdependence between Tregs and Teff cells [37] may mean that any therapy that blocks Teff cell stimulation and expansion also leads to reduced Treg population and function. Ideally, immunomodulation in T1DM would specifically inhibit Teff activation while also preserving or even promoting Treg function. The currently ongoing T1DAL trial is investigating that premise. In the evolving field of T cell biology, it is becoming clear that Teff and Tregs are not mutually exclusive and there is a significant interdependence their numbers and function. In T1DM, the resultant balance for a number of processes acting in concert will promote self-tolerance and protecting the β cells or shift to facilitating β cell destruction and T1DM. Therefore, the goal of restoring Teff/Treg homeostasis remains an exciting and novel approach to T1DM management.
Acknowledgments
This work was supported by grants R01 DK083583 (to RGM) and T32DK065549 (to SMC) from the National Institutes of Health, by an Investigator Initiated Study from Merck & Co., Inc. (to RGM), and by a grant from the Juvenile Diabetes Research Foundation (to RGM). The authors thank Dr. Bernhard Maier for thoughtful assistance with manuscript preparation and review.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005(23):447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010 Jul;10(7):501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 3.Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) The DCCT Research Group. J Clin Endocrinol Metab. 1987 Jul;65(1):30–36. doi: 10.1210/jcem-65-1-30. [DOI] [PubMed] [Google Scholar]
- 4.Pozzilli P, Visalli N, Buzzetti R, Cavallo MG, Marietti G, Hawa M, et al. Metabolic and immune parameters at clinical onset of insulin-dependent diabetes: a population-based study. IMDIAB Study Group. Immunotherapy Diabetes. Metabolism. 1998 Oct;47(10):1205–1210. doi: 10.1016/s0026-0495(98)90324-9. [DOI] [PubMed] [Google Scholar]
- 5.Rother KI, Spain LM, Wesley RA, Digon BJ, 3rd, Baron A, Chen K, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009 Dec;32(12):2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mordes JP, Bortell R, Doukas J, Rigby M, Whalen B, Zipris D, et al. The BB/Wor rat and the balance hypothesis of autoimmunity. Diabetes Metab Rev. 1996 Jul;12(2):103–109. doi: 10.1002/(SICI)1099-0895(199607)12:2<103::AID-DMR161>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008 Nov;28(6):677–684. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 8.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004 Oct;114(7):979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector t-cell inhibition and regulatory T-cell expansion. Diabetes. 2008 Oct;57(10):2672–2683. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1–2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985 Jan 1;161(1):72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008 Mar;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev. 2005 Aug;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005 Nov;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003 Feb 14;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003 Apr;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001 Jan;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 18.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003 Jul;15(4):430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. Feb 203;2005:156–164. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 20.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009 May;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009 May;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997 Oct 16;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003 Dec 15;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006 Sep;25(3):455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005 Apr 4;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999 Oct 4;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005 Mar 7;201(5):737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001 Sep 3;194(5):629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007 Oct;27(4):635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006 May 15;107(10):3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002 Nov 1;169(9):4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 32.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002 Sep 16;196(6):851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 34.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007 Aug 6;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004 Aug;21(2):267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001 Apr;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 37.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010 Dec 1;120(12):4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010 Aug 30;207(9):1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997 Mar 15;158(6):2947–2954. [PubMed] [Google Scholar]
