Abstract
The incidence of invasive infections due to Haemophilus influenzae has decreased significantly in developed countries with high rates of vaccination against H. influenzae serotype b (Hib). This vaccine provides no protection against H. influenzae serotype f (Hif), typically associated with invasive infections in adults with chronic disease and/or immunodeficiency, and rarely in otherwise healthy adults and children. The specific properties of Hif associated with virulence remain largely uncharacterized. A panel of 26 Hif strains consisting of both invasive disease-associated and mucosal surface non-invasive disease-associated isolates was surveyed by DNA fingerprinting, biotyping and PCR detection of hmw1, hmw2, hsf, the hif fimbrial locus and the lipo-oligosaccharide (LOS) biosynthetic island, and assessment of β-lactamase expression and determination of resistance to the bactericidal activity of normal adult human serum. Repetitive sequence-based PCR fingerprinting differentiated the 26 strains into three clusters, with the majority of isolates (22/26, 84.6 %) clustered into a single indistinguishable group. Most isolates (24/26, 92.3 %) were of biotype I and two isolates produced β-lactamase with detection of a conjugative plasmid, and the isolates displayed a range of resistances to the bactericidal activity of human serum. All 26 isolates carried the adhesin hsf, 21 carried a partial hif fimbrial operon and 4 had the adhesin genes hmw1/2. A LOS biosynthetic island was detected in 20 isolates consisting of the genes lic2BC. It was concluded that Hif has many recognized virulence properties and comprises a relatively homogeneous group independent of the anatomical source from which it was isolated.
Introduction
Haemophilus influenzae, a human-restricted Gram-negative coccobacillus, is a commensal of the upper respiratory mucosa and a pathogen commonly causing airway mucosal disease and occasional invasive disease. Since Margaret Pittman’s original description of capsular serotypes among H. influenzae isolates in 1931 (Pittman, 1931), H. influenzae serotype b (Hib) had been the most clinically significant strain causing invasive disease (e.g. meningitis, epiglottitis, septicaemia and osteomyelitis) in previously well infants and children (Aubrey & Tang, 2003). Since the early 1990s, routine administration of the Hib conjugate vaccines (which induce protective levels of anti-capsular antibody) has virtually eliminated Hib disease among infants and young children in developed countries (Agrawal & Murphy, 2011; Ladhani, 2012). However, the vaccine provides no protection from infection due to non-b serotypes, including H. influenzae serotype f (Hif), which remains a rare but significant cause of invasive infection. Given the reduction in Hib carriage among Hib conjugate vaccine recipients, concern exists as to whether invasive disease due to Hif or other non-b encapsulated strains will become more prevalent due to serotype replacement (Takala et al., 1991; Urwin et al., 1996; Ward, 1996; Tsang, 2007). In fact, several investigations in North America and Europe in the post-conjugate vaccine era have indicated an increased incidence, albeit small, of invasive infections due to non-b H. influenzae, with a predominance of non-typable and Hif strains, primarily in older age groups (Urwin et al., 1996; Ladhani et al., 2010, 2012; MacNeil et al., 2011; Resman et al., 2011a; Rubach et al., 2011; Ladhani, 2012).
Invasive disease associated with Hif appears to disproportionately affect individuals with underlying co-morbidities, including adults suffering from chronic obstructive pulmonary disease (COPD), ethanol abuse, chronic renal disease and human immunodeficiency virus/AIDS, and children with immunoglobulin deficiencies, severe combined immunodeficiency, human immunodeficiency virus/AIDS, malignancy and sickle-cell disease (Nitta et al., 1995; Urwin et al., 1996; Fickweiler et al., 2004; Resman et al., 2011b; Watson et al., 2011). This is in contrast to Hib, which is considered a primary pathogen able to cause disease in otherwise healthy and immunocompetent individuals, predominantly children (Morris et al., 2008). This discrepancy in disease epidemiology suggests that Hif and other non-b encapsulated strains may be intrinsically less virulent than Hib, a hypothesis that is supported by animal model studies of systemic infection showing decreased virulence of capsular serotype f strains relative to strains expressing capsular serotype b (Zwahlen et al., 1983).
Compared with Hib, there is comparatively little knowledge and understanding of the epidemiology and virulence factors and features of serotype f strains accounting for invasive disease. In this study, a panel of 26 serotype f strains was characterized by biotyping and DNA fingerprinting, determination of β-lactamase production, PCR detection of a panel of known adhesins important for adherence and colonization, comprising hmw1, hmw2, hsf and the hif fimbriae (St Geme et al., 1993, 1996, 1998; Mhlanga-Mutangadura et al., 1998), PCR analysis of variability at a lipo-oligosaccharide (LOS) biosynthetic island, and assessment of their ability to resist the bactericidal activity of adult normal human serum (NHS). Importantly, we report that isolates of Hif, regardless of their anatomical source or history of causing invasive disease, comprise a relatively homogeneous group that, taken as a whole, contains a high prevalence of known virulence factors and properties.
Methods
Bacterial strains, media and growth conditions.
Reference H. influenzae isolates with known serotypes were obtained from the American Type Culture Collection: serotype a (ATCC 9006); Eagan, a division I type b; type c (ATCC 9007); type d (ATCC 9008); type e (ATCC 8142); and type f (ATCC 9833). For certain experiments, the unencapsulated and serum-sensitive laboratory strain Rd KW20 [named R652 by our laboratory (Center for Childhood Infections, Seattle Children′s Hospital Research Institute); see Table 1] (Wilcox & Smith, 1975), non-typable and serum-resistant strain INT-1 (R2866) (Nizet et al., 1996), non-typable strain 11 (R3945) (Barenkamp & St Geme, 1996), non-typable strain 12 (R2846) (Barenkamp & Leininger, 1992), fimbriated serotype b strain C54 (Pichichero et al., 1982) and serum-resistant strain Eagan (E1a) (Weller et al., 1977) were used as controls. Bacteria were stored at −80 °C in sterile skimmed milk and cultured at 37 °C in 5 % CO2 on chocolate agar supplemented with 1 % (v/v) IsovitaleX (Becton Dickinson) or in Difco brain–heart infusion broth (Becton Dickinson) supplemented with haemin/HCl (10 µg ml−1; Sigma Chemical) (sBHI) and β-NAD+ (10 µg ml−1; Sigma) and with agar (Bacto-Agar; Difco) for solid sBHI plates. The strains are held in the collection of one of the authors (A. L. S.) consisting of isolates from clinical bacteriological laboratories where the author was located (C numbers) or strains referred to A. L. S. (R numbers). Strains isolated prior to 1982 (R1068–R1072) were a gift from Dr John Philpott-Howard, who reported on antibiotic resistance in H. influenzae in the UK since 1977 (Philpott-Howard & Williams, 1982).
