Abstract
Intercalated cells in the collecting duct system express V-type H+-ATPases which participate in acid extrusion, bicarbonate secretion, and chloride absorption depending on the specific subtype. The activity of H+-ATPases is regulated by acid-base status and several hormones, including angiotensin II and aldosterone. Angiotensin II stimulates chloride absorption mediated by pendrin in type B intercalated cells and this process is energized by the activity of H+-ATPases. Moreover, angiotensin II stimulates bicarbonate secretion by the connecting tubule (CNT) and early cortical collecting duct (CCD). In the present study we examined the effect of angiotensin II (10 nM) on H+-ATPase activity and localization in isolated mouse connecting tubules and cortical collecting ducts. Angiotensin II stimulated Na+-independent intracellular pH recovery about 2-3 fold, and this was abolished by the specific H+-ATPase inhibitor concanamycin. The effect of angiotensin II was mediated through type 1 angiotensin II receptors (AT1-receptors) because it could be blocked by saralasin. Stimulation of H+-ATPase activity required an intact microtubular network - it was completely inhibited by colchicine. Immunocytochemistry of isolated CNT/CCDs incubated in vitro with angiotensin II suggests enhanced membrane associated staining of H+-ATPases in pendrin expressing intercalated cells. In summary, angiotensin II stimulates H+-ATPases in CNT/CCD intercalated cells, and may contribute to the regulation of chloride absorption and bicarbonate secretion in this nephron segment.
Key Words: Pendrin, Bicarbonate secretion, Chloride absorption
Introduction
Vacuolar type H+-ATPases are expressed along the entire nephron in various cell types and are involved in proton secretion into the urine thereby promoting bicarbonate reabsorption in the proximal tubule and thick ascending limb of Henle [1, 2]. In the segments of the collecting duct system (e.g. connectin tubule, cortical and medullary collecting ducts), H+-ATPases are abundant in intercalated cells and can be found at the luminal, basolateral, or both membranes depending on the subtype of intercalated cells [1, 3]. In type A intercalated cells, H+-ATPases are found at the luminal membrane secreting protons into urine and thereby energizing ammonium secretion and bicarbonate regeneration [1]. These cells are characterized by the additional presence of the basolateral chloride/bicarbonate exchanger AE1 [3, 4]. In contrast, in non-type A intercalated cells (i.e. type B and non-A/non-B intercalated cells), H+-ATPases can be localized at the luminal, basolateral or both membranes and act in concert with the apical chloride/bicarbonate exchanger pendrin [1, 3, 5]. Several subtypes of nontype A intercalated cells may exist but all forms express pendrin and vary with respect to their subcellular distribution of H+-ATPases [6, 7]. Non-type A intercalated cells mediate bicarbonate secretion (in the absence of luminal H+-ATPases) and reabsorb chloride (independently of the subcellular distribution of H+-ATPases).
The activity of intercalated cells is tightly regulated by a variety of factors including acid-base status, dietary electrolyte intake, and various hormones including angiotensin II, aldosterone, and endothelin [3]. Angiotensin II is a potent regulator of urinary acidification in vivo [8, 9, 10, 11] and has been shown by us and others to stimulate H+-ATPase activity in proximal tubule cells [12], type A intercalated cells in the outer medullary collecting ducts [13, 14], and renal cell lines [15, 16, 17]. Recent studies by Pech and Wall demonstrated that angiotensin II stimulates chloride absorption in isolated mouse cortical collecting ducts. Chloride absorption was dependent on the presence of pendrin and blocked by an H+-ATPase inhibitor suggesting that H+-ATPases in non-type A intercalated cells may also be stimulated by angiotensin II [18]. Along the same lines, angiotensin II enhances bicarbonate secretion in rabbit early cortical collecting ducts [19]. Taken together, these observations suggest that angiotensin II may have a direct stimulatory effect on H+-ATPases in type B intercalated cells and may thereby drive bicarbonate secretion and chloride absorption.
We examined in the present study whether angiotensin II directly stimulates H+-ATPase activity in isolated mouse cortical collecting duct intercalated cells and which receptor subtype may be involved. Our results reveal a potent stimulation of H+-ATPases by angiotensin II via AT1 receptors and suggest that stimulated H+-ATPases may be important for type B intercalated cell function.
Materials and Methods
Animals
Male C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME, USA), 12-14 weeks old, were kept under standard conditions with free access to food and water.
The generation, breeding, and genotyping of mice expressing eGFP under the control of the B1 H+-ATPase (ATP6V1B1) promoter has been described previously [20]. B1-eGFP mice were kindly provided by Dr. Lance Miller and Rauol Nelson, University of Utah, Salt Lake City, USA, and bred in Zurich.
The use of mice was according to local Animal Welfare Laws and approved by the Yale University Committee for the Use of Animals and the Zurich Veterinary Office (Kantonales Veterinäramt).