- 40.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004 Mar;4(3):390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 42.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005 Jun;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993 Nov 4;366(6450):69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgouroudis E, Piccirillo CA. Control of type 1 diabetes by CD4+Foxp3+ regulatory T cells: lessons from mouse models and implications for human disease. Diabetes Metab Res Rev. 2009 Mar;25(3):208–218. doi: 10.1002/dmrr.945. [DOI] [PubMed] [Google Scholar]
- 45.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008 Jan;57(1):113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 46.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005 Apr 18;201(8):1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumanogoh A, Wang X, Lee I, Watanabe C, Kamanaka M, Shi W, et al. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001 Jan 1;166(1):353–360. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]
- 48.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000 Apr;12(4):431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 49.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003 Oct 1;171(7):3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 50.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995 Nov;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 51.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995 Nov 10;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 52.Bending D, De la Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 Mar;119(3):565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Brandt J, Fischer HJ, Walter L, Hunig T, Kloting I, Reichardt HM. Type 1 diabetes in BioBreeding rats is critically linked to an imbalance between Th17 and regulatory T cells and an altered TCR repertoire. J Immunol. 2010 Aug 15;185(4):2285–2294. doi: 10.4049/jimmunol.1000462. [DOI] [PubMed] [Google Scholar]
- 54.Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011 Dec;23(6):739–745. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke A. Th17 cells in inflammatory conditions. Rev Diabet Stud. 2006 Summer;3(2):72–75. doi: 10.1900/RDS.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009 Jun;58(6):1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dendrou CA, Wicker LS. The IL-2/CD25 pathway determines susceptibility to T1D in humans and NOD mice. J Clin Immunol. 2008 Nov;28(6):685–696. doi: 10.1007/s10875-008-9237-9. [DOI] [PubMed] [Google Scholar]
- 58.Herold KC, Brooks-Worrell B, Palmer J, Dosch HM, Peakman M, Gottlieb P, et al. Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes. 2009 Nov;58(11):2588–2595. doi: 10.2337/db09-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janson PC, Winerdal ME, Marits P, Thorn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One. 2008;3(2):e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagar M, Vernitsky H, Cohen Y, Dominissini D, Berkun Y, Rechavi G, et al. Epigenetic inheritance of DNA methylation limits activation-induced expression of FOXP3 in conventional human CD25-CD4+ T cells. Int Immunol. 2008 Aug;20(8):1041–1055. doi: 10.1093/intimm/dxn062. [DOI] [PubMed] [Google Scholar]
- 61.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007 Sep;37(9):2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 62.Tree TI, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the Immunology of Diabetes Society T Cell Workshop. Ann N Y Acad Sci. 2006 Oct;1079:9–18. doi: 10.1196/annals.1375.002. [DOI] [PubMed] [Google Scholar]
- 63.Petrich de Marquesini LG, Fu J, Connor KJ, Bishop AJ, McLintock NE, Pope C, et al. IFN-gamma and IL-10 islet-antigen-specific T cell responses in autoantibody-negative first-degree relatives of patients with type 1 diabetes. Diabetologia. 2010 Jul;53(7):1451–1460. doi: 10.1007/s00125-010-1739-3. [DOI] [PubMed] [Google Scholar]
- 64.Hughson A, Bromberg I, Johnson B, Quataert S, Jospe N, Fowell DJ. Diabetes. 2011 Jun 29; doi: 10.2337/db10-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008 May;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003 Jul 21;198(2):235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vuckovic S, Withers G, Harris M, Khalil D, Gardiner D, Flesch I, et al. Decreased blood dendritic cell counts in type 1 diabetic children. Clin Immunol. 2007 Jun;123(3):281–288. doi: 10.1016/j.clim.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Badami E, Sorini C, Coccia M, Usuelli V, Molteni L, Bolla AM, et al. Defective Differentiation of Regulatory FoxP3+ T Cells by Small-Intestinal Dendritic Cells in Patients With Type 1 Diabetes. Diabetes. 2011 Jun 6; doi: 10.2337/db10-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, et al. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010 Oct 1;120(10):3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012 Jan 10;10(1):3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009;4(1):e4226. doi: 10.1371/journal.pone.0004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 73.Wilczynski JR, Radwan M, Kalinka J. The characterization and role of regulatory T cells in immune reactions. Front Biosci. 2008;13:2266–2274. doi: 10.2741/2840. [DOI] [PubMed] [Google Scholar]