Table 1. Panel of Hif isolates used in this study: source information and PCR analysis results.
| Strain* | Original designation† | Anatomical site | Year | infA–ksgA (lic2BC)‡ | infA–ksgA (losAB)§ | purE–pepN (hif)|| | hmw1/2¶ | hsf# |
| R232 | Boston | Blood | 1972 | + | − | + | − | + |
| R543 | ATCC 9833 | Blood | 1975 | + | − | + | − | + |
| R1068 | 3 | Sputum | Before 1982 | + | − | + | − | + |
| R1069 | 210 | Sputum | Before 1982 | + | − | + | − | + |
| R1070 | 236 | Nasopharynx | Before 1982 | − | − | − | − | + |
| R1071 | 256 | Sinus aspirate | Before 1982 | + | − | + | − | + |
| R1073 | 409 | Sputum | Before 1982 | + | − | + | − | + |
| R1074 | 1235 | Sputum | Before 1982 | + | − | + | + | + |
| R1075 | 3738 | Sputum | Before 1982 | + | − | + | + | + |
| R1527 | Seattle | CSF | 1985 | − | − | + | − | + |
| R1831 | Seattle | Blood | 1986 | + | − | + | − | + |
| R2978 | 11OU35 | Blood | 1996 | − | − | + | + | + |
| R3072 | – | Blood | 1997 | + | − | + | − | + |
| R3073 | Seattle | CSF | 1991 | + | − | + | − | + |
| R3779 | 2006−14 | Blood | 2006 | + | − | − | − | + |
| R3788 | Seattle | Sinus aspirate | 2008 | + | − | +** | − | + |
| R3827 | St Louis | Thigh abscess | 2009 | + | − | +** | − | + |
| R3905 | 91 : 31 | Blood | 2010 | − | − | + | − | + |
| R3906 | 106 : 29 | Blood | 2010 | + | − | + | − | + |
| R3907 | Seattle | Blood | 1994 | + | − | + | − | + |
| R3915 | Seattle | Joint aspirate | 2010 | + | − | + | − | + |
| R3944 | Seattle | Blood | 2011 | + | − | +** | + | + |
| C10 | Boston | CSF | 1970 | + | − | + | − | + |
| C581 | Seattle | Sputum | 1978 | + | − | + | − | + |
| C633 | 13643 | Sputum | 1979 | − | − | + | − | + |
| C909 | Seattle | CSF | 1982 | − | − | + | − | + |
Strain names identify the individual who sent the strain: R1068–R1075 were from J. Philpott-Howard (Guy’s, King’s & St Thomas’ School of Medicine, London, UK); R2978 was from L. Van Alphen (Utrecht University, Utrecht, The Netherlands); R3072 was from S. Kaplan (Baylor College of Medicine, Infectious Disease Service, Texas Children’s Hospital, TX, USA); R3779 was from R. Tsang (National Microbiology Laboratory, Public Health Agency of Canada, Manitoba, Canada); R3788 was from X. Quin (University of Washington School of Medicine, Washington, DC, USA); and R3905 and R3906 were from J. Clarridge (University of Washington School of Medicine, Washington, DC, USA). Boston refers to Children’s Hospital of Boston, Seattle is the Children’s Hospital and Regional Medical Center of Seattle, and St Louis refers to St Louis Children’s Hospital.
The original description refers to the coding of the isolate when received or the source clinical laboratory.
PCR amplification of the LOS island within the infA–ksgA locus and subsequent PCR for lic2BC. +, Both amplifications were positive; −, one or other of the amplicons was not detected.
PCR amplification of the LOS island within the infA–ksgA locus and subsequent PCR for losAB. +, Both amplifications were positive; −, one or other of the amplicons was not detected.
PCR amplification of the hif fimbrial locus at purE–pepN. +, Amplification was positive for a ~4 kb amplicon matching that of the partial hif allele of ATCC 9833 (R543); −, the amplicon was not detected.
Amplification by PCR of hmw. +, Production of an ~1200 bp hmw amplicon, except for strain R2978, which produced a partial ~400 bp amplicon.
Amplification by PCR of hsf. A ‘+’ indicates the production of ~400 bp hsf amplicon.
Partial-length amplicons at the purE–pepN locus of ~800 bp containing hicAB genes.
Capsular typing by multiplex PCR.
The primers used for PCR analysis are listed in Table 2. Genomic DNA was prepared using a DNeasy Tissue kit (Qiagen). Routine PCR conditions and DNA manipulations were performed as described previously (Erwin et al., 2005). Initial screening of putative type f strains was with a multiplex PCR, using primers and methods reported previously (Nelson & Smith, 2010). Encapsulated control strains were obtained from the American Type Culture Collection comprising capsular types a–f. Strains with the amplicon pattern of type f in the multiplex assay were tested with primers specific for the capsule export locus bexA (bexA F and bexA R; Table 2) and separate specific primers (f1 and f3; Table 2) for serotype f for confirmation (Falla et al., 1994, 1995). All strains identified as serotype f produced a 400 bp amplicon with the confirmatory type f-specific primers identical to that produced by the Hif reference strain ATCC 9833.
Table 2. Primers used for PCR.
| Designation | Sequence (5′→3′) | Reference |
| bexA F | ATGATTCGCGTAAATAATGTATGTAAGAAG | Nelson & Smith (2010) |
| bexA R | TTGCGTCTCGTTGTAGTATTGATAC | |
| ERIC1 | ATGTAAGCTCCTGGGGATTCAC | Hulton et al. (1991) |
| ERIC2 | AAGTAAGTGACTGGGGTGAGCG | |
| f1 | GCTACTATCAAGTCCAAATC | Falla et al. (1994) |
| f3 | AATGCTGGAGTATCTGGTTC | |
| hia_hsf F | TGTCCGCCTTGATCCGAACAATCA | This study |
| hia_hsf R | ACTGGTAACCAACACCTGCTGCAA | |
| HMWP1F | GCGTCGAAGGAGGTCAACGAACG | St Geme et al. (1998) |
| HMWP3R | GCCCCACACAATAGCGC | |
| infA-ksgA F | TATGTGCAGTAACCACGTGACCGT | Erwin et al. (2005) |
| infA-ksgA R | TCGACAAGTTCACCAACAGGCTCT | |
| lic2B-F | TAAGTATGATCCTCAAATGCAT | Erwin et al. (2005) |
| lic2BA | CAATTTAGCGATGAGTTCC | |
| losB-R | GGTTGAGCCATCTTGTGGAATGTC | Erwin et al. (2005) |
| purE_pepN F | GACCCCATCACAACGCGAATTTGTG | Erwin et al. (2005) |
| purE_pepN R | CTGTGACCGTAAAATCTGGTTGTTTGTAATC | |
| topoF | GAGACTCACAAAGCGACAACC | Erwin et al. (2005) |
| topoR | GGCTTGAGGTGCGTCATCATCTTC |
Biochemical assays for biotype assignment.
Biotypes were assigned by detection of indole production, ornithine decarboxylase activity and urea hydrolysis, using methods and a classification system described elsewhere (Kilian, 1976; Murray et al., 2003). We originally intended to use the API NH strips (bioMérieux) for biotype assignment; however, in our experience, this assay does not yield reproducible results with these strains so the reactions were performed in test tubes as originally described by Kilian (1976).
Detection of β-lactamase activity.
β-Lactamase expression was detected by testing for ampicillin hydrolysis with a modified phenol red assay (Scheifele et al., 1976), adapted for a microtitre plate. Strain R2866 (INT-1) carries a plasmid-encoded β-lactamase and served as a positive control, with strain R652 (Rd KW20) as a negative control for β-lactamase expression.
Strain typing.
DNA was extracted from H. influenzae isolates using a MoBio bacteraemia DNA kit (MoBio Laboratories) in the laboratory of Carey-Ann Burnham (Washington University, St Louis, MO, USA). Repetitive sequence-based PCR (repPCR) was performed using ~100 ng DNA, and primers ERIC1 and ERIC2 (Table 2), in a final reaction volume of 25 µl using Ready-To-Go RAPD analysis beads (GE Healthcare Biosciences) (Hulton et al., 1991; Kang & Dunne, 2003). Following an initial denaturation step at 95 °C for 7 min, 30 cycles of PCR were performed (90 °C for 15 s, 51 °C for 30 s and 65 °C for 7 min), with a final elongation step at 65 °C for 14 min. The resultant PCR products were resolved using an Agilent 2100 (Agilent Technologies) with a Diversilab DNA chip (bioMérieux) to produce virtual gel electropherograms. Diversilab Bacterial Barcodes software (bioMérieux) was used to analyse the banding patterns and create a similarity index for the isolates (Healy et al., 2005a, b). Isolates with a similarity index of ≥85 % were considered identical for the purpose of this analysis. A non-typable strain, R465, was included as an unrelated control and was characterized previously by enterobacterial repetitive intergenic consensus (ERIC) sequence-based PCR fingerprinting (Watson et al., 2004).