Preparation of isolated cortical collecting duct fragments and intracellular pH measurements
C57Bl/6J mice were sacrificed with pentobarbital (150 mg/ kg), both kidneys removed and transferred into ice-cold HEPES solution. After the removal of the capsula, vessels and pelvis, the kidneys were cut into slices 2-3 mm in thickness. The cortex was prepared under a stereo microscope (5x magnification) and the cortical slices were then incubated in a digestion solution (4 ml Minimum Essential Medium, 5 mM glycine, 6 mg/ml trypsin inhibitor, and 250 μg/ml collagenase (Sigma)) at 37 °C in a water bath for 15 min. After 15 min the digestion was stopped by transferring the tubules onto ice, gently removing the supernatant and replacing it with 4 ml ice-cold 1 % BSA HEPES solution. The BSA HEPES solution was replaced by ice-cold HEPES solution after 10 min and tubules kept on ice for the experiments. Cortical collecting ducts were selected under the preparation microscope and transferred to a perfusion chamber containing coverslips precoated with the cell-adhesive Cell-Tak (Becton-Dickinson). The temperature of the chamber was maintained at 37 ± 0.5 °C by an electronic feedback circuit. The control bath solution was initially a HEPES solution (sol. 1), flowing continuously at ∼ 3 ml/ min. The chamber volume was ∼ 180 μl. Single tubule fragments were loaded with the pH-sensitive dye 2’,7’-bis(2carboxylethyl)-5(6)-carboxyflourescein (BCECF) (10 μM) for 20 min at room temperature as described [12, 21, 22, 23]. pHi was measured microflourometrically by alternately exciting the dye with a 10 μm diameter spot of light at 440 and 490 nm while monitoring the emission at 532 nm [12, 21, 22, 23]. Each experiment was calibrated for pHi using the nigericin/ high K+ method [24] that converted the obtained ratios to pHi.
The solutions used are given in Table 1. H+-ATPase activity was measured as described previously [12, 14, 21, 22, 23, 25, 26, 27, 28]. Briefly, bicarbonate free solutions were used and Na+ removed to abolish Na+/H+ exchanger activity. For these experiments Na+ was replaced by equimolar amounts of NMDG (N-Methyl-D-Glucamine). To induce a strong intracellular acidification and elicit H+-ATPase activation, NH4Cl pulses were performed in the absence of Na+ as described previously [22, 29]. All chemicals were obtained from Sigma. Stimulators and inhibitors were added to the BCECF incubation solution and all other solutions at the concentrations stated.
Table 1.
Composition of solutions used for functional experiments.
| Standard | Na+-free | Na+-free | Na+ and K+-free | High K+ | |
|---|---|---|---|---|---|
| HEPES | HEPES | HEPES + NH4CI | HEPES | calibration | |
| NaCl | 125 | - | - | - | - |
| NMDG | – | 125 | 105 | 130 | 32.8 |
| NH4CI | – | – | 20 | – | – |
| KC1 | 3 | 3 | 3 | 3 | 105 |
| MgS04 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| CaCl2 | 1 | 1 | 1 | 1 | 1 |
| KH2P04 | 2 | 2 | 2 | 2 | – |
| Glucose | 5 | 5 | 5 | 5 | – |
| HEPES | 32.2 | 32.2 | 32.2 | 32.2 | 32.2 |
| pH | 7.4 | 7.4 | 7.4 | 7.4 | 6.0, 7.0, 8.0 |
NMDG is N-Methyl-D-Glucamine, all solutions were titrated to pH 7.4 at 37 °C using either NaOH or KOH. NMDG was titrated with HCl.
Immunostaining
Tubules were prepared as described above, eGFP positive CNT/CCD fragments collected under a dissection microscope equipped with a fluorescent lamp, and staining was performed as described previously [22]. Briefly, isolated tubules were transferred onto cover-slips precoated with Cell-Tak and allowed to adhere for 15 min. Next, isolated tubules were incubated with 10 nM Angiotensin II for 15 min at 37 °C and then fixed with 2% paraformaldehyde in PBS for 5 min at room temperature, tubules were washed 3 times with PBS, permeabilized with 0.1% Triton X, washed twice with PBS, treated with 1% SDS [30], washed 3 times with PBS, and incubated with PBS containing 1% bovine serum albumin for 15 min prior to addition of the primary antibodies. The primary antibodies were diluted in PBS and applied overnight at 4 °C: guinea-pig anti-mouse pendrin (1:2000 raw serum) [31], and rabbit anti-human ATP6V0B1 (B1) (1:500 whole serum) [22]. Tubules were then washed twice for 5 min with high-NaCl PBS (PBS+2.7% NaCl), once with PBS, and incubated with the secondary antibodies (goat anti-guinea-pig Alexa 647 1:500, donkey anti-rabbit Alexa 594 1:1000, Jackson ImmunoResearch Laboratories Europe Ltd., Suffolk, UK), and DAPI at a dilution of 1:500 for 1 h at room temperature. Tubules were again washed twice with high-NaCl PBS and once with PBS. Cover-slips were mounted with Glycergel mounting medium (DakoCytomation, Glostrup, Denmark). Slides were examined using a Leica SP2 confocal microscope (Zurich Center for Microscopy and Imaging), and the images assembled with Photoshop (Adobe, San Jose, Calif., USA) software.