- 74.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003 Mar;51(2):59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 76.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007 Jan 22;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004 Jun 7;199(11):1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol. 2009 May;131(2):260–270. doi: 10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007 Sep 15;179(6):3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 81.Li Q, Nacion K, Bu H, Lin F. The complement inhibitor FUT-175 suppresses T cell autoreactivity in experimental autoimmune encephalomyelitis. Am J Pathol. 2009 Aug;175(2):661–667. doi: 10.2353/ajpath.2009.081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008 May 1;180(9):5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao X, Liu H, Ding G, Wang Z, Fu H, Ni Z, et al. Complement C3 deficiency prevent against the onset of streptozotocin-induced autoimmune diabetes involving expansion of regulatory T cells. Clin Immunol. 2011 Sep;140(3):236–243. doi: 10.1016/j.clim.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, et al. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood. 2008 Feb 15;111(4):2452–2461. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 85.Weaver DJ, Jr., Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010 Mar;40(3):710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin M, Yin N, Murphy B, Medof ME, Segerer S, Heeger PS, et al. Immune cell-derived c3 is required for autoimmune diabetes induced by multiple low doses of streptozotocin. Diabetes. 2010 Sep;59(9):2247–2252. doi: 10.2337/db10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holler E, Roncarolo MG, Hintermeier-Knabe R, Eissner G, Ertl B, Schulz U, et al. Prognostic significance of increased IL-10 production in patients prior to allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000 Feb;25(3):237–241. doi: 10.1038/sj.bmt.1702126. [DOI] [PubMed] [Google Scholar]
- 88.Baker KS, Roncarolo MG, Peters C, Bigler M, DeFor T, Blazar BR. High spontaneous IL-10 production in unrelated bone marrow transplant recipients is associated with fewer transplant-related complications and early deaths. Bone Marrow Transplant. 1999 Jun;23(11):1123–1129. doi: 10.1038/sj.bmt.1701780. [DOI] [PubMed] [Google Scholar]
- 89.Lin MT, Storer B, Martin PJ, Tseng LH, Gooley T, Chen PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003 Dec 4;349(23):2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 90.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000 Jul;106(1):145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004 Feb;113(3):451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Durinovic-Bello I, Schlosser M, Riedl M, Maisel N, Rosinger S, Kalbacher H, et al. Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 Type 1 diabetes. Diabetologia. 2004 Mar;47(3):439–450. doi: 10.1007/s00125-003-1315-1. [DOI] [PubMed] [Google Scholar]
- 93.Sanda S, Roep BO, von Herrath M. Islet antigen specific IL-10+ immune responses but not CD4+CD25+FoxP3+ cells at diagnosis predict glycemic control in type 1 diabetes. Clin Immunol. 2008 May;127(2):138–143. doi: 10.1016/j.clim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 2000 Dec;7(24):2099–2104. doi: 10.1038/sj.gt.3301334. [DOI] [PubMed] [Google Scholar]
- 95.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994 Apr 1;179(4):1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tai N, Yasuda H, Xiang Y, Zhang L, Rodriguez-Pinto D, Yokono K, et al. IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin Immunol. 2011 Jun;139(3):336–349. doi: 10.1016/j.clim.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Lee MS, Wogensen L, Shizuru J, Oldstone MB, Sarvetnick N. Pancreatic islet production of murine interleukin-10 does not inhibit immune-mediated tissue destruction. J Clin Invest. 1994 Mar;93(3):1332–1338. doi: 10.1172/JCI117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azar ST, Salti I, Zantout MS, Major S. Alterations in plasma transforming growth factor beta in normoalbuminuric type 1 and type 2 diabetic patients. J Clin Endocrinol Metab. 2000 Dec;85(12):4680–4682. doi: 10.1210/jcem.85.12.7073. [DOI] [PubMed] [Google Scholar]
