Abstract
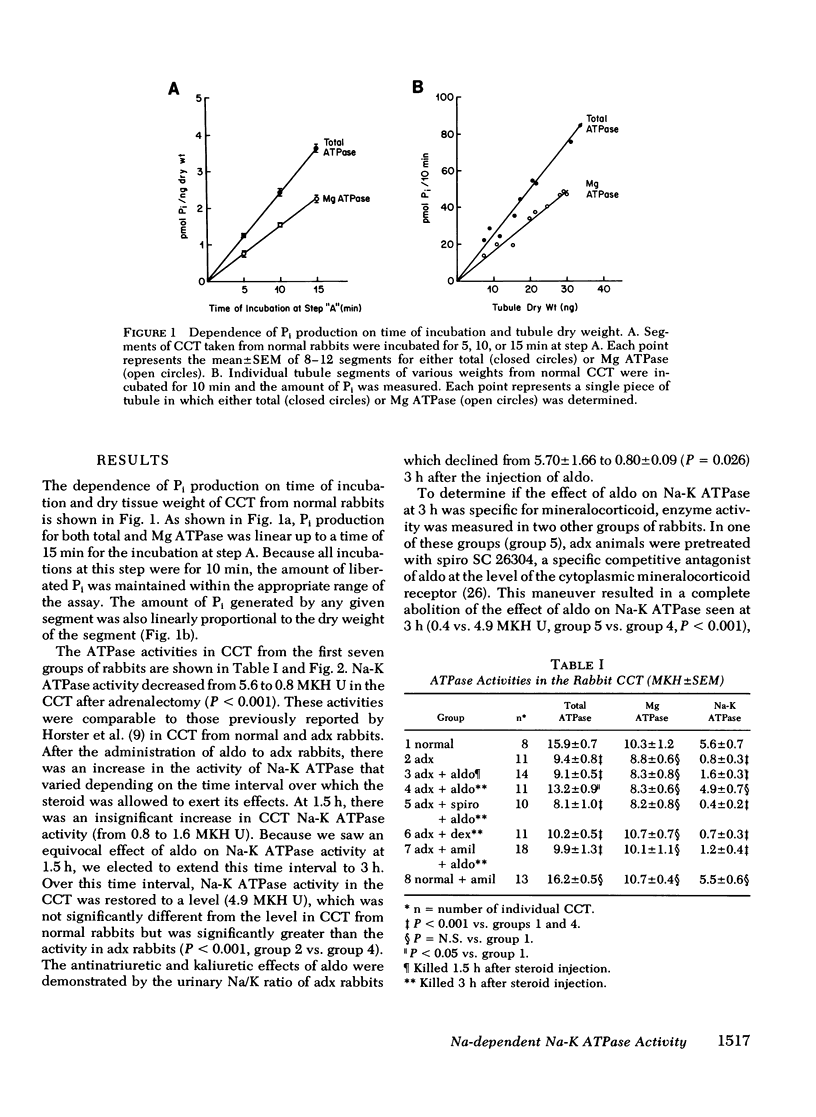
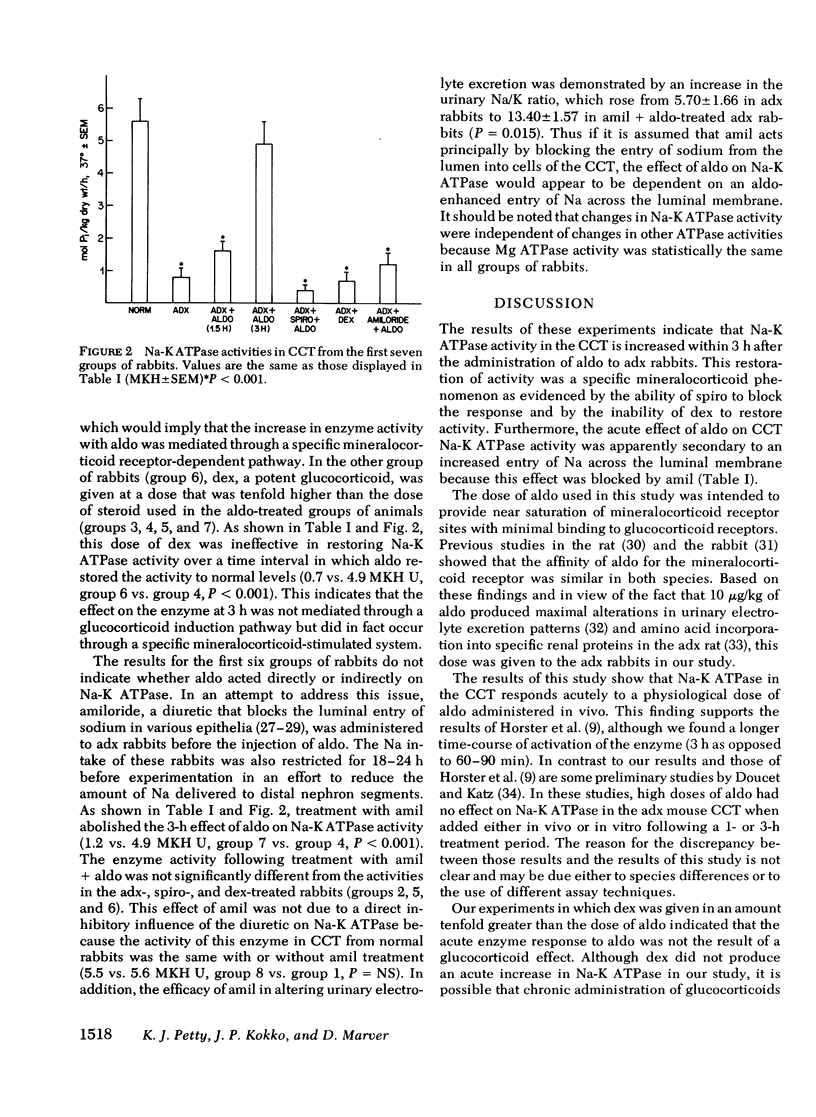
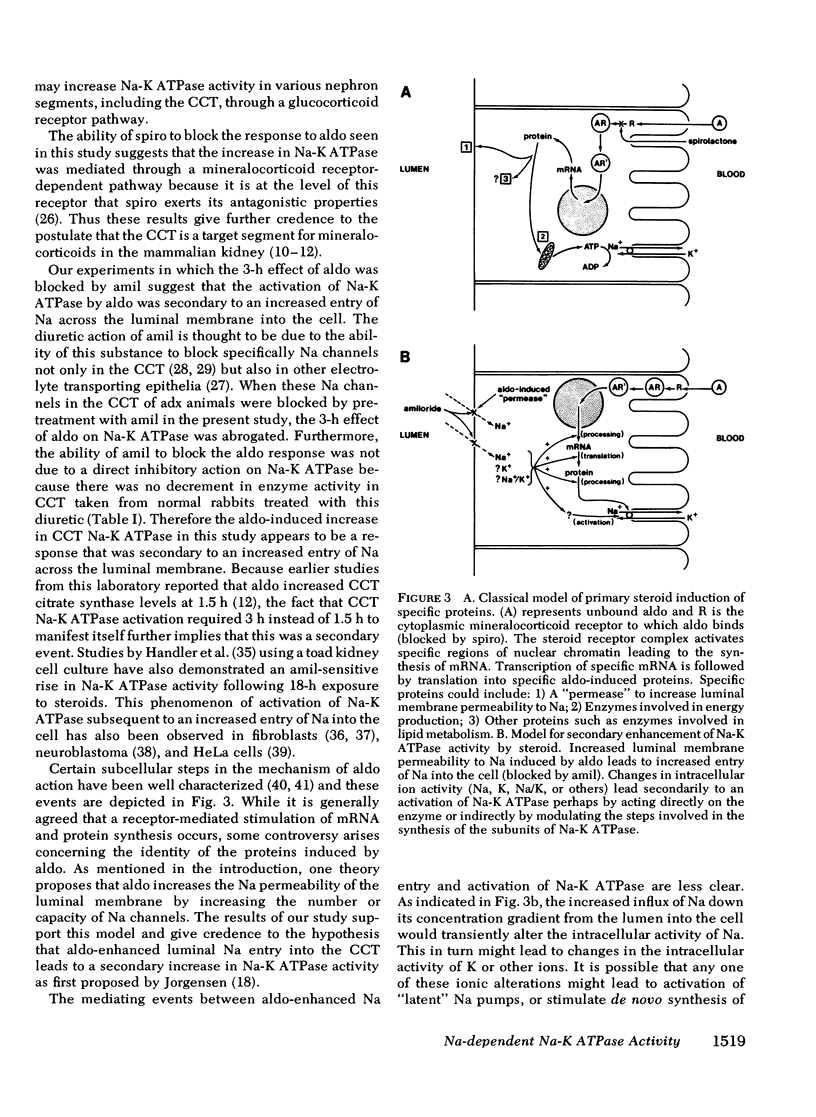
The possibility that mineralocorticoids have a direct influence on renal Na-K ATPase activity has been the focus of intense research effort and some controversy for a number of years. Early studies were hindered by an inability to differentiate between possible glucocorticoid vs. mineralocorticoid effects on this enzyme within the multitude of cells that comprise the heterogeneous mammalian nephron. This study attempts to circumvent this problem by monitoring Na-K ATPase activity in the rabbit renal cortical collecting tubule (CCT), a proposed target epithelium for mineralocorticoids. Using an ultramicro assay, Na-K ATPase activity was measured in CCT from normal, adrenalectomized (adx), and adx rabbits subjected to one of several corticosteroid treatment protocols. The results indicate that Na-K ATPase activity in the CCT decreased by 86% subsequent to adrenalectomy. Injection of physiological doses of aldosterone (10 micrograms/kg) but not dexamethasone (100 micrograms/kg) restored CCT Na-K ATPase activity in adx rabbits to normal levels within 3 h after injection. An insignificant rise in activity was observed 1.5h after aldosterone treatment. In addition, spirolactone SC 26304, a specific mineralocorticoid antagonist, blocked the action of aldosterone on Na-K ATPase.. Therefore an acute increase in Na-K ATPase activity participates in the action of aldosterone on Na transport in this segment. To differentiate between primary vs. secondary activation of this enzyme, adx animals were treated with amiloride before the injection of aldosterone with the intent of blocking luminal membrane Na entry into CCT. In these animals, pretreatment with amiloride blocked the increase in CCT Na-K ATPase act activity seen with aldosterone alone at 3 h. Thus the increase in activity with aldosterone appears to be a secondary adaptation that is dependent on an aldosterone-enhanced increase in the passive entry of Na across the luminal membrane. The subcellular mechanism by which Na modulates Na-K ATPase activity remains obscure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONTING S. L., CANADY M. R. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE AND SODIUM TRANSPORT IN TOAD BLADDER. Am J Physiol. 1964 Nov;207:1005–1009. doi: 10.1152/ajplegacy.1964.207.5.1005. [DOI] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L., Huett M., Lamb J. F., Newton J. P., Polson J. M. Evidence for the genetic control of the sodium pump density in HeLa cells. J Physiol. 1974 Sep;241(3):771–794. doi: 10.1113/jphysiol.1974.sp010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE J. SITE OF ACTION OF ALDOSTERONE ON THE BLADDER OF THE TOAD. Nature. 1963 Nov 23;200:787–788. doi: 10.1038/200787a0. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Silva P., Besarab A., Epstein F. H. Separate effects of aldosterone, DOCA, and methylprednisolone on renal Na-K-ATPase,. Am J Physiol. 1974 Aug;227(2):345–350. doi: 10.1152/ajplegacy.1974.227.2.345. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Titus E. Effect of adrenal steroids on a Na+- and K+-requiring adenosine triphosphatase from rat kidney. J Biol Chem. 1966 Nov 10;241(21):5083–5089. [PubMed] [Google Scholar]
- Crabbé J. Decreased sensitivity to amiloride of amphibian epithelia treated with aldosterone. Further evidence for an apical hormonal effect. Pflugers Arch. 1980 Jan;383(2):151–158. doi: 10.1007/BF00581876. [DOI] [PubMed] [Google Scholar]
- EDELMAN I. S., BOGOROCH R., PORTER G. A. ON THE MECHANISM OF ACTION OF ALDOSTERONE ON SODIUM TRANSPORT: THE ROLE OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1169–1177. doi: 10.1073/pnas.50.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Funder J. W., Edelman I. S. Subcellular mechanisms in the action of adrenal steroids. Am J Med. 1972 Nov;53(5):545–560. doi: 10.1016/0002-9343(72)90152-0. [DOI] [PubMed] [Google Scholar]
- Fimognari G. M., Fanestil D. D., Edelman I. S. Induction of RNA and protein synthesis in the action of aldosterone in the rat. Am J Physiol. 1967 Oct;213(4):954–962. doi: 10.1152/ajplegacy.1967.213.4.954. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology. 1973 Apr;92(4):994–1004. doi: 10.1210/endo-92-4-994. [DOI] [PubMed] [Google Scholar]