Statistics
Data are presented as mean and standard error of the mean (SEM). All data were tested for statistical significance using unpaired student's t-test and results were considered significant if P < 0.05.
Results
Angiotensin II stimulates H+-ATPase activity
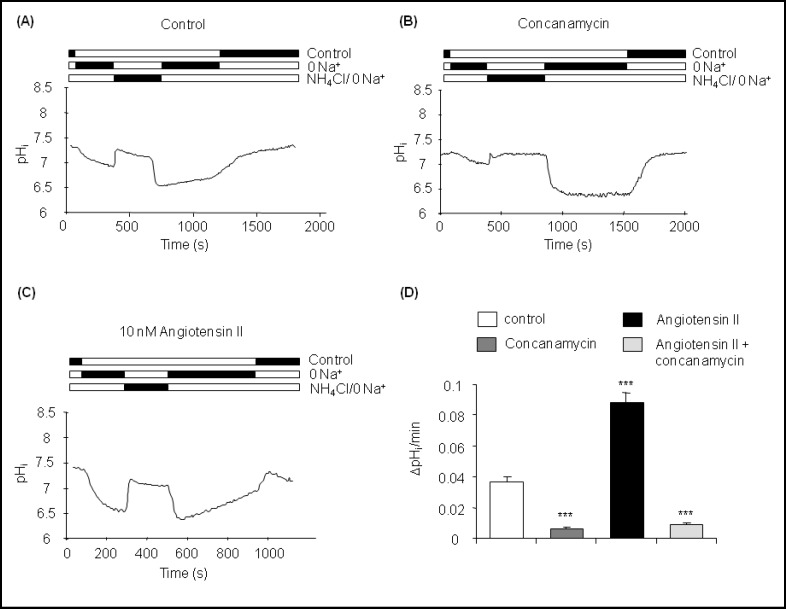
CNT-CCD intercalated cells had an initial intracellular pH (pHi) under control conditions of pH 7.29 ± 0.01 (n = 36 cells in 7 CNT-CCDs) (Table 2). Removal of Na+ from the bath caused a slow acidification of pHi to pH 6.90 ± 0.02 which was further acidified after removal of an NH4Cl (20 mM) load from the bath to pH 6.54 ± 0.04 (Fig. 1A). A slow alkalinization of 0.037 ± 0.003 units pH/ min. was observed in the nominal absence of Na+ and K+ from the bath. We and others have previously shown that this type of intracellular pH recovery under these conditions is mostly mediated by the activity of plasma membrane H+-ATPases [12, 13, 14, 21, 22, 23, 26, 27, 28].
Table 2.
Summary of functional data from pH measurements.
| Initial pHi | Na+- independent pH, recovery (ΔpH/min) | Na'-dependent pH, recovery (ΔpH/min) | Final pHi | Number Cells (tubules) | |
|---|---|---|---|---|---|
| Control | 7.29 ± 0.01 | 0.037 ± 0.003 | 0.184 ±0.012 | 7.24 ± 0.02 | 36(7) |
| Concanamycin | 7.31 ±0.01 | 0.006 ± 0.001 | 0.262 ±0.018 | 7.23 ± 0.02 | 53(5) |
| Angiotensin II | 7.27 ± 0.03 | 0.088 ± 0.007 | 0.351 ±0.031 | 7.26 ± 0.04 | 20(6) |
| Angiotensin II Concanamycin | 7.27 ± 0.01 | 0.009 ± 0.001 | 0.262 ± 0.039 | 7.23 ± 0.04 | 35(5) |
| Saralasin Angiotensin II | 7.29 ± 0.02 | 0.031 ±0.007 | 0.189 ±0.023 | 7.19 ±0.03 | 24(4) |
| Colchicine Angiotensin II | 7.33 ± 0.02 | 0.032 ± 0.002 | 0.119 ±0.055 | 7.18 ±0.02 | 22(4) |
Data are summarized as mean ± SEM. Shown are initial pHi in the presence of sodium, pHi recovery rates after removal of NH4Cl in the absence of sodium and after readdition of sodium to the bath, final pHi before calibration, and the number of intercalated cells and isolated CNT-CCDs investigated.
Fig. 1.