- 99.Olivieri A, De Angelis S, Dionisi S, D'Annunzio G, Locatelli M, Marinaro M, et al. Serum transforming growth factor beta1 during diabetes development in non-obese diabetic mice and humans. Clin Exp Immunol. 2010 Dec;162(3):407–414. doi: 10.1111/j.1365-2249.2010.04253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA, Jr., et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002 Aug-Sep;19(1–2):9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 101.King C, Davies J, Mueller R, Lee MS, Krahl T, Yeung B, et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity. 1998 May;8(5):601–613. doi: 10.1016/s1074-7613(00)80565-8. [DOI] [PubMed] [Google Scholar]
- 102.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006 Mar;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008 Mar;149(3):1338–1349. doi: 10.1210/en.2007-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Tokui Y, Yamagata K, Kozawa J, Sayama K, Iwahashi H, et al. Continuous stimulation of human glucagon-like peptide-1 (7–36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia. 2007 Sep;50(9):1900–1909. doi: 10.1007/s00125-007-0737-6. [DOI] [PubMed] [Google Scholar]
- 105.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007 Nov;148(11):5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- 106.Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998 Feb;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 107.Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol. 2003 Jun;33(6):1519–1527. doi: 10.1002/eji.200323469. [DOI] [PubMed] [Google Scholar]
- 108.Reinhold D, Bank U, Buhling F, Lendeckel U, Faust J, Neubert K, et al. Inhibitors of dipeptidyl peptidase IV induce secretion of transforming growth factor-beta 1 in PWM-stimulated PBMC and T cells. Immunology. 1997 Jul;91(3):354–360. doi: 10.1046/j.1365-2567.1997.d01-2258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reinhold D, Bank U, Buhling F, Tager M, Born I, Faust J, et al. Inhibitors of dipeptidyl peptidase IV (DP IV, CD26) induces secretion of transforming growth factor-beta 1 (TGF-beta 1) in stimulated mouse splenocytes and thymocytes. Immunol Lett. 1997 Jun;58(1):29–35. doi: 10.1016/s0165-2478(97)02716-8. [DOI] [PubMed] [Google Scholar]
- 110.Preller V, Gerber A, Wrenger S, Togni M, Marguet D, Tadje J, et al. TGF-beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J Immunol. 2007 Apr 1;178(7):4632–4640. doi: 10.4049/jimmunol.178.7.4632. [DOI] [PubMed] [Google Scholar]
- 111.Tian L, Gao J, Hao J, Zhang Y, Yi H, O'Brien TD, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010 Jul;151(7):3049–3060. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- 112.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003 Sep;9(9):1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 113.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005 Oct;115(10):2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bisikirska BC, Herold KC. Use of anti-CD3 monoclonal antibody to induce immune regulation in type 1 diabetes. Ann N Y Acad Sci. 2004 Dec;1037:1–9. doi: 10.1196/annals.1337.001. [DOI] [PubMed] [Google Scholar]
- 115.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002 May 30;346(22):1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 116.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005 Jun 23;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 117.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010 Oct;40(10):2891–2901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gitelman SE, editor. American Diabetes Association's 71st Scientific Sessions. California: San Diego; Jun, 2011. Treatment of type 1 diabetes -- update on clinical trials; pp. 24–28. 2011. [Google Scholar]
- 119.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr., et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011 Aug 6;378(9790):487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009 Aug;132(2):166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006 Mar 15;107(6):2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005 Nov;11(11):1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 123.Wolf M, Schimpl A, Hunig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(−) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001 Jun;31(6):1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 124.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007 Feb;119(2):482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 125.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007 Mar;39(3):329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010 Feb;59(2):407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006 Sep 7;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 128.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009 Jun 18;113(25):6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 129.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002 Aug 1;100(3):1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 130.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005 Jun 15;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 131.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006 Dec 15;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 132.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, et al. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008 Sep;57(9):2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006 Jan;55(1):40–49. [PubMed] [Google Scholar]