Detection of adhesins by PCR.
The primers used for the detection of adhesin genes are listed in Table 2. Genomic DNA was prepared using a DNeasy Tissue kit (Qiagen). Routine PCR conditions and DNA manipulations were performed as described previously (Erwin et al., 2005). For classification purposes, the absence of an amplicon, or an amplicon of <100 bp, was considered negative for that locus. The isolates were examined for the adhesins hmw1/2 (St Geme et al., 1993) and hsf, including its smaller allele hia (St Geme et al., 1996), using strain R2846 (strain 12; GenBank accession no. CP002276) as a positive control. For detection of hsf, strain C54 was used as a positive control and produced a ~400 bp amplicon band using the hia_hsf F and hia_hsf R primer set. The adhesin genes hsf and hia are identical at the N and C termini, such that the hia_hsf primer set identified both alleles. For amplification of the high-molecular-weight adhesion genes hmw1 and hmw2 (primers HMWP1F and HMWP3R; Table 2), strain R2846 (strain 12) was used as a positive control with production of an ~1200 bp amplicon, and strain R2866 (INT-1) whose genomic sequence is available (GenBank accession no. CP002277) was used as a negative control with no amplicon detected. The fimbrial gene cluster lies between purE and pepN in strains expressing the structure, also called haemagglutinating pili. The gene cluster required for the expression of fimbriae, hifABCDE, is ~7.5 kb in serotype b strains, but in certain strains hicAB is also present, whose function is unknown (Mhlanga-Mutangadura et al., 1998). The Hif reference strain ATCC 9833 (R543; Table 1) has a partial fimbrial cluster containing hifA, hifC and parts of hifD and hifE, which total ~3.7 kb (Mhlanga-Mutangadura et al., 1998). For amplification of the fimbriae locus, isolates were screened using the purE_pepN primer set, and the fimbriated serotype b strain C54 was used as a full-length ~7 kb positive control and ATCC 9833 (R543) as a positive control for the partial ~3.7 kb allele.
Determination of the composition of the LOS biosynthetic island.
Prior experiments indicated that the genes for glycosyltransferases lic2B and lic2C are between infA and ksgA in a type b genome (strain 7004; Hood et al., 2004). In strains Rd KW20 (R652) and INT-1 (R2866), infA and ksgA are adjacent, whilst in strain 86-028NP, the locus contains only lic2C. In R2846 (strain 12) and ~20 % of other non-typable strains, the locus contains the glycosyltransferases losA and losB, whose expression is phase variable due to the presence of the octameric repeat 5′-CGAGCATA-3′, with the number of repeats varying between two and ten, modifying the translational reading frame; expression of these enzymes alters the structure of the surface LOS, affecting resistance to NHS (Erwin et al., 2006b). Both the lic2B–lic2C and the losA–losB regions are within the 2.2 kb amplicon found with the infA–ksgA primers; these regions can be differentiated by subsequent PCR and/or sequencing using allele-specific primers as reported previously (Erwin et al., 2005). Because of the heterogeneity of this island we examined the composition of 26 type f strains by PCR for the infA–ksgA locus using the infA–ksgA primer set (Table 2) and subsequent PCR using the lic2B-F and lic2BA primer pair to identify the lic2BC allele, or the infA–ksgA F and losB–R primer pair to identify the losAB allele.
Resistance to serum bactericidal activity.
Resistance testing to human serum bactericidal activity was assayed using pooled NHS and assay conditions reported previously (Erwin et al., 2005). NHS was obtained by venepuncture from healthy, adult volunteers according to an approved human studies protocol. Serial dilutions of serum in 10 mM PBS with 4 mM KCl and 0.1 % (w/v) gelatine, ranging from 0.02 to 50 % (v/v) serum, were tested. The reported IC50 was the concentration of serum required to kill 50 % of the inoculum (~ 2000 c.f.u.) within 30 min of incubation at 37 °C in room air. The IC50 was calculated using XLfit version 4.1 software (ID Business Solutions), which unweights the lowest and highest values. The IC50 values of the unencapsulated and serum-sensitive Rd KW20 strain (R652), the non-typable and serum-resistant INT-1 strain (R2866) and the serotype b encapsulated strain Eagan (E1a) are shown in Table 3 for reference.
Table 3. NHS bactericidal activity for Hif isolates.
| Type of isolate & strain | Mean IC50 (range) | sd | n* |
| Controls | |||
| R652 (Rd KW20) | 1.08 (0.32–2.09) | 0.48 | 20 |
| R2866 (INT-1) | 47.79 (39.95–50.00) | 3.63 | 16 |
| E1a (Eagan) | 50.00 (50.00–50.00) | 0.00 | 3 |
| Invasive disease-associated isolates | |||
| R232 | >50.00† | 5 | |
| R543 | 35.01 (25.04–50.00) | 13.60 | 7 |
| R1527 | 35.01 (25.00–>50.00) | 13.69 | 5 |
| R1831 | 3.37 (3.06–3.94) | 0.49 | 3 |
| R2978 | 37.32 (24.92–>50.00) | 9.04 | 5 |
| R3072 | 2.49 (1.29–3.68) | 1.06 | 5 |
| R3073 | 2.49 (1.29–2.82) | 1.05 | 7 |
| R3779 | 16.23 (11.24–24.75) | 7.41 | 5 |
| R3827 | 5.97 (4.33–6.80) | 1.01 | 7 |
| R3905 | 8.64 (7.28–10.56) | 1.31 | 7 |
| R3906 | 16.56 (14.00–18.76) | 2.40 | 5 |
| R3907 | 5.62 (3.45–8.58) | 2.65 | 5 |
| R3915 | 41.25 (23.76–>50.00) | 15.15 | 5 |
| R3944 | 24.68 (23.68–25.55) | 0.94 | 3 |
| C10 | >50.00† | 5 | |
| C909 | 12.79 (8.95–14.48) | 2.23 | 5 |
| Group‡ | 21.71 | 4.32 | |
| Mucosal carriage and non-invasive disease-associated isolates§ | |||
| R1068 | 48.12 (44.37–50.00) | 3.25 | 5 |
| R1069 | >50.00† | 7 | |
| R1070 | 45.33 (26.63–50.00) | 10.45 | 7 |
| R1071 | 45.86 (26.63–50.00) | 7.16 | 5 |
| R1073 | 47.46 (44.33–50.00) | 2.87 | 5 |
| R1074 | 47.15 (41.45–50.00) | 4.93 | 5 |
| R1075 | 37.32 (24.93–50.00) | 9.04 | 7 |
| R3788 | 4.33 (2.15–6.89) | 1.99 | 5 |
| C581 | 45.66 (28.33–>50.00) | 9.69 | 5 |
| C633 | 46.41 (32.04–>50.00) | 8.03 | 5 |
| Group‡ | 41.76 | 4.29 |
Number of replicates used to calculate the reported values.
IC50 values greater than the upper limit of detection (>50.00), range and sd could not be calculated. For group mean calculations, these were considered to be 50.00.
Group analysis represents mean IC50 and sem.
Mucosal isolates collected mostly from non-invasive disease specimens.
Statistical analysis.