Angiotensin II stimulates H+-ATPase activity in CNT-CCD intercalated cells. (A-C) Original tracings of intracellular pH measurements in intercalated cells in isolated CNT-CCD fragments under control conditions (A), during incubation with the H+-ATPase inhibitor con-canamycin (100 nM) (B), and during exposure to 10 nM angiotensin II (C). The rate of intracellular pH recovery (alkalinization) after removal of NH4Cl from the bath and in the absence of sodium was analyzed. (D) Summary of Na+-independent pHi recovery rates. Mean ± SEM, ***indicates p < 0.001 compared to control.
Accordingly, preincubation with the H+-ATPase inhibitor concanamycin (100 nM) [32, 33, 34] significantly reduced the rate of Na+-independent pHi recovery to 0.006 ± 0.001 units pH/min. demonstrating that most of the alkalinization rate was due to H+-ATPase activity (Table 2, Fig. 1A and 1D). The small remaining rate may be mediated by H+/K+-ATPase activity [35, 36].
When the collecting tubules were preincubated with 10 nM angiotensin II for 15 min, the initial pH (7.27 ± 0.03), the extent of acidification after Na+ removal and after the NH4Cl pulse were not altered (data not shown). However, the rate of pHi recovery under nominally Na+ and K+ free conditions was significantly accelerated to 0.088 ± 0.007 pH units/ min, 2-3 times control rates (Table 2, Fig. 1C,D). Again, inhibition of H+-ATPase activity with concanamycin (100 nM) reduced the rate of pHi recovery to 0.009 ± 0.001 units pH/min demonstrating that increased H+-ATPase activity was responsible for the accelerated alkalinization in the presence of angiotensin II (Table 2, Fig. 1D).
Angiotensin II stimulates H+-ATPase via the AT1 receptor
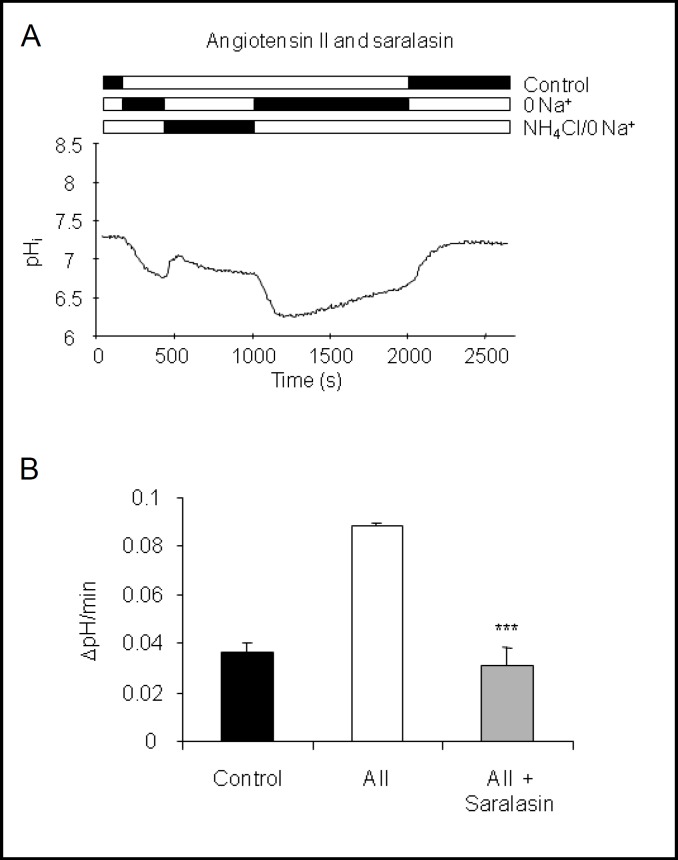
Bicabonate reabsorptipon and urinary acidification along the nephron and collecting duct is stimulated by angiotensin II involving the AT1 receptor subtype [13, 14, 17, 37, 38]. Similarly, AT1 receptors are involved in the stimulation of bicarbonate secretion by the initial cortical collecting duct by angiotensin II [19]. Thus, we tested whether angiotensin II stimulated H+-ATPase activity via AT 1 receptors in our preparation. Isolated CNT-CCDs were preincubated in vitro with the AT1 receptor antagonist saralasin (1 μM) for 5 min before angiotensin II (10 nM) was added. Preincubation with saralasin completely abolished the stimulatory effect of angiotensin II on H+-ATPase activity. The Na+-independent pHi recovery rate in the presence of saralasin and angiotensin II was 0.031 ± 0.007 units pH/min (Table 2, Fig. 2), not significantly different from control.
Fig. 2.
The AT1 receptor antagonist saralasin prevents the stimulation by angiotensin II. CNT-CCDs were preincubated with the AT1 receptor antagonist saralasin (1 μM) and incubated with 10 nM angiotensin II. (A) Original pHi tracing of an intercalated cell incubated with saralasin and angiotensin II. (B) Summary of pHi recovery rates of intercalated cells under control conditions, angiotensin II, and saralasin with angiotensin II. pHi recovery rates from control and angiotensin II are taken from Fig. 1 and shown for comparison. Mean ± SEM, ***indicates p < 0.001 compared to angiotensin II alone.