- 134.Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- 135.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996 Apr 1;183(4):1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med. 1996 Dec;2(12):1348–1353. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- 137.Tisch R, Liblau RS, Yang XD, Liblau P, McDevitt HO. Induction of GAD65-specific regulatory T-cells inhibits ongoing autoimmune diabetes in nonobese diabetic mice. Diabetes. 1998 Jun;47(6):894–899. doi: 10.2337/diabetes.47.6.894. [DOI] [PubMed] [Google Scholar]
- 138.Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005 Jul-Aug;19(4):238–246. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Hjorth M, Axelsson S, Ryden A, Faresjo M, Ludvigsson J, Casas R. GAD-alum treatment induces GAD65-specific CD4+CD25highFOXP3+ cells in type 1 diabetic patients. Clin Immunol. 2011 Jan;138(1):117–126. doi: 10.1016/j.clim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008 Oct 30;359(18):1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 141.Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011 Jul 23;378(9788):319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krueger GG, Gottlieb AB, Sterry W, Korman N, Van De Kerkhof P. A multicenter, open-label study of repeat courses of intramuscular alefacept in combination with other psoriasis therapies in patients with chronic plaque psoriasis. J Dermatolog Treat. 2008;19(3):146–155. doi: 10.1080/09546630701846103. [DOI] [PubMed] [Google Scholar]
- 143.Menter A, Cather JC, Baker D, Farber HF, Lebwohl M, Darif M. The efficacy of multiple courses of alefacept in patients with moderate to severe chronic plaque psoriasis. J Am Acad Dermatol. 2006 Jan;54(1):61–63. doi: 10.1016/j.jaad.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 144.Krueger GG. Selective targeting of T cell subsets: focus on alefacept - a remittive therapy for psoriasis. Expert Opin Biol Ther. 2002 Apr;2(4):431–441. doi: 10.1517/14712598.2.4.431. [DOI] [PubMed] [Google Scholar]
- 145.Krueger GG, Callis KP. Development and use of alefacept to treat psoriasis. J Am Acad Dermatol. 2003 Aug;49(2 Suppl):S87–S97. doi: 10.1016/mjd.2003.552. [DOI] [PubMed] [Google Scholar]
- 146.da Silva AJ, Brickelmaier M, Majeau GR, Li Z, Su L, Hsu YM, et al. Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cells. J Immunol. 2002 May 1;168(9):4462–4471. doi: 10.4049/jimmunol.168.9.4462. [DOI] [PubMed] [Google Scholar]
- 147.Chamian F, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, et al. Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J Transl Med. 2007;5:27. doi: 10.1186/1479-5876-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001 Jul 26;345(4):248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 149.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009 Jul;15(7):746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jul 30;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, et al. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011 Aug 15;187(4):1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng Q, Xu Y, Liu Y, Zhang B, Li X, Guo F, et al. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J Mol Med (Berl) 2009 Dec;87(12):1191–1205. doi: 10.1007/s00109-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 153.Chilton PM, Rezzoug F, Fugier-Vivier I, Weeter LA, Xu H, Huang Y, et al. Flt3-ligand treatment prevents diabetes in NOD mice. Diabetes. 2004 Aug;53(8):1995–2002. doi: 10.2337/diabetes.53.8.1995. [DOI] [PubMed] [Google Scholar]
- 154.Morin J, Faideau B, Gagnerault MC, Lepault F, Boitard C, Boudaly S. Passive transfer of flt-3L-derived dendritic cells delays diabetes development in NOD mice and associates with early production of interleukin (IL)-4 and IL-10 in the spleen of recipient mice. Clin Exp Immunol. 2003 Dec;134(3):388–395. doi: 10.1111/j.1365-2249.2003.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O'Keeffe M, Brodnicki TC, Fancke B, Vremec D, Morahan G, Maraskovsky E, et al. Fms-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development. Int Immunol. 2005 Mar;17(3):307–314. doi: 10.1093/intimm/dxh210. [DOI] [PubMed] [Google Scholar]