Abstract
Background
Iron is an essential mineral for both cellular and pathogen survival and is essential for viral replication. In turn, iron metabolism has been shown to be altered by several viral infections. However, little is known regarding the association between iron status and HPV natural history. We hypothesize iron to be an HPV-cofactor that is associated with longer duration of infection.
Methods
Ferritin and soluble transferrin receptor (sTfR) were measured in baseline serum samples from 327 women enrolled in the Ludwig-McGill Cohort. Incident HPV clearance rates (any-type, oncogenic HPV, non-oncogenic HPV, and HPV-16) over 36 months were estimated from Cox-proportional hazard models accounting for correlations between multiple infections.
Results
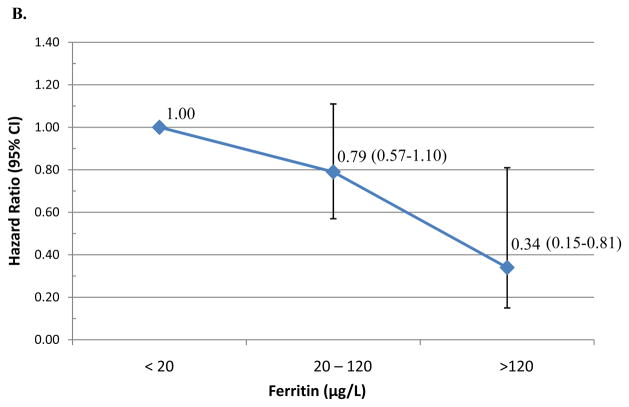
Women with ferritin levels above the median were less likely to clear an incident oncogenic HPV (AHR=0.73; 95%CI 0.55–0.96) and HPV-16 infections (AHR=0.29; 95%CI 0.11–0.73). Using physiological cut-points, women with enriched iron stores (≥120ug/L) were less likely to clear incident oncogenic HPV infections compared to those with low-levels of iron (<20ug/L)(AHR=0.34; 95%CI 0.15–0.81).
Conclusion
This study observed that women with the highest ferritin levels were less likely to clear incident oncogenic and HPV-16 infections compared to women with low ferritin. Rising iron stores may decrease probability of clearing new HPV infection, possibly by promoting viral activity and contributing to oxidative DNA damage.
Impact
This novel study suggests that elevated iron stores may put women at risk for persistent HPV infection, an early event in cervical carcinogenesis. Further examination of the association between iron status and HPV natural history is warranted.
Keywords: Human Papillomavirus, clearance, Ferritin, Iron, Transferrin
INTRODUCTION
Iron is an essential mineral that is required for oxygen transfer as well as cellular metabolism. Iron can exist in several oxidation states, a property that supports electron transfer for ATP generation as well as promotion of reactive oxygen species (ROS). Iron status has been examined as a contributor to carcinogenesis (1, 2) and associated with increased risk of cancer in several epidemiological studies.(1, 2) Several studies have documented that elevated iron promotes cancer cell proliferation and causes oxidative DNA damage through its interaction with oxygen and hydrogen peroxide.(3) ROS produced by elevated iron has been shown to directly alter the viral activity of several cancer-causing viruses, including Human papillomavirus (HPV), such as by directly influencing HPV transcriptional activity.(4)
Epidemiological studies have reported an association between longer duration of HPV infections and factors that induce ROS, such as smoking, co-infection with Chlamydia and reduced antioxidant intake.(5–7) Thus, we hypothesize that iron may be an HPV-cofactor that is associated with longer duration of infection and HPV-associated carcinogenesis. To date, the association between iron status and early events in cervical carcinogenesis, such as the inability to clear HPV infections, has not been investigated. Serum ferritin, an iron storage protein, and soluble transferrin receptor (sTfR), a cellular iron transport protein, have been shown to be reliable markers of iron status.(8) In addition, they can be used to calculate the sTfR-ferritin index (molar ratio of sTfR per ferritin), which is a robust biomarker for determining iron deficiency.(9, 10) Nested within the Ludwig-McGill study, we examined the association between biomarkers of iron status (ferritin, sTfR, and sTfR-F index) and clearance of incident HPV infection among 327 women contributing 494 infections (249 oncogenic, 245 non-oncogenic and 64 HPV-16 infections).