Angiotensin II stimulates the trafficking of H+-ATPases to the membrane
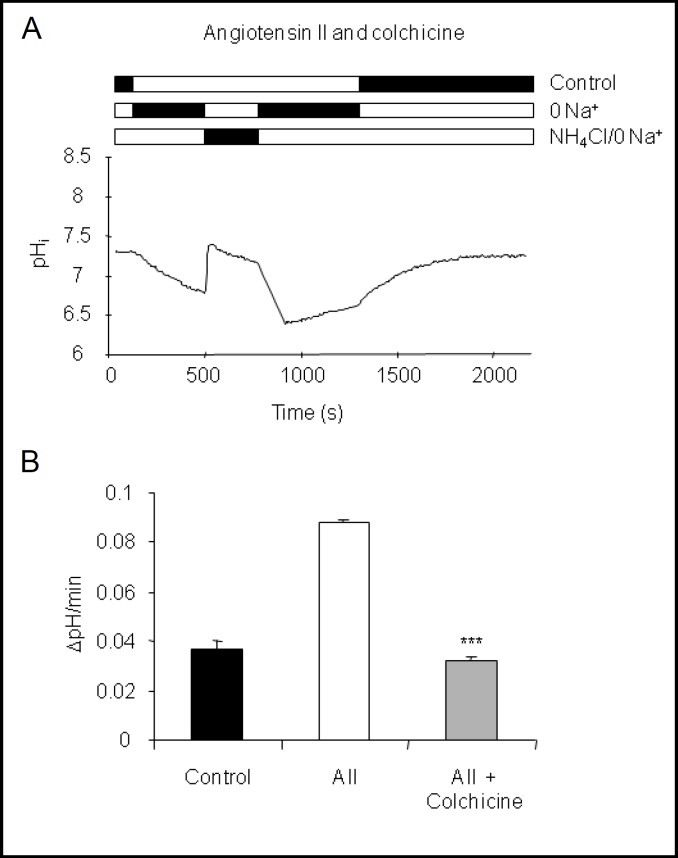
Stimulation of H+-ATPase activity in intercalated cells by various hormones, acid, or CO2 is at least in part mediated by trafficking and increased expression of H+-ATPases at the plasma membrane [1]. Disruption of the microtubular network with colchicine has been shown to prevent the increased expression and activity of H+-ATPases [12, 14, 23, 39, 40] in similar preparations. Here, preincubation of CNT-CCDs in vitro with colchicine (10 μM) for 20 min before application of 10 nM angiotensin II completely and significantly abolished the stimulatory effect of angiotensin II on H+-ATPase activity (0.032 ± 0.002 units pH/min) (Table 2, Fig. 3).
Fig. 3.
Stimulation of H+-ATPase activity requires an intact microtubular network. Isolated CNT-CCDs were preincubated with colchicine (20 μM) for 20 min prior to stimulation with 10 nM angiotensin II. (A) Original pHi tracing of an intercalated cell incubated with colchicine and angiotensin II. (B) Summary of pHi recovery rates of intercalated cells under control conditions, angiotensin II, and colchicine with angiotensin II. pHi recovery rates from control and angiotensin II are taken from Fig. 1 and shown for comparison. Mean ± SEM, ***indicates p < 0.001 compared to angiotensin II alone.
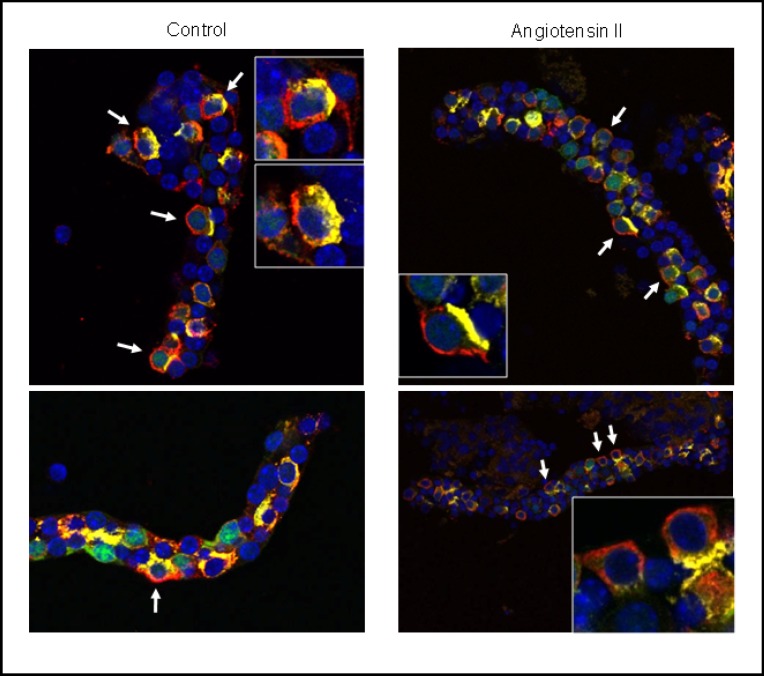
We performed additional experiments with CNT-CCDs from mice expressing eGFP under the promoter of the B1 H+-ATPase subunit to facilitate identification of the respective segments. CNT-CCDs were incubated in vitro with angiotensin II and stained with antibodies against the B1 H+-ATPase subunit and pendrin (Fig. 4). In control CNT/CCDs, localization of the B1 H+-ATPase in pendrin positive cells was mostly at the basolateral pole of cells with dispersed staining at the membrane and in the cytosol. In CNT/CCDs incubated in vitro with angiotensin II, staining for the B1 H+-ATPase appeared to be more membrane associated whereas no difference in pendrin staining was noted consistent with previous experiments [18].