The VassarStats website for statistical computation (http://faculty.vassar.edu/lowry/VassarStats.html) was used for analysis. A Mann–Whitney U test was used to compare mean IC50 values for NHS resistance capacity between mucosal and invasive disease-associated isolates.
Results
Confirmation of capsular serotype f
Initially, 36 strains previously identified as type f on the basis of serotyping in a clinical microbiology laboratory were subcultured from the collection of one of the authors (A. L. S.). These 36 isolates were examined using a multiplex PCR for capsule types a–f, and in separate reactions with primers for bexA, the capsule export gene conserved among all encapsulated H. influenzae (Nelson & Smith, 2010). Among those isolates identified as serotype f using the multiplex assay and among those that were also bexA positive, 25 isolates and the ATCC 9833 Hif reference strain were subsequently confirmed to contain a specific type f capsular gene by further PCR amplification (Falla et al., 1994). Eleven of the thirty-six original isolates did not test positive for bexA and/or for serotype f using confirmatory primers, and as these strains could not be absolutely confirmed as Hif strains, they were not used in subsequent analyses. The 25 Hif strains and the ATCC 9833 Hif strain confirmed as type f were originally isolated from diverse sources: blood (n = 10), cerebrospinal fluid (CSF) (n = 4), sputum (n = 7), sinus aspirates (n = 2), nasopharynx (n = 1), joint aspirate (n = 1), and thigh abscess (n = 1) between 1972 and the 2011. The ATCC 9833 type f reference strain was isolated from blood and CSF by Pittman (1931). The site of isolation, the date isolated and the number assigned in our laboratory to each strain is shown in Table 1.
General features and colony morphology of confirmed Hif isolates
Isolates were assayed for indole production, ornithine decarboxylase activity and urea hydrolysis for biotype assignment. Among the panel of isolates, 24/26 Hif strains (92.3 %) were identified as biotype I (positive for all three of the biotyping reactions), with only 2 isolates, R1068 and R3944, identified as biotype II (ornithine decarboxylase activity was not detectable). We noted a striking variation in colony morphology on sBHI agar: the majority of the isolates had a ‘smooth’ texture, whilst the CSF isolate C10 had a small flat colony and the sputum isolate C581 had a large ‘mucoid’ colony phenotype. One isolate, R3944, produced both a smooth colony and a wrinkled colony on subculture; each morphotype had identical results in the phenotypic analysis. β-Lactamase activity was detected in 2/26 (7.7 %) Hif strains, R3788 and R3907, by an ampicillin hydrolysis assay. Consistent with the detected β-lactamase activity, strain R3907, a blood isolate, and R3788, a sinus isolate, were the only isolates determined to carry a large conjugative plasmid associated with β-lactamase activity, as they produced an ~450 bp amplicon by PCR with primers topoF and topoR (Erwin et al., 2005).
Hif isolate typing by repPCR fingerprinting
DNA fingerprinting for the confirmed Hif isolates was performed using repPCR with primers detecting ERIC sequences (Healy et al., 2005a), and amplicons were resolved using bioMérieux Diversilab Bacterial Barcodes software to generate a similarity index. Isolates with similarity indices >85 % were considered identical in this study (Healy et al., 2005a, b). A dendrogram of Hif strains with virtual electropherograms is shown in Fig. 1, illustrating three distinct and unrelated clusters of isolates. Cluster A was the largest group with 22/26 (84.6 %) isolates, followed by clusters B and C with only 2/26 (7.7 %) isolates each. There was no correlation between cluster assignments with respect to geographical origin, clinical disease association or biotype. Both of the β-lactamase-producing isolates, R3788 and R3907, were within cluster A. A non-typable strain, R465, was included as an unrelated control and was characterized previously by ERIC sequence-based PCR fingerprinting.
Fig. 1.
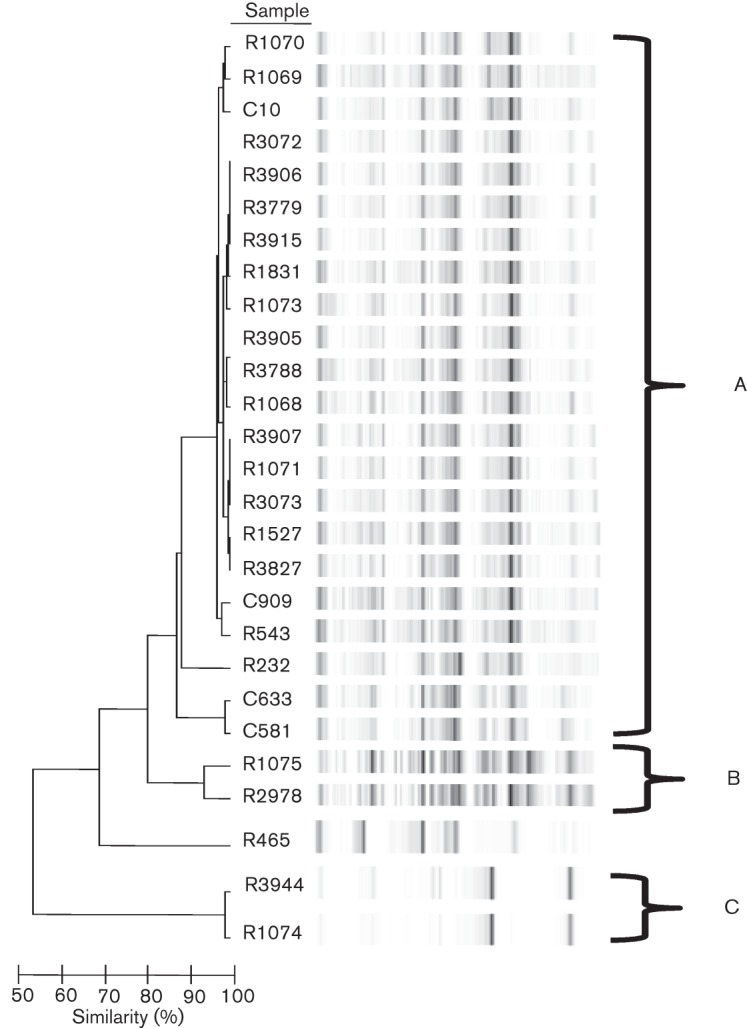
Dendrogram of Hif isolates. DNA was isolated from 26 Hif isolates and used as template DNA for repPCR with primers ERIC1 and ERIC2 (Table 2). The resultant amplicons were resolved on an Agilent 2100 with Diversilab DNA chips to create virtual electropherograms and Bacterial Barcodes software was used to calculate a similarity index for the isolates. Isolates with a similarity index of ≥85 % were considered to be identical. Banding patterns are shown for the 26 isolates, organized into three unrelated clusters, A–C, with the majority of isolates (22/26, 85 %) in cluster A. The non-typable strain R465, analysed previously by ERIC sequence-based PCR fingerprinting in our laboratory (Watson et al., 2004), was included as a control and was confirmed to be unrelated to the other strains in the study.
