Abstract
A 59-year-old man with a history of fever, unsteadiness, hemiparesis, motor aphasia and consciousness disturbance was hospitalized for Streptococcus equi subsp. zooepidemicus meningitis. He denied contact with farm animals, but had a practice of consuming unpasteurized goats’ cheese from an uncertain source.
Introduction
Streptococcus equi subsp. zooepidemicus is an uncommon human pathogen that belongs to the Lancefield group C streptococci. This organism most often causes respiratory and various secondary infections in horses, mastitis in cows, lymphadenitis in guinea pigs and jaw abscesses in swine (Salata et al., 1989). Group C streptococci also include S. equi subsp. equi, S. equi subsp. ruminatorum, Streptococcus dysgalactiae subsp. dysgalactiae and S. dysgalactiae subsp. equisimilis (Rajasekhar & Clancy, 2010).
Group C streptococci have been reported to cause central nervous system infection, including meningitis, brain abscess and subdural empyema (Salata et al., 1989), most often in people over 70 years of age or neonates, and the condition is rarely reported in young adults (Jovanović et al., 2008). The majority of reported cases are from the UK, Europe and North America (Eyre et al., 2010). To our knowledge, this is the first report describing an individual in South America with no known risk factors who developed otitis media complicated by mastoiditis and meningitis secondary to S. equi subsp. zooepidemicus.
Case report
A 59-year-old right-handed male, a shoe seller by profession, with a history of hypertension and ischaemic stroke 11 years prior, presented to an emergency department (at the Hospital Nacional Daniel A. Carrion) in Callao, Lima, complaining of an earache in the right ear, headache, fever and altered mental status. The symptoms developed insidiously 2 weeks prior to presentation, and were characterized by flu symptoms without fever that lasted 3 days, followed by an earache in the right ear with whitish discharge and no disturbance of consciousness. The patient was assessed by an otorhinolaryngologist, who prescribed cefaclor (a second generation cephalosporin) (500 mg every 8 h for 14 days) and non-steroidal anti-inflammatories (NSAIDs) (550 mg naproxen sodium every 12 h for 7 days) for suspected mastoiditis; however, 4 days after antibiotic treatment was started, otalgia and right purulent otorrhoea continued, and the patient’s symptoms worsened, with him developing unsteadiness, fever, right hemiparesis and motor aphasia. He was admitted to the hospital (Hospital Nacional Daniel A. Carrion), where he had generalized tonic–clonic seizures and delirium leading to loss of consciousness.
On admission, the patient’s vital signs were unremarkable and he was afebrile. Physical examination revealed neck stiffness and right middle-ear effusion. Cardiac, respiratory and abdominal examinations were normal. The patient scored a 6 on the Glasgow coma scale. Notable findings on neurological assessment included anisocoria, bilateral Babinski sign and increased deep tendon reflexes. The patient’s peripheral white blood cell count was 25 700 cells mm−3 (87.5 % neutrophils, 3.7 % lymphocytes, 8.7 % monocytes), and his erythrocyte sedimentation rate was 60 mm h−1. His serum fibrinogen level was 691 mg dl−1. Cerebrospinal fluid (CSF) was cloudy and yellow in colour, and contained 1.08 leukocytes ml−1 (91 % neutrophils), but examination of a gram-stained smear demonstrated no organisms. His CSF glucose level was 47 mg dl−1; his concurrent plasma glucose level was 171 mg dl−1. His total CSF protein level was 129 mg dl−1. Computed tomography of the head demonstrated right-sided mastoiditis with no evidence of extension into the adjacent brain parenchyma (Fig. 1).
Fig. 1.
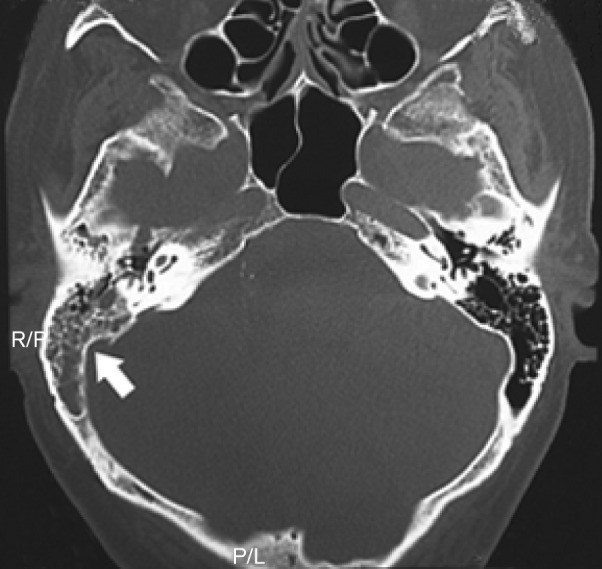
Computed tomography scan showing findings consistent with right-sided mastoiditis (indicated by the arrow). No evidence of extension of the mastoiditis into the adjacent brain parenchyma was seen in the present image. No evidence of petrositis was seen.