Fig. 4.
Immunolocalization of H+-ATPases in pendrin expressing CNT/ CCD intercalated cells. CNT-CCDs expressing eGFP (green) in intercalated cells were incubated without (left panel) or with 10 nM angiotensin II (right panel) and stained with antibodies against the B1 H+-ATPase subunit (red) and pendrin (yellow). Nuclei were visualized with DAPI (blue). Inserts show higher magnification of selected intercalated cells, arrows point to intercalated cells with typical patterns of H+-ATPase staining. Original magnification 400 x.
Discussion
Angiotensin II is a potent regulator of blood pressure and acid-base homeostasis. Angiotensin II acts on blood pressure by inducing vasoconstriction and stimulating renal NaCl reabsorption in the proximal tubule, distal convoluted tubule, and collecting duct system [37, 41, 42]. In addition, angiotensin II has multiple effects on renal acid-base handling. Angiotensin II stimulates bicarbonate reabsorption by the proximal tubule, the thick ascending limb of the loop of Henle, and the distal convoluted tubule [10, 11, 43, 44, 45, 46, 47, 48]. In the late cortical collecting duct and outer medullary collecting duct, angiotensin II enhances proton secretion by type A intercalated cells [13, 14, 19]. Moreover, angiotensin II also increases luminal alkalinization in the connecting tubule and early cortical collecting duct and directly stimulates chloride absorption by type B intercalated cells [18, 19]. Moreover, stimulation of pendrin activity may also indirectly enhance electroneutral NaCl reabsorption via the recently described Na+-driven Cl−/HCO3− exchanger (NDCBE/ SLC4A8) [49].
Here we demonstrate that angiotensin II in the physiological range [42, 50] stimulates the activity of H+-ATPases in intercalated cells in isolated mouse CNT/ CCDs. Angiotensin II most likely acts via AT1 receptors since the AT1 receptor antagonist saralasin completely abolished the effect. The distribution and localization of angiotensin II receptors along the nephron is not completely documented but AT1 receptors may be present both on luminal and basolateral membranes of almost all cells along the collecting duct system as evident from various functional experiments and immunolocalization studies [9, 13, 14, 18, 19, 37, 38, 41, 42, 44, 50, 51, 52].
Angiotensin II acting in the collecting duct may come from various sources including circulating and filtered angiotensin II, angiotensin II produced in the proximal tubule, as well as angiotensin II activated locally in the collecting duct [53]. Rohrwasser et al. demonstrated the existence of all major components of a local renin-angiotensin system in the collecting duct [52] including angiotensin-converting enzyme (ACE).
Regulation of H+-ATPase activity may involve various mechanisms including assembly and disassembly of proton pumps, trafficking of pumps into the membrane, and changes in the ATP: H+-pumping coupling ratio [1, 54, 55]. In type A intercalated cells, angiotensin II stimulates H+-ATPases by a process that requires an intact microtubular network sensitive to colchicine [14] and leads to an accumulation of H+-ATPases at the luminal membrane [13]. Similarly, many other hormones or stimuli increase H+-ATPase trafficking and abundance at the membrane in the collecting duct, along the nephron, or in similar preparations [12, 23, 26, 39, 56, 57, 58]. Colchicine prevented the stimulation of H+-ATPase activity by angiotensin II in the CNT/CCD intercalated cells suggesting a similar mechanism. Immunocytochemistry of isolated mouse CNT/CCD fragments stimulated in vitro with angiotensin II suggested a more membrane associated H+-ATPase staining in cells expressing pendrin consistent with retention or increased trafficking of pumps into the membrane and enhanced pump activity. The stimulated H+-ATPase activity would then energize enhanced chloride absorption or luminal alkalinization via pendrin as observed in earlier studies [18, 19].
In summary, angiotensin II stimulates H+-ATPase activity in isolated mouse CNT/CCD intercalated cells involving AT1 receptors and leading to enhanced membrane abundance of H+-ATPases. Increased H+-ATPase activity is a prerequisite for enhancing pendrin activity by altering driving forces for bicarbonate secretion and chloride absorption.