PCR detection of adhesins
The panel of Hif isolates was subjected to PCR analysis to determine the presence of genes encoding the following adhesins notable in other encapsulated and non-typable H. influenzae for their role in adherence and colonization: the high-molecular-weight adhesin (hmw1 and hmw2 genes), the Hsf adhesin (hsf gene) and the hif gene cluster (between purE and pepN) encoding surface fimbriae (St Geme et al., 1993, 1998; Mhlanga-Mutangadura et al., 1998). The results are shown in Table 1. Among this panel of Hif strains, all 26 isolates (100 %) were PCR positive for amplification at the hsf adhesin locus using the hia_hsf primer pair. For the high-molecular-weight adhesins (hmw1 and hmw2), three isolates (11.5 %; R1074, R1075 and R3944) produced amplicons of ~1200 bp correlating to the presence of hmw, whilst one isolate, R2978, produced a ~400 bp partial hmw amplicon. Interestingly, the four isolates producing a full-length or partial amplicon for hmw were grouped into clusters B (R1075 and R2978) and C (R3944 and R1074), further distinguishing these two clusters from the majority of the isolates in cluster A testing negative for hmw carriage. In examination of the Hif fimbrial locus by PCR amplifying the region between purE and pepN, all strains except R1070 and R3779 displayed an insert at this locus. Whilst most of the strains testing positive for an insert exhibited a ~4 kb amplicon consistent with the partial-length hif allele previously identified in ATCC 9833 (R543) containing hifA, hifC and parts of hifD and hifE, strains R3728, R3827 and R3944 all produced an 800 bp amplicon consistent with our previous work in non-typable strains in which this 800 bp amplicon was sequenced and determined to contain the hic genes (‘hif contiguous’ DNA) hicAB, two putative conserved genes of unknown function (Mhlanga-Mutangadura et al., 1998). Digestion of the ~4 kb hif amplicon in the 21 strains with this insert with AseI and DraI yielded three identically sized fragments of ~2.7 kb among all of the positive isolates, suggesting that the hif sequence was relatively conserved among the isolates, although it is conceivable that sequence polymorphisms existed outside the restriction enzyme sites, masking the ability to detect differences by this method. With regard to the presence or absence of hif or hicAB, there were no correlations with the DNA fingerprinting clustering assignments, as these alleles were distributed across all three clusters (Fig. 1).
Mapping of the LOS biosynthesis island
Previous work has indicated that the LOS modification genes lic2B and lic2C are located in a genetic island between infA and ksgA in a type b genome (strain 7004; Hood et al., 2004). In strains Rd KW20 (R652) and INT-1 (R2866), infA and ksgA are adjacent, whilst in the non-typable strain 86-028NP, the locus only contains lic2C (Harrison et al., 2005). In the non-typable strain 12 (R2846), the infA–ksgA locus contains a different pair of glycosyltransferase genes, losA and losB, whose expression is phase variable due to the presence of the octameric repeat 5′-CGAGCATA-3′ varying between two and ten copies (Erwin et al., 2006b). Because of the heterogeneity of this island, we examined the 26 type f strains by PCR for the infA–ksgA locus. Twenty of the twenty-six isolates (76.9 %) produced an amplicon of 2.2 kb at the infA–ksgA locus, which, when examined by a subsequent PCR using primers specific for lic2BC, were all positive (Table 1). Carriage of the lic2BC insert at the infA–ksgA locus did not correlate with a specific fingerprinting cluster. PCR analysis of all 26 strains for losAB did not yield an amplicon (Table 1), suggesting that the losAB allele may be absent from serotype f strains. These findings at the LOS modification locus further support the relative homogeneity of Hif strains, regardless of their clinical source.
Resistance to the bactericidal activity of NHS
The presence of a polysaccharide capsule promotes virulence by imparting resistance to the complement-mediated antibacterial properties of NHS. Unencapsulated strains, such as Rd KW20 (R652), are relatively serum sensitive compared with capsule-producing strains (e.g. Eagan), which can replicate in 50 % NHS and whole blood, or the rare serum-resistant non-typable strain INT-1 (R2866) (Table 3) (Williams et al., 2001). In the serum-resistance assay, the reported IC50 was the mean (±sd) concentration of serum required to kill 50 % of the inoculum within a 30 min incubation period (Table 3). Five of the sixteen strains isolated from normally sterile anatomical sites had mean IC50 values of <6.24 %, the minimum value previously found with type b isolates (Table 3). Two of the sixteen invasive group isolates (the blood isolate R232 and CSF isolate C10) exhibited high-level resistance with mean IC50 values of ≥50 % NHS (Table 3). The mean IC50 value for the invasive group taken as a whole was 21.71±4.32 % with a wide range of resistance to NHS (Table 3). The mean IC50 for the ten strains isolated from mucosal airway secretions taken as a whole was 41.76±4.29 % (mean±sem), but only one of those strains (the sputum isolate R1069) exhibited high-level resistance with a mean IC50 of ≥50 % NHS (Table 3). Interestingly, the mean IC50 values of the mucosal isolate group were significantly greater than the invasive disease-associated isolate group (Mann–Whitney U test, P = 0.0114), suggesting that the ability of Hif strains to produce invasive infections is not entirely dependent on serum-resistance capacity.
Discussion
Before vaccination against Hib became widely adopted in developed countries in the mid-1990s, serotype f was the second most common capsular serotype causing invasive H. influenzae infection (Berndsen et al., 2011; Rubach et al., 2011). In the post-Hib vaccine era, invasive disease in children due to H. influenzae is uncommon, with the majority of these infections due to unencapsulated strains, with the remainder being non-b encapsulated strains, predominantly serotypes f and a in North America and serotypes f and e in Europe (Tsang et al., 2006; Adam et al., 2010; Ladhani et al., 2010, 2012; Agrawal & Murphy, 2011; Resman et al., 2011a; Rubach et al., 2011). To understand better the ability of serotype f strains to produce invasive infection, we assembled a panel of Hif isolates from a diversity of clinical sources for factors related to pathogenesis. Importantly, we have reported here that isolates of Hif seem to comprise a relatively homogeneous group that, taken as a whole, contain a high prevalence of known virulence factors and properties that potentially contribute to pathogenesis.
Prior investigation of Hif isolates collected from North America and Europe and typed by multilocus enzyme electrophoresis indicated that Hif strains were remarkably clonal in origin with limited genetic diversity, possibly emerging from a single original clone continuing to undergo expansion (Bruun et al., 2004). Similar conclusions have been reached by studies using alternative methods including multilocus sequence typing (MLST) (Meats et al., 2003), PFGE (Campos et al., 2003) and ribotyping (Urwin et al., 1996). There is a suggestion that most invasive infections due to non-b encapsulated strains are caused by a limited number of circulating clones that are intrinsically more pathogenic than the clones associated more often with non-invasive disease (Omikunle et al., 2002). Funding for the present study was not sufficient to permit MLST of all 26 Hif strains, so DNA fingerprinting using repPCR was performed to examine the relative diversity of these isolates. The majority (~85 %) of our isolates were indistinguishable by this method and clustered together, independent of the two other unrelated clusters containing two isolates each. The fact that clusters B and C contained the only four isolates carrying hmw alleles suggests that acquisition and carriage of hmw among serotype f strains is relatively uncommon and that hmw may be a marker for less-dominant Hif clones in circulation. Importantly, even among isolates grouping together in the same cluster, we were able to detect differences with regard to the carriage of β-lactamase-encoding conjugative plasmids, different adhesin alleles and inserts in the LOS biosynthetic island, suggesting that there may be slightly more diversity in our isolates than is suggested by DNA fingerprinting alone. In our sample, all of our isolates were biotype I except for two that were biotype II; biotype assignment did not correlate with the isolate having been an invasive or mucosal-associated isolate. A previous report found only biotype I among Hif isolates (Bruun et al., 2004). This is reminiscent of serotype b strains where the majority of isolates are biotype I, especially those from invasive disease, with a smaller subset of Hib isolates of different biotypes occasionally associated with specific diseases (e.g. conjunctival Hib isolates are usually biotype III) (Kilian, 1976). Taken as a whole, the results from DNA fingerprinting and biotype assignment of our current sample of 26 Hif isolates are congruent with previous studies in showing relatively limited diversity within this serotype, despite Hif being implicated in a wide variety of clinically significant infections from mucosal surfaces and normally sterile sites.