On arrival, empiric therapy (2 g ceftriaxone every 12 h and 1 g vancomycin every 12 h, both for 4 weeks) was begun. The patient was comatose during the first 5 days of treatment. On the 9th hospital day the patient developed ventilator-associated pneumonia and the antibiotic regimen was changed to intravenous meropenem (1g every 8 h) for 21 days. His admission CSF culture became positive, yielded a penicillin-sensitive group C β-haemolytic streptococcus, which was later identified as S. equi subsp. zooepidemicus by API-20 Strep test (bioMérieux). Antimicrobial susceptibility to penicillin G, gentamicin, tetracycline, ampicillin, azithromycin, ceftriaxone, trimethoprim–sulfamethoxazole, erythromycin, vancomycin and levofloxacin in the group C β-haemolytic streptococcus was determined by the agar dilution method, as described by the Clinical and Laboratory Standards Institute guidelines (CLSI, 2012), using Mueller–Hinton agar (BBL Sensi-Disc; BD) supplemented with 5 % defibrinated sheep blood as culture media in an atmosphere of 5 % CO2. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as quality controls. Blood and urine cultures were negative.
Discussion
Contact with animals and consumption of animal products are risk factors for infection with S. equi subsp. zooepidemicus. This organism is part of the natural gastrointestinal flora of animals including horses, pigs, sheep, cows, goats, foxes, birds, rabbits, guinea pigs and monkeys (Barnham et al., 1987). Our patient denied contact with animals, but reported having consumed unpasteurized goat’s cheese from an uncertain source, a common practice in Peru. Given that consumption of unpasteurized dairy products has been reported in one third of cases worldwide, we believe this was the probable source of infection for our patient (Eyre et al., 2010; Jovanović et al., 2008).
Meningitis secondary to S. equi subsp. zooepidemicus has a mortality rate of approximately 24 %; estimated mortality rates associated with group C streptococcal bacteraemia range from 25 to 40 %, with the highest rates reported in the elderly (Berenguer et al., 1992; Bradley et al., 1991). Among survivors, only 38 % typically make a complete recovery (Eyre et al., 2010). For all previously reported cases for which antibiotic therapy was specified, treatment regimens have included benzylpenicillin and a third-generation cephalosporin. Our patient received a 28 day course of ceftriaxone; however, beginning on the 9th day of hospitalization, meropenem was also administered because the patient developed a ventilator-associated pneumonia. Hearing loss in the right ear persisted 6 months after the patient’s initial presentation for care; hearing loss is reported as a complication in 19 % of cases (Eyre et al., 2010).
To our knowledge, there are only 20 reported cases of meningitis due to S. equi subsp. zooepidemicus (Eyre et al., 2010), and this is the third report of otitis complicated by mastoiditis and secondary meningitis caused by S. equi subsp. zooepidemicus worldwide (Minces et al., 2011), and the first in Peru. Clinicians should consider group C streptococcus in the differential diagnosis of bacterial meningitis, especially in patients who have consumed unpasteurized dairy products or who have mastoiditis accompanying meningitis.
Acknowledgements
We thank Nilda Gadea for performing the API 20 test. This work was funded by US Department of Defense's Global Emerging Infection Systems (DoD-GEIS), a division of the Armed Forces Health Surveillance Center (AFHSC), award number C0476_11_L, and the National Institutes of Health Fogarty International Center, award number RO1NS55627 to Joseph R. Zunt. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. The study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Lieutenant Commander Tilley is a military service member and Dr Montano is an employee of the US Government. This work was prepared as part of their official duties.
Abbreviations:
- CSF
cerebrospinal fluid
References
- Barnham M., Ljunggren A., McIntyre M. (1987). Human infection with Streptococcus zooepidemicus (Lancefield group C): three case reports. Epidemiol Infect 98, 183–190 10.1017/S0950268800061896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer J., Sampedro I., Cercenado E., Baraia J., Rodríguez-Créixems M., Bouza E. (1992). Group-C β-hemolytic streptococcal bacteremia. Diagn Microbiol Infect Dis 15, 151–155 10.1016/0732-8893(92)90040-Z [DOI] [PubMed] [Google Scholar]
- Bradley S. F., Gordon J. J., Baumgartner D. D., Marasco W. A., Kauffman C. A. (1991). Group C streptococcal bacteremia: analysis of 88 cases. Rev Infect Dis 13, 270–280 10.1093/clinids/13.2.270 [DOI] [PubMed] [Google Scholar]
- CLSI (2012). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard, 8th edn, M11–A8. Wayne, PA: Clinical and Laboratory Standards Institute; [PubMed] [Google Scholar]
- Eyre D. W., Kenkre J. S., Bowler I. C., McBride S. J. (2010). Streptococcus equi subspecies zooepidemicus meningitis–a case report and review of the literature. Eur J Clin Microbiol Infect Dis 29, 1459–1463 10.1007/s10096-010-1037-5 [DOI] [PubMed] [Google Scholar]
- Jovanović M., Stevanović G., Tosić T., Stosović B., Zervos M. J. (2008). Streptococcus equi subsp. zooepidemicus meningitis. J Med Microbiol 57, 373–375 10.1099/jmm.0.47487-0 [DOI] [PubMed] [Google Scholar]
- Minces L. R., Brown P. J., Veldkamp P. J. (2011). Human meningitis from Streptococcus equi subsp. zooepidemicus acquired as zoonoses. Epidemiol Infect 139, 406–410 10.1017/S0950268810001184 [DOI] [PubMed] [Google Scholar]
- Rajasekhar A., Clancy C. J. (2010). Meningitis due to group C streptococcus: a case report and review of the literature. Scand J Infect Dis 42, 571–578 10.3109/00365541003754428 [DOI] [PubMed] [Google Scholar]
- Salata R. A., Lerner P. I., Shlaes D. M., Gopalakrishna K. V., Wolinsky E. (1989). Infections due to Lancefield group C streptococci. Medicine (Baltimore) 68, 225–239 [DOI] [PubMed] [Google Scholar]