Acknowledgements
This study was supported by NIH grants HD40793 to S. Breton, DK42956 to D. Brown, and DK-17433 to J.P. Geibel and the Swiss National Science Foundation (3100-068318 and 31003A-122217) to C.A. Wagner. C.A. Wagner was a Feodor Lynen fellow of the Humboldt foundation, Germany.
References
- 1.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 2.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988;82:2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflugers Arch. 2009;458:137–156. doi: 10.1007/s00424-009-0657-z. [DOI] [PubMed] [Google Scholar]
- 4.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA. 1989;86:5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol. 1999;10:1–12. doi: 10.1681/ASN.V1011. [DOI] [PubMed] [Google Scholar]
- 7.Teng-umnuay P, Verlander JW, Yuan W, Tisher CC, Madsen KM. Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. J Am Soc Nephrol. 1996;7:260–274. doi: 10.1681/ASN.V72260. [DOI] [PubMed] [Google Scholar]
- 8.Henger A, Tutt P, Riesen WF, Hulter HN, Krapf R. Acid-base and endocrine effects of aldosterone and angiotensin II inhibition in metabolic acidosis in human patients. J Lab Clin Med. 2000;136:379–389. doi: 10.1067/mlc.2000.110371. [DOI] [PubMed] [Google Scholar]
- 9.Levine DZ, Iacovitti M, Buckman S, Harrison V. In vivo modulation of rat distal tubule net HCO3 flux by VIP, iso-proterenol, angiotensin II, and ADH. Am J Physiol. 1994;266:F878–883. doi: 10.1152/ajprenal.1994.266.6.F878. [DOI] [PubMed] [Google Scholar]
- 10.Levine DZ, Iacovitti M, Buckman S, Burns KD. Role of angiotensin II in dietary modulation of rat late distal tubule bicarbonate flux in vivo. J Clin Invest. 1996;97:120–125. doi: 10.1172/JCI118378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine DZ, Iacovitti M, Buckman S, Hincke MT, Luck B, Fryer JN. Ang II-dependent HCO3- reabsorption in surviving rat distal tubules: Expression/activation of H+-ATPase. Am J Physiol. 1997;272:F799–808. doi: 10.1152/ajprenal.1997.272.6.F799. [DOI] [PubMed] [Google Scholar]
- 12.Wagner CA, Giebisch G, Lang F, Geibel JP. Angiotensin II stimulates vesicular H+-ATPase in rat proximal tubular cells. Proc Natl Acad Sci USA. 1998;95:9665–9668. doi: 10.1073/pnas.95.16.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol. 2007;18:2085–2093. doi: 10.1681/ASN.2006070753. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira-Souza M, Malnic G, Mello-Aires M. Atrial natriuretic peptide impairs the stimulatory effect of angiotensin II on H+-ATPase. Kidney Int. 2002;62:1693–1699. doi: 10.1046/j.1523-1755.2002.00604.x. [DOI] [PubMed] [Google Scholar]
- 16.Carraro-Lacroix LR, Girardi AC, Malnic G. Long-term regulation of vacuolar H+-atpase by angiotensin II in proximal tubule cells. Pflugers Arch. 2009;458:969–979. doi: 10.1007/s00424-009-0668-9. [DOI] [PubMed] [Google Scholar]
- 17.Carraro-Lacroix LR, Malnic G. Signaling pathways involved with the stimulatory effect of angiotensin II on vacuolar H+-ATPase in proximal tubule cells. Pflugers Arch. 2006;452:728–736. doi: 10.1007/s00424-006-0085-2. [DOI] [PubMed] [Google Scholar]
- 18.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]
- 19.Weiner ID, New AR, Milton AE, Tisher CC. Regulation of luminal alkalinization and acidification in the cortical collecting duct by angiotensin II. Am J Physiol. 1995;269:F730–738. doi: 10.1152/ajprenal.1995.269.5.F730. [DOI] [PubMed] [Google Scholar]
- 20.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of eGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005;288:C1134–1144. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- 21.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1 subunit of the H+ATPase is required for maximal urinary acidification. Proc Nat Acad Sci USA. 2005;102:13616–13621. doi: 10.1073/pnas.0506769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner CA, Lukewille U, Valles P, Breton S, Brown D, Giebisch GH, Geibel JP. A rapid enzymatic method for the isolation of defined kidney tubule fragments from mouse. Pflugers Arch. 2003;446:623–632. doi: 10.1007/s00424-003-1082-3. [DOI] [PubMed] [Google Scholar]
- 23.Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Nat Acad Sci USA. 2004;101:2636–2641. doi: 10.1073/pnas.0307321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 25.Lang K, Wagner CA, Haddad G, Burnekova O, Geibel J. Intracellular pH activates membrane-bound Na+/H+ exchanger and vacuolar H+-ATPase in human embryonic kidney (HEK) cells. Cell Physiol Biochem. 2003;13:257–262. doi: 10.1159/000074540. [DOI] [PubMed] [Google Scholar]
- 26.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, micro-villar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol. 2010;298:F643–654. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paunescu TG, Russo LM, Da Silva N, Kovacikova J, Mohebbi N, Van Hoek AN, McKee M, Wagner CA, Breton S, Brown D. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am J Physiol Renal Physiol. 2007;293:F1915–1926. doi: 10.1152/ajprenal.00160.2007. [DOI] [PubMed] [Google Scholar]