Different protein adhesins are carried to varying extents among H. influenzae isolates. Most encapsulated strains contain genes for the hsf fibril adhesin or high-molecular-weight adhesin HMW, but rarely both, and some strains are also able to synthesize fimbriae (St Geme et al., 1993, 1996, 1998). We took advantage of this to explore Hif diversity further. All of our Hif isolates were PCR positive for the hsf adhesin, with 4 of the 26 isolates also PCR positive for hmw1/hmw2 and 21 containing a partial hif fimbrial gene cluster. A prior report determined that only 1 of 21 type f strains contained hmw1A/1B/1C, and 13 of these 21 isolates were hia positive (Rodriguez et al., 2003). The same study also found that hifB was absent in all type f strains; this was surprising in that 17 of the type f strains contained hifA, hifC and hifD detected by probes on colony blots in that study (Rodriguez et al., 2003). Our finding of an ~4 kb amplicon at the purE–pepN locus in the majority of our isolates is consistent with our prior description of the partial fimbrial gene cluster in the type f ATCC 9833 (Mhlanga-Mutangadura et al., 1998), and is in agreement with the prior study showing that serotype f strains lack hifB (Rodriguez et al., 2003). Restriction of the ~4 kb product with AseI and DraI produced an identical pattern in the 21 strains producing an ~4 kb hif amplicon, suggesting sequence similarity. The complete fimbrial cluster found in the fimbriated serotype b strain C54 is 7.76 kb and contains hicAB in addition to hifABCDE (Pichichero et al., 1982). The expression of haemagglutinating fimbriae only requires hifABCDE, so the function of hicAB in this strain is unknown. One of three of the type f strains in our study producing the 800 bp amplicon at the purE–pepN locus, previously sequenced as hicAB (Mhlanga-Mutangadura et al., 1998), exhibited a wrinkled colonial morphology (blood isolate R3944), suggesting that hicAB alone was not sufficient for this phenotype, and therefore the function of the hicAB allele remains undefined. A limitation of DNA PCR surveys for virulence genes, as performed in this study, is the inability to determine whether carriage of a particular gene correlates with protein expression, as this method does not consider transcriptional or translational regulatory features potentially influencing expression of a certain gene. Verification of protein expression from these genes was beyond the scope of this study but would be important for future investigations to characterize further the virulence factors expressed in serotype f strains.
The prevalence of β-lactamase expression in this panel at 7.7 % of 26 isolates was less than that reported by two prior investigations of Hif samples, where activity was detected in 20.9 % of 43 isolates and in 24.5 % of 49 isolates (Urwin et al., 1996; Campos et al., 2003); both of these studies had larger sample sizes and assayed for β-lactamase activity using a nitrocefin-based assay for detection. We have had inconsistent results with the nitrocefin disk and therefore measured the hydrolysis of ampicillin colorimetrically, which may account for some differences. A side-by-side comparison of these two methods has not been performed. An obvious limitation of this method is the inability to detect β-lactamase-negative ampicillin-resistant (BLNAR) isolates encoding altered penicillin-binding proteins through mutation in the ftsI gene conferring resistance (Ubukata et al., 2001). A review of the literature failed to find reports of BLNAR isolates among Hif isolates, and the BLNAR phenotype is rare in North America in general, but evidence does suggest that, in areas of Europe and Asia, there is increasing prevalence of BLNAR isolates among non-typable and serotype b isolates, suggesting that future studies on antimicrobial susceptibility patterns should include methods to capture these strains as well (Hasegawa et al., 2006; Dabernat & Delmas, 2012).
Serum resistance varied greatly across our sample group of Hif isolates and did not correlate with potential virulence as predicted by specimen source. For example, some blood isolates were relatively serum sensitive, whilst some isolates from sputum were equally serum resistant. Surprisingly, the mean IC50 value of the mucosal surface-isolated group was significantly greater than that of the invasive isolate group. Previously, we have shown that low-level serum resistance of oropharyngeal non-typable H. influenzae isolates permits them to establish a lower respiratory tract infection (Nakamura et al., 2011), indicating that low-level serum resistance in some sputum isolates is not surprising. Serum resistance of an encapsulated strain is imparted largely by the presence of the polysaccharide capsule (Moxon & Kroll, 1988), as evidenced by the serum resistance of a bexA− and phenotypically unencapsulated strain Eagan with an IC50 value of 1.20 % (K. L. Nelson and A. L. Smith, unpublished data). In an invasive unencapsulated strain, such as R2866 (INT-1), serum resistance results from phase-variable expression of the lgtC-mediated αGal1-4βGal epitope, which mimics the pK blood group, with the resulting phase variant not being recognized as foreign (Weiser & Pan, 1998; Erwin et al., 2006a). The lic2B and lic2C LOS biosynthetic genes flanked by the infA and ksgA genes are associated with a serum-resistance phenotype in a serotype b strain (High et al., 1996; Hood et al., 2004). Approximately 20 % of non-typable H. influenzae isolates contain phase-variable losA and losB genes, which, when expressed, mediate low-level serum resistance (Erwin et al., 2006b). None of our panel of isolates was positive for losAB, and only 20/26 isolates were positive for lic2BC. Interestingly, several of the lic2BC-negative Hif isolates were highly serum resistant in our assay, suggesting that other mechanisms may account for their phenotype in vitro. Phase variation of other surface structures, such as LOS, may compete with the binding by bactericidal antibody and complement, decreasing the level of serum resistance. It is entirely possible that some strains in our panel that tested serum sensitive may be so due to selection of a phase variant exhibiting that phenotype, and thus serum resistance as assayed in vitro may not always reflect the true serum-resistance potential of an isolate in vivo. We demonstrated previously a lack of correspondence between in vitro serum resistance and the isolation of non-typable H. influenzae strains from normally sterile sites (Erwin et al., 2005). These data suggest that serum resistance is multifactorial and is not a specific requirement for a particular strain to produce an invasive infection. Rather, an invasive infection is probably due to a combination of factors (e.g. impaired host defences or bacterial strains with a slight competitive advantage) all occurring simultaneously to result in disease.
In summary, Hif isolates have multiple traits and known virulence factors associated with pathogenesis, and as a whole remain a rare but significant cause of morbidity and mortality, which may be slowly increasing in incidence worldwide in the post-Hib-conjugate-vaccine era. The finding that Hif strains seem to have many properties in common with other non-b serotypes, in addition to some non-typable strains, suggests that future vaccine strategies could be developed potentially to target multiple different serotypes of H. influenzae simultaneously.
Acknowledgements
M. E. W. is supported by a Physician Scientist Training Grant (2T32HD043010-10) within the Washington University Department of Pediatrics. Prior support was by a Pediatric Infectious Diseases Society fellowship award generously supplied by GlaxoSmithKline. The laboratory of A. L. S., MD, at Seattle Children’s Hospital is supported in part by the NIAID (AI46512). The majority of this work was performed in the laboratory of A. L. S., Seattle Children’s Research Institute, WA, USA. Strain typing by ERIC PCR fingerprinting was performed in the laboratory of Carey-Ann D. Burnham, Washington University School of Medicine, Pathology and Immunology, St Louis, MO, USA.
Abbreviations:
- BLNAR
β-lactamase-negative ampicillin-resistant
- CSF
cerebrospinal fluid
- ERIC
enterobacterial repetitive intergenic consensus
- Hib
Haemophilus influenzae serotype b
- Hif
Haemophilus influenzae serotype f
- IC50
concentration of serum required to kill 50 % of the inoculum
- LOS
lipo-oligosaccharide
- MLST
multilocus sequence typing
- NHS
normal human serum
- repPCR
repetitive sequence-based PCR
References
- Adam H. J., Richardson S. E., Jamieson F. B., Rawte P., Low D. E., Fisman D. N. (2010). Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 28, 4073–4078 10.1016/j.vaccine.2010.03.075 [DOI] [PubMed] [Google Scholar]
- Agrawal A., Murphy T. F. (2011). Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 49, 3728–3732 10.1128/JCM.05476-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey R., Tang C. (2003). The pathogenesis of disease due to type b Haemophilus influenzae. Methods Mol Med 71, 29–50 [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Leininger E. (1992). Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun 60, 1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., St Geme J. W., III (1996). Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol 19, 1215–1223 10.1111/j.1365-2958.1996.tb02467.x [DOI] [PubMed] [Google Scholar]
- Berndsen M. R., Erlendsdottir H., Gottfredsson M. (2011). Evolving epidemiology of invasive Haemophilus infections in the post-vaccination era: results from a long-term population-based study. Clin Microbiol Infect 18, 918–923 [DOI] [PubMed] [Google Scholar]
- Bruun B., Gahrn-Hansen B., Westh H., Kilian M. (2004). Clonal relationship of recent invasive Haemophilus influenzae serotype f isolates from Denmark and the United States. J Med Microbiol 53, 1161–1165 10.1099/jmm.0.45749-0 [DOI] [PubMed] [Google Scholar]
- Campos J., Román F., Pérez-Vázquez M., Aracil B., Oteo J., Cercenado E., the Spanish Study Group for H. influenzae type f (2003). Antibiotic resistance and clinical significance of Haemophilus influenzae type f. J Antimicrob Chemother 52, 961–966 10.1093/jac/dkh004 [DOI] [PubMed] [Google Scholar]
- Dabernat H., Delmas C. (2012). Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001–2008: a retrospective database analysis. Eur J Clin Microbiol Infect Dis 31, 2745–2753 10.1007/s10096-012-1623-9 [DOI] [PubMed] [Google Scholar]
- Erwin A. L., Nelson K. L., Mhlanga-Mutangadura T., Bonthuis P. J., Geelhood J. L., Morlin G., Unrath W. C., Campos J., Crook D. W. & other authors (2005). Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect Immun 73, 5853–5863 10.1128/IAI.73.9.5853-5863.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Allen S., Ho D. K., Bonthuis P. J., Jarisch J., Nelson K. L., Tsao D. L., Unrath W. C., Watson M. E., Jr & other authors (2006a). Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum. Infect Immun 74, 6226–6235 10.1128/IAI.00722-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Bonthuis P. J., Geelhood J. L., Nelson K. L., McCrea K. W., Gilsdorf J. R., Smith A. L. (2006b). Heterogeneity in tandem octanucleotides within Haemophilus influenzae lipopolysaccharide biosynthetic gene losA affects serum resistance. Infect Immun 74, 3408–3414 10.1128/IAI.01540-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falla T. J., Crook D. W., Brophy L. N., Maskell D., Kroll J. S., Moxon E. R. (1994). PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol 32, 2382–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falla T. J., Crook D. W., Anderson E. C., Ward J. I., Santosham M., Eskola J., Moxon E. R. (1995). Characterization of capsular genes in Haemophilus influenzae isolates from H. influenzae type b vaccine recipients. J Infect Dis 171, 1075–1076 10.1093/infdis/171.4.1075 [DOI] [PubMed] [Google Scholar]
- Fickweiler K., Borte M., Fasshauer M., Spencker F. B., Handrick W., Rodloff A. C. (2004). Meningitis due to Haemophilus influenzae type f in an 8-year-old girl with congenital humoral immunodeficiency. Infection 32, 112–115 10.1007/s15010-004-3040-1 [DOI] [PubMed] [Google Scholar]
- Harrison A., Dyer D. W., Gillaspy A., Ray W. C., Mungur R., Carson M. B., Zhong H., Gipson J., Gipson M. & other authors (2005). Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol 187, 4627–4636 10.1128/JB.187.13.4627-4636.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Kobayashi R., Takada E., Ono A., Chiba N., Morozumi M., Iwata S., Sunakawa K., Ubukata K. on behalf of the Working Group of the Nationwide Surveillance for Bacterial Meningitis (2006). High prevalence of type b β-lactamase-non-producing ampicillin-resistant Haemophilus influenzae in meningitis: the situation in Japan where Hib vaccine has not been introduced. J Antimicrob Chemother 57, 1077–1082 10.1093/jac/dkl142 [DOI] [PubMed] [Google Scholar]
- Healy M., Huong J., Bittner T., Lising M., Frye S., Raza S., Schrock R., Manry J., Renwick A. & other authors (2005a). Microbial DNA typing by automated repetitive-sequence-based PCR. J Clin Microbiol 43, 199–207 10.1128/JCM.43.1.199-207.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy M., Reece K., Walton D., Huong J., Frye S., Raad I. I., Kontoyiannis D. P. (2005b). Use of the Diversi Lab System for species and strain differentiation of Fusarium species isolates. J Clin Microbiol 43, 5278–5280 10.1128/JCM.43.10.5278-5280.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- High N. J., Jennings M. P., Moxon E. R. (1996). Tandem repeats of the tetramer 5′-CAAT-3′ present in lic2A are required for phase variation but not lipopolysaccharide biosynthesis in Haemophilus influenzae. Mol Microbiol 20, 165–174 10.1111/j.1365-2958.1996.tb02498.x [DOI] [PubMed] [Google Scholar]
- Hood D. W., Deadman M. E., Cox A. D., Makepeace K., Martin A., Richards J. C., Moxon E. R. (2004). Three genes, lgtF, lic2C and lpsA, have a primary role in determining the pattern of oligosaccharide extension from the inner core of Haemophilus influenzae LPS. Microbiology 150, 2089–2097 10.1099/mic.0.26912-0 [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Higgins C. F., Sharp P. M. (1991). ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol 5, 825–834 10.1111/j.1365-2958.1991.tb00755.x [DOI] [PubMed] [Google Scholar]
- Kang H. P., Dunne W. M. (2003). Stability of repetitive-sequence PCR patterns with respect to culture age and subculture frequency. J Clin Microbiol 41, 2694–2696 10.1128/JCM.41.6.2694-2696.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. (1976). A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol 93, 9–62 10.1099/00221287-93-1-9 [DOI] [PubMed] [Google Scholar]
- Ladhani S. N. (2012). Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clin Ther 34, 385–399 10.1016/j.clinthera.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Ladhani S., Slack M. P., Heath P. T., Von Gottberg A., Chandra M., Ramsay M. E., European Union Invasive Bacterial Infection Surveillance participants (2010). Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg Infect Dis 16, 455–463 10.3201/eid1603.090290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S. N., Collins S., Vickers A., Litt D. J., Crawford C., Ramsay M. E., Slack M. P. (2012). Invasive Haemophilus influenzae serotype e and f disease, England and Wales. Emerg Infect Dis 18, 725–732 10.3201/eid1805.111738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil J. R., Cohn A. C., Farley M., Mair R., Baumbach J., Bennett N., Gershman K., Harrison L. H., Lynfield R. & other authors (2011). Current epidemiology and trends in invasive Haemophilus influenzae disease – United States, 1989–2008. Clin Infect Dis 53, 1230–1236 10.1093/cid/cir735 [DOI] [PubMed] [Google Scholar]
- Meats E., Feil E. J., Stringer S., Cody A. J., Goldstein R., Kroll J. S., Popovic T., Spratt B. G. (2003). Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 41, 1623–1636 10.1128/JCM.41.4.1623-1636.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhlanga-Mutangadura T., Morlin G., Smith A. L., Eisenstark A., Golomb M. (1998). Evolution of the major pilus gene cluster of Haemophilus influenzae. J Bacteriol 180, 4693–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. K., Moss W. J., Halsey N. (2008). Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect Dis 8, 435–443 10.1016/S1473-3099(08)70152-X [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Kroll J. S. (1988). Type b capsular polysaccharide as a virulence factor of Haemophilus influenzae. Vaccine 6, 113–115 10.1016/S0264-410X(88)80011-2 [DOI] [PubMed] [Google Scholar]
- Murray P. R., Baron E. J., Jorgensen J. H., Pfaller M. A., Yolken R. H. (eds) (2003). Manual of Clinical Microbiology Washington, DC: American Society for Microbiology.