MATERIALS AND METHODS
Study Sample
The current analysis included women participating in the Ludwig-McGill cohort study, an HPV natural history study of 2528 low-income women living in São Paulo, Brazil recruited between 1993 and 1997. Study design, clinical sampling, and HPV testing for the Ludwig-McGill cohort study have been previously detailed(11). All HPV analyses were performed using standard polymerase chain reaction (PCR) of the L1 gene with MY09/11 consensus primers and the β-globin gene as an internal control as previously reported(11, 12). Study visits were every 4 months in the first year and twice yearly thereafter for up to 5 years. All participants signed an approved informed consent before entering the study. The study was approved by the institutional review board at each participating institutions. Women with normal cytology who were enrolled during the first 2 years of the Ludwig-McGill study that had an incident HPV infection over 3-years of follow-up and baseline serum available were included (N=327).
Serum Iron Marker Testing
Non-fasting blood samples were processed for serum and stored at −20°C. Ferritin (μg/L) and soluble transferrin receptor (sTfR; g/L) were measured in the baseline serum specimen as previously described.(9). The sTfR-ferritin index was calculated using the following formula: sTfR (μg/L)/log(ferritin(μg/L).(9, 10) sTfR and sTfR-F index are inversely associated with iron level, thus a high sTfR-F index reflects lower iron status. The percent coefficient of variability was 9% for ferritin and 4% for sTfR.(9) Serum levels of ferritin and sTfR are relatively stable over time and a single measurement from a subject reflects long-term average level.(10)
Statistical analysis
Time-to-clearance of HPV infection was defined as the time between the first positive HPV DNA test and the subsequent first negative test (clearance event). Clearance time was censored at the woman’s last visit if she did not clear the infection within 3-years of follow-up or at the first of two consecutive visits with missing HPV results. All clearance events were determined on a type-specific basis and then grouped as any HPV, oncogenic infections (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) or non-oncogenic infections (6/11, 26, 32, 34, 40, 42, 44, 53, 54, 55, 57, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, ref(13)). Analyses were conducted based on individual HPV infections; therefore, women infected with multiple HPV types contributed multiple outcome events (37%).
Iron status biomarkers were evaluated as continuous measures (log transformed for the skewed data) and dichotomized at the median. Since women in the cohort are mostly young premenopausal, we have used levels of ferritin <20μg/L as iron deficient, 20–120μg/L as iron adequate and >120μg/L as iron rich.(14) Differences in ferritin, sTfR, and sTfR-F index by baseline characteristics were tested by the Wilcoxon rank-sum or Kruskal-Wallis test. Median clearance time was estimated using the Kaplan-Meier method and evaluated using the log-rank test. We examined associations of baseline iron status with type-specific clearance of incident HPV infections during the first 3-years of follow-up using Cox proportional hazard models, with a robust sandwich estimator taking into account within-subject correlations.(15) Covariates in the final model were selected using backward selection based on models run individually for any type, oncogenic, non-oncogenic and HPV-16. Variables that were significant at 0.10 level were then adjusted for in the final models, including age, age at menarche, smoking status, education, number of lifetime sexual partners, oral contraceptive use, alcohol, age at first intercourse, and number of pregnancies. The proportional hazard assumption for each Cox model was met, as determined by the Kolmogorov-type Supremum test. All statistical tests were two-sided and considered as statistically significant at the level of 0.05. All analyses were performed with SAS (SAS9.2., SAS Institute).
RESULTS AND DISCUSSSION
Iron biomarker levels are presented by demographic and risk-factor characteristics (Table 1). The median levels of ferritin, sTfR, and sTfR-F index were 26.6 μg/L (range 1.0–391.5 μg/L), 2.0 (0.09–8.17 μg/L), and 1.08 (0.72–6.21), respectively. Among this population of pre-menopausal Brazilian women, median ferritin differed across age categories (≤20, 21–30, 31–40 and ≥40 years of age; p-value=0.05)). These data were similar to that previously reported among pre-menopausal women in the US (16) and a wide age-range of women in Brazil(18–81years).(17) Median sTfR and sTfR-index were significantly lower (reflecting higher iron status) among white women (p-value=0.003 and p-values=0.004), smokers (p-value=0.04 and p-value=0.09) and alcohol drinkers (p-value=0.04 and p-value=0.005), respectively. These observations of lower median sTfR levels among smokers and alcohol drinkers were consistent with a study by Pynaert et al,(18) which reported that never smokers has significantly higher sTfR levels compared to current smokers (1.12 vs. 1.05 μg/L).(18) Finally, iron levels were elevated, as measured by lower median sTfR-F index, with increasing duration of oral contraceptive use (p-value=0.01), with the largest difference between women taking oral contraceptive for over 6-years. Casabellata as colleagues reported similar finding however the duration of OC use was 3-months.(19)
Table 1.
Demographic characteristics