- 28.Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol. 2009;20:1705–1713. doi: 10.1681/ASN.2008111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 30.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS) Histochem Cell Biol. 1996;105:261–267. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 31.Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl- excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol. 2008;295:C1658–1667. doi: 10.1152/ajpcell.00419.2008. [DOI] [PubMed] [Google Scholar]
- 32.Dröse S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Dröse S, Boddien C, Gassel M, Ingenhorst G, Zeeck A, Altendorf K. Semisynthetic derivatives of concanamycin A and C, as inhibitors of V- and P-type ATPases: Structure-activity investigations and developments of photoaffinity probes. Biochem. 2001;40:2816–2825. doi: 10.1021/bi001759q. [DOI] [PubMed] [Google Scholar]
- 34.Huss M, Ingenhorst G, Konig S, Gassel M, Drose S, Zeeck A, Altendorf K, Wieczorek H. Concanamycin A, the specific inhibitor of V-ATPases, binds to the Vo subunit c. J Biol Chem. 2002;277:40544–40548. doi: 10.1074/jbc.M207345200. [DOI] [PubMed] [Google Scholar]
- 35.Younes-Ibrahim M, Barlet-Bas C, Buffin-Meyer B, Cheval L, Rajerison R, Doucet A. Ouabain-sensitive and -insensitive K-ATPases in rat nephron: Effect of K depletion. Am J Physiol. 1995;268:F1141–1147. doi: 10.1152/ajprenal.1995.268.6.F1141. [DOI] [PubMed] [Google Scholar]
- 36.Silver RB, Soleimani M. H+-K+-ATPases: Regulation and role in pathophysiologi-cal states. Am J Physiol. 1999;276:F799–811. doi: 10.1152/ajprenal.1999.276.6.F799. [DOI] [PubMed] [Google Scholar]
- 37.Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31S–38S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 38.Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:1658–1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 39.Brown D, Sabolic I, Gluck S. Colchicine-induced redistribution of proton pumps in kidney epithelial cells. Kidney Int. 1991;Suppl 33:S79–83. [PubMed] [Google Scholar]
- 40.Tsuruoka S, Schwartz GJ. Adaptation of the outer medullary collecting duct to metabolic acidosis in vitro. Am J Physiol. 1998;275:F982–990. doi: 10.1152/ajprenal.1998.275.6.F982. [DOI] [PubMed] [Google Scholar]
- 41.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock D G, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 42.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–195. [PubMed] [Google Scholar]
- 43.Capasso G, Unwin R, Ciani F, De Santo N G, De Tommaso G, Russo F, Giebisch G. Bicarbonate transport along the loop of Henle. II. Effects of acid-base, dietary, and neurohumoral determinants. J Clin Invest. 1994;94:830–838. doi: 10.1172/JCI117403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barreto-Chaves ML, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol. 1996;271:F977–984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 45.Baum M, Quigley R, Quan A. Effect of luminal angiotensin II on rabbit proximal convoluted tubule bicarbonate absorption. Am J Physiol. 1997;273:F595–600. doi: 10.1152/ajprenal.1997.273.4.F595. [DOI] [PubMed] [Google Scholar]
- 46.du Cheyron D, Chalumeau C, Defontaine N, Klein C, Kellermann O, Paillard M, Poggioli J. Angiotensin II stimulates NHE3 activity by exocytic insertion of the transporter: Role of PI 3-kinase. Kidney Int. 2003;64:939–949. doi: 10.1046/j.1523-1755.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 47.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci USA. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu FY, Cogan MG. Angiotensin II: A potent regulator of acidification in the rat early proximal convoluted tubule. J Clin Invest. 1987;80:272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger Slc4a8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–1158. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 51.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT 2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 52.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 53.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest. 1996;97:2878–2882. doi: 10.1172/JCI118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forgac M. Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 55.Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflugers Arch. 2009;457:589–598. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- 56.Cannon C, van Adelsberg J, Kelly S, Al-Awqati Q. Carbon-dioxide-induced exocytotic insertion of H+ pumps in turtle-bladder luminal membrane: Role of cell pH and calcium. Nature. 1985;314:443–446. doi: 10.1038/314443a0. [DOI] [PubMed] [Google Scholar]
- 57.Gluck S, Cannon C, Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci USA. 1982;79:4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest. 1985;75:1638–1644. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]