- Nakamura S., Shchepetov M., Dalia A. B., Clark S. E., Murphy T. F., Sethi S., Gilsdorf J. R., Smith A. L., Weiser J. N. (2011). Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog 7, e1001247 10.1371/journal.ppat.1001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. L., Smith A. L. (2010). Determination of capsulation status in Haemophilus influenzae by multiplex polymerase chain reaction. Diagn Microbiol Infect Dis 66, 235–240 10.1016/j.diagmicrobio.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta D. M., Jackson M. A., Burry V. F., Olson L. C. (1995). Invasive Haemophilus influenzae type f disease. Pediatr Infect Dis J 14, 157–160 10.1097/00006454-199502000-00019 [DOI] [PubMed] [Google Scholar]
- Nizet V., Colina K. F., Almquist J. R., Rubens C. E., Smith A. L. (1996). A virulent nonencapsulated Haemophilus influenzae. J Infect Dis 173, 180–186 10.1093/infdis/173.1.180 [DOI] [PubMed] [Google Scholar]
- Omikunle A., Takahashi S., Ogilvie C. L., Wang Y., Rodriguez C. A., St Geme J. W., III, Adderson E. E. (2002). Limited genetic diversity of recent invasive isolates of non-serotype b encapsulated Haemophilus influenzae. J Clin Microbiol 40, 1264–1270 10.1128/JCM.40.4.1264-1270.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott-Howard J., Williams J. D. (1982). Increase in antibiotic resistance in Haemophilus influenzae in the United Kingdom since 1977: report of study group. Br Med J (Clin Res Ed) 284, 1597–1599 10.1136/bmj.284.6329.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. (1982). Do pili play a role in pathogenicity of Haemophilus influenzae type b? Lancet 320, 960–962 10.1016/S0140-6736(82)90161-1 [DOI] [PubMed] [Google Scholar]
- Pittman M. (1931). Variation and type specificity in the bacterial species Hemophilus influenzae. J Exp Med 53, 471–492 10.1084/jem.53.4.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resman F., Ristovski M., Ahl J., Forsgren A., Gilsdorf J. R., Jasir A., Kaijser B., Kronvall G., Riesbeck K. (2011a). Invasive disease caused by Haemophilus influenzae in Sweden 1997-2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin Microbiol Infect 17, 1638–1645 10.1111/j.1469-0691.2010.03417.x [DOI] [PubMed] [Google Scholar]
- Resman F., Svensjö T., Ünal C., Cronqvist J., Brorson H., Odenholt I., Riesbeck K. (2011b). Necrotizing myositis and septic shock caused by Haemophilus influenzae type f in a previously healthy man diagnosed with an IgG3 and a mannose-binding lectin deficiency. Scand J Infect Dis 43, 972–976 10.3109/00365548.2011.589079 [DOI] [PubMed] [Google Scholar]
- Rodriguez C. A., Avadhanula V., Buscher A., Smith A. L., St Geme J. W., III, Adderson E. E. (2003). Prevalence and distribution of adhesins in invasive non-type b encapsulated Haemophilus influenzae. Infect Immun 71, 1635–1642 10.1128/IAI.71.4.1635-1642.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubach M. P., Bender J. M., Mottice S., Hanson K., Weng H. Y., Korgenski K., Daly J. A., Pavia A. T. (2011). Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA. Emerg Infect Dis 17, 1645–1650 10.3201/eid1709.101991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele D. W., Syriopoulou V. P., Harding A. L., Emerson B. B., Smith A. L. (1976). Evaluation of a rapid β-lactamase test for detecting ampicillin-resistant strains of Hemophilus influenzae type b. Pediatrics 58, 382–387 [PubMed] [Google Scholar]
- St Geme J. W., III, Falkow S., Barenkamp S. J. (1993). High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A 90, 2875–2879 10.1073/pnas.90.7.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., III, Cutter D., Barenkamp S. J. (1996). Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol 178, 6281–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., III, Kumar V. V., Cutter D., Barenkamp S. J. (1998). Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun 66, 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala A. K., Eskola J., Leinonen M., Käyhty H., Nissinen A., Pekkanen E., Mäkelä P. H. (1991). Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis 164, 982–986 10.1093/infdis/164.5.982 [DOI] [PubMed] [Google Scholar]
- Tsang R. (2007). Capsule switching and capsule replacement in vaccine-preventable bacterial diseases. Lancet Infect Dis 7, 569–570 10.1016/S1473-3099(07)70191-3 [DOI] [PubMed] [Google Scholar]
- Tsang R. S., Mubareka S., Sill M. L., Wylie J., Skinner S., Law D. K. (2006). Invasive Haemophilus influenzae in Manitoba, Canada, in the postvaccination era. J Clin Microbiol 44, 1530–1535 10.1128/JCM.44.4.1530-1535.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Shibasaki Y., Yamamoto K., Chiba N., Hasegawa K., Takeuchi Y., Sunakawa K., Inoue M., Konno M. (2001). Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother 45, 1693–1699 10.1128/AAC.45.6.1693-1699.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin G., Krohn J. A., Deaver-Robinson K., Wenger J. D., Farley M. M., the Haemophilus influenzae Study Group (1996). Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiologic characteristics in the H. influenzae serotype b vaccine era. Clin Infect Dis 22, 1069–1076 10.1093/clinids/22.6.1069 [DOI] [PubMed] [Google Scholar]
- Ward J. I. (1996). Invasive infections due to Haemophilus influenzae serotype f (Hif) – is Hif an emerging pathogen? Clin Infect Dis 22, 1077–1078 10.1093/clinids/22.6.1077 [DOI] [PubMed] [Google Scholar]
- Watson M. E., Jr, Burns J. L., Smith A. L. (2004). Hypermutable Haemophilus influenzae with mutations in mutS are found in cystic fibrosis sputum. Microbiology 150, 2947–2958 10.1099/mic.0.27230-0 [DOI] [PubMed] [Google Scholar]
- Watson M. E., Jr, Davila S. Z., Burnham C. A., Nguyen V., Nelson K. L., Smith A. L., Storch G. A. (2011). Thigh abscess due to Haemophilus influenzae type f in a human immunodeficiency virus-positive child. Infect Dis Clin Pract 19, e21–e23 10.1097/IPC.0b013e31821896af [DOI] [Google Scholar]
- Weiser J. N., Pan N. (1998). Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol 30, 767–775 10.1046/j.1365-2958.1998.01108.x [DOI] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. (1977). The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis 135, 34–41 10.1093/infdis/135.1.34 [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Smith H. O. (1975). Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5′-triphosphate-dependent deoxyribonuclease activity. J Bacteriol 122, 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. J., Morlin G., Valentine N., Smith A. L. (2001). Serum resistance in an invasive, nontypeable Haemophilus influenzae strain. Infect Immun 69, 695–705 10.1128/IAI.69.2.695-705.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. (1983). Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis 148, 385–394 10.1093/infdis/148.3.385 [DOI] [PubMed] [Google Scholar]