- Hendler E. D., Torretti J., Kupor L., Epstein F. H. Effects of adrenalectomy and hormone replacement on Na- K-ATPase in renal tissue. Am J Physiol. 1972 Mar;222(3):754–760. doi: 10.1152/ajplegacy.1972.222.3.754. [DOI] [PubMed] [Google Scholar]
- Hill J. H., Cortas N., Walser M. Aldosterone action and sodium- and potassium-activated adenosine triphosphatase in toad bladder. J Clin Invest. 1973 Jan;52(1):185–189. doi: 10.1172/JCI107163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster M., Schmid H., Schmidt U. Aldosterone in vitro restores nephron Na-K-ATPase of distal segments from adrenalectomized rabbits. Pflugers Arch. 1980 Apr;384(3):203–206. doi: 10.1007/BF00584554. [DOI] [PubMed] [Google Scholar]
- Imai M. The connecting tubule: a functional subdivision of the rabbit distal nephron segments. Kidney Int. 1979 Apr;15(4):346–356. doi: 10.1038/ki.1979.46. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Regulation of the (Na+ equals K+)-activated ATP hydrolyzing enzyme system in rat kidney. II. The effect of aldosterone on the activity in kidneys of adrenalectomized rats. Biochim Biophys Acta. 1969 Nov 18;192(2):326–334. [PubMed] [Google Scholar]
- Jorgensen P. L. The role of aldosterone in the regulation of (Na + + K + )-ATPase in rat kidney. J Steroid Biochem. 1972 Feb;3(2):181–191. doi: 10.1016/0022-4731(72)90049-0. [DOI] [PubMed] [Google Scholar]
- Kinne R., Kirsten R. Der Einfluss von Aldosteron auf die Aktivität mitochondrialer und cytoplasmatischer Enzyme in der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(4):244–254. [PubMed] [Google Scholar]
- Knox W. H., Sen A. K. Mechanism of action of aldosterone with particular reference to (Na plus K)-ATPase. Ann N Y Acad Sci. 1974;242(0):471–488. doi: 10.1111/j.1749-6632.1974.tb19111.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- Landon E. J., Jazab N., Forte L. Aldosterone and sodium-potassium-dependent ATPase activity of rat kidney membranes. Am J Physiol. 1966 Oct;211(4):1050–1056. doi: 10.1152/ajplegacy.1966.211.4.1050. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Edelman I. S. Effect of aldosterone on incorporation of amino acids into renal medullary proteins. J Membr Biol. 1978 Jun 22;41(1):15–40. doi: 10.1007/BF01873338. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Edelman I. S. Induction of citrate synthase by aldosterone in the rat kidney. J Membr Biol. 1978 Jun 22;41(1):41–64. doi: 10.1007/BF01873339. [DOI] [PubMed] [Google Scholar]
- Marver D. Aldosterone receptors in rabbit renal cortex and red medulla. Endocrinology. 1980 Feb;106(2):611–618. doi: 10.1210/endo-106-2-611. [DOI] [PubMed] [Google Scholar]
- Marver D., Schwartz M. J. Identification of mineralocorticoid target sites in the isolated rabbit cortical nephron. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3672–3676. doi: 10.1073/pnas.77.6.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver D., Stewart J., Funder J. W., Feldman D., Edelman I. S. Renal aldosterone receptors: studies with (3H)aldosterone and the anti-mineralocorticoid (3H)spirolactone (SC-26304). Proc Natl Acad Sci U S A. 1974 Apr;71(4):1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Boulpaep E. L. Effect of amiloride on the apical cell membrane cation channels of a sodium-absorbing, potassium-secreting renal epithelium. J Membr Biol. 1979 Nov 30;50(3-4):365–387. doi: 10.1007/BF01868898. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Gelehrter T. D., Legg A., Pettican P. Melittin stimulates Na entry, Na-K pump activity and DNA synthesis in quiescent cultures of mouse cells. Cell. 1981 Mar;23(3):781–788. doi: 10.1016/0092-8674(81)90442-6. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Schmid J., Schmid H., Dubach U. C. Sodium- and potassium-activated ATPase. A possible target of aldosterone. J Clin Invest. 1975 Mar;55(3):655–660. doi: 10.1172/JCI107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Schwartz M. J., Kokko J. P. Urinary concentrating defect of adrenal insufficiency. Permissive role of adrenal steroids on the hydroosmotic response across the rabbit cortical collecting tubule. J Clin Invest. 1980 Aug;66(2):234–242. doi: 10.1172/JCI109849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Coggins C. H., Lichtenstein N. S., Leaf A. Evidence for a mucosal effect of aldosterone on sodium transport in the toad bladder. J Clin Invest. 1966 Oct;45(10):1640–1647. doi: 10.1172/JCI105471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]


