Abstract
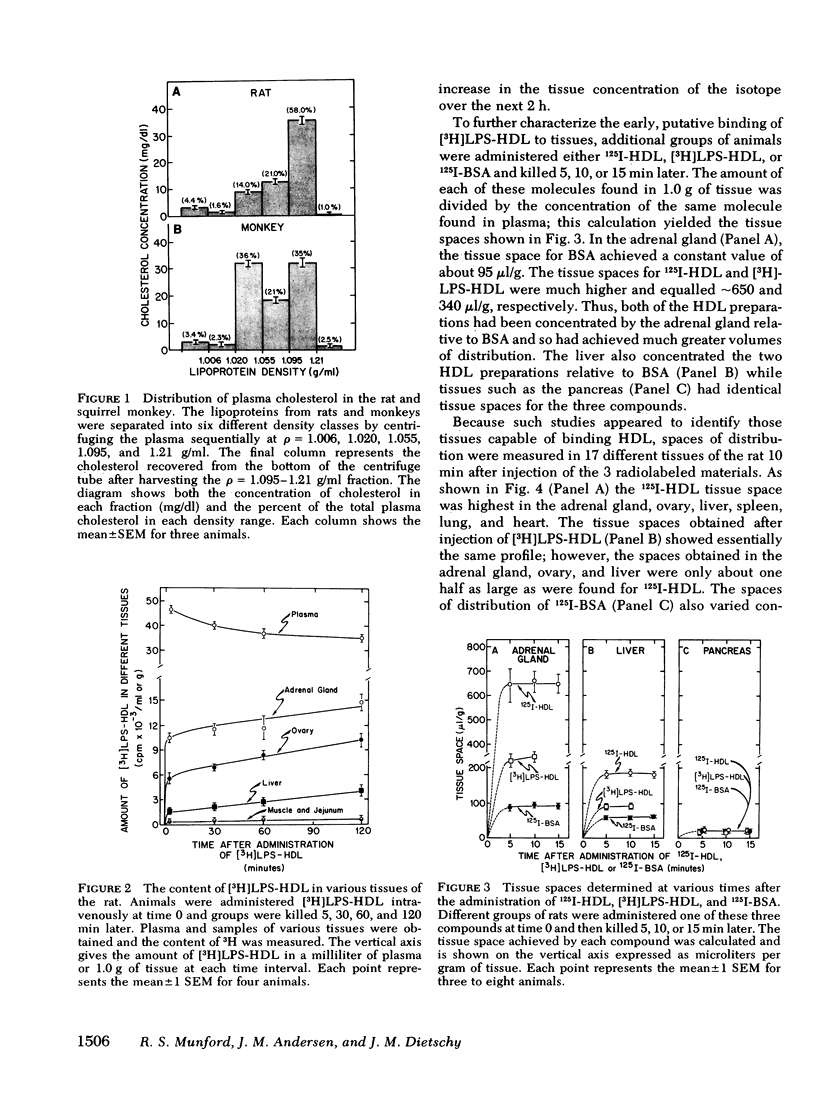
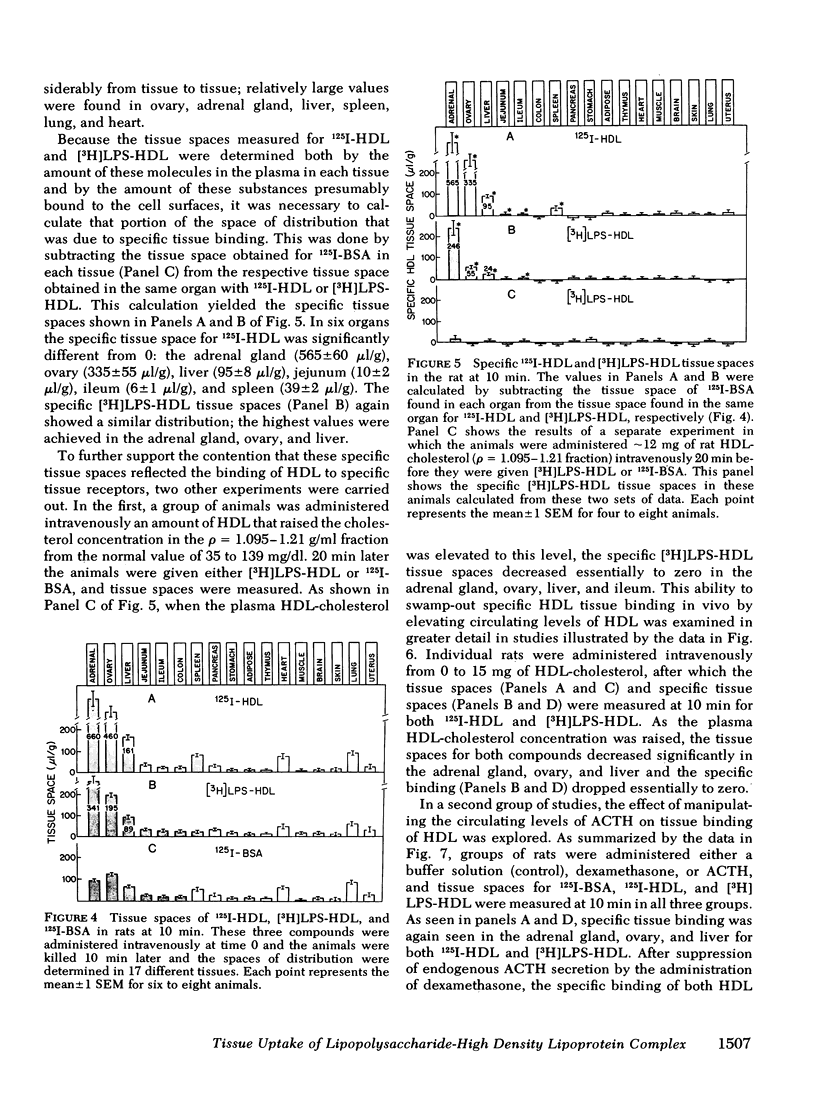
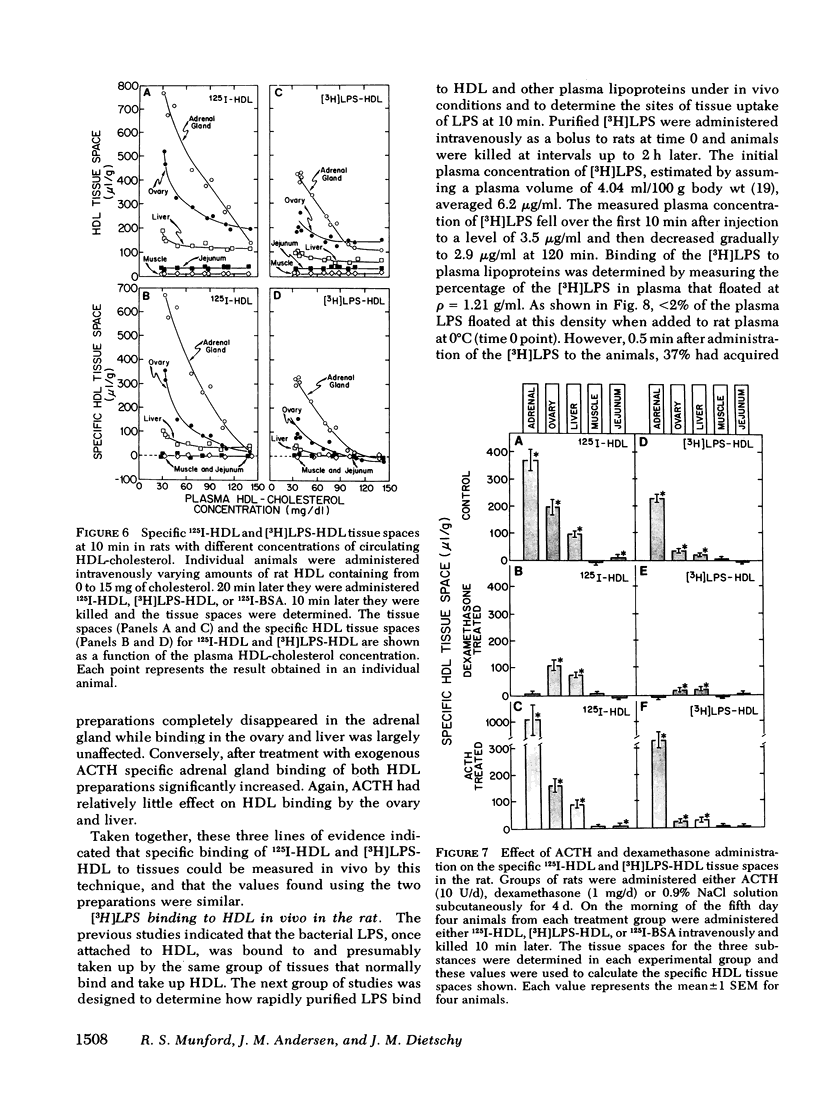
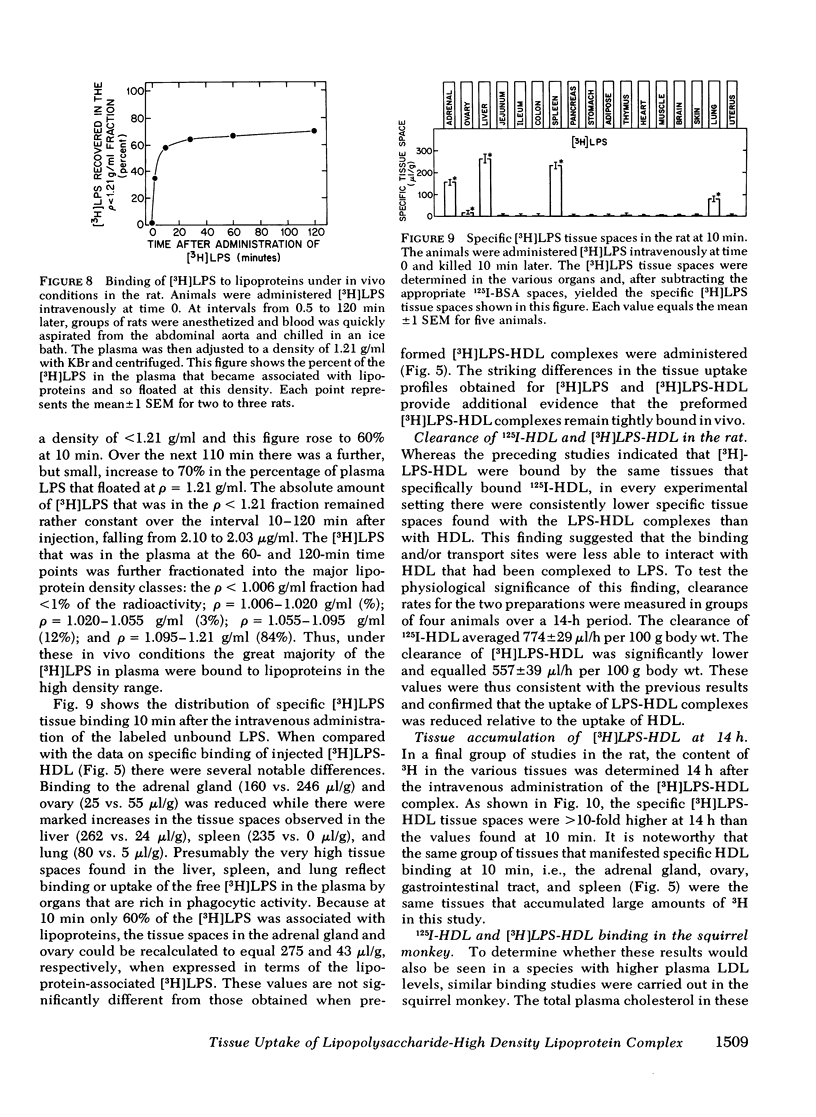
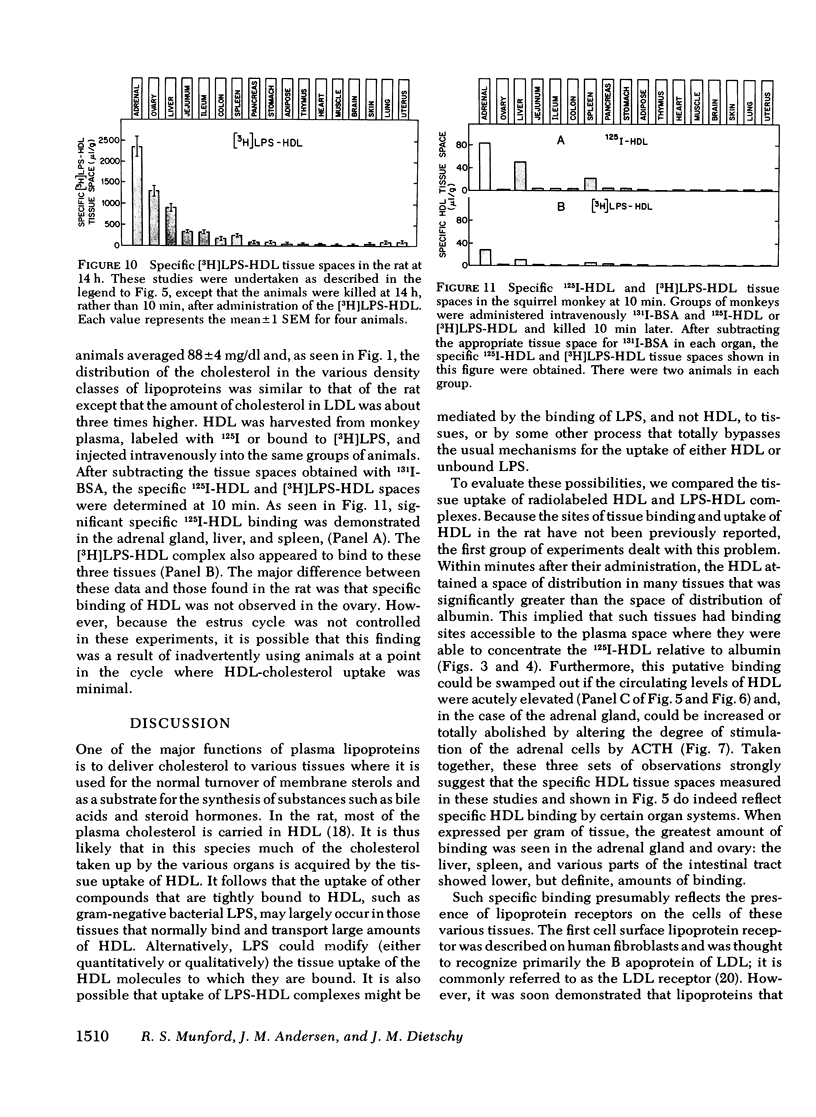
When gram-negative bacterial lipopolysaccharides (LPS) are injected intravenously into the rabbit or rat, they bind to plasma lipoproteins, particularly high density lipoproteins (HDL). The present studies were performed to examine the mechanisms by which LPS-HDL complexes are removed from the circulation and taken up by various tissues. Our approach was to compare the sites of specific tissue binding and uptake of HDL and of LPS-HDL complexes in the rat and squirrel monkey. In the rat, binding of homologous 125I-HDL was demonstrated principally in the adrenal gland, ovary, liver, and spleen. [3H]LPS-HDL complexes (produced in vitro by incubating Salmonella typhimurium [3H]LPS with rat HDL and lipoprotein-free plasma) bound to the same tissues, but with apparently lower affinities. The specificity of binding of both 125I-HDL and [3H]LPS-HDL to these organs was demonstrated in two ways. First, tissue binding of both radiolabeled preparations was swamped out by raising the circulating levels of HDL-cholesterol from 32 to 140 mg/dl. Second, treatment of the animals with dexamethasone abolished specific binding of both HDL preparations to the adrenal gland while administration of adrenocorticotropin increased the specific adrenal binding of the two preparations. The steady-state plasma clearance rate for 125I-HDL equaled 774 +/- 29 microliters/h and was significantly lower (557 +/- 39 microliters/h) for the LPS-HDL complex, a finding that presumably reflected the lesser ability of the various tissues to bind the LPS-HDL complex. Binding studies were also done in the squirrel monkey, an animal that has the same level of circulating HDL cholesterol as the rat, but nearly three times more cholesterol in low density lipoproteins. Specific binding of homologous 125I-HDL and [3H]LPS-HDL was again found principally in the adrenal gland and liver. The results indicate that the sites of tissue uptake of bacterial LPS are strongly influenced by binding of LPS to HDL. In particular, LPS-HDL binding may be an important determinant of the extent to which LPS are taken up by the adrenal gland during bacterial sepsis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Kinetic parameters of the lipoprotein transport systems in the adrenal gland of the rat determined in vivo. Comparison of low and high density lipoproteins of human and rat origin. J Biol Chem. 1981 Jul 25;256(14):7362–7370. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne J. T., Hess B. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem. 1980 Nov 25;255(22):10875–10883. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W., Weisgraber K. H., Bersot T. P. Apoprotein (E--A-II) complex of human plasma lipoproteins. II. Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E--A-II) complex. J Biol Chem. 1978 Sep 10;253(17):6289–6295. [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Disparities in the interaction of rat and human lipoproteins with cultured rat fibroblasts and smooth muscle cells. Requirements for homology for receptor binding activity. J Biol Chem. 1980 Dec 10;255(23):11163–11172. [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- LEVIN J., CLUFF L. E. ENDOTOXEMIA AND ADRENAL HEMORRHAGE. A MECHANISM FOR THE WATERHOUSE-FRIDERICHSEN SYNDROME. J Exp Med. 1965 Feb 1;121:247–260. doi: 10.1084/jem.121.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Migeon C. J., Kenny F. M., Hung W., Voorhess M. L. Study of adrenal function in children with meningitis. Pediatrics. 1967 Aug;40(2):163–183. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Sibbald W. J., Short A., Cohen M. P., Wilson R. F. Variations in adrenocortical responsiveness during severe bacterial infections. Unrecognized adrenocortical insufficiency in severe bacterial infections. Ann Surg. 1977 Jul;186(1):29–33. doi: 10.1097/00000658-197707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney J. B., Braithwaite F., Eder H. A. Characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1977 Jan 25;16(2):271–278. doi: 10.1021/bi00621a018. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Xarli V. P., Steele A. A., Davis P. J., Buescher E. S., Rios C. N., Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore) 1978 May;57(3):211–221. doi: 10.1097/00005792-197805000-00002. [DOI] [PubMed] [Google Scholar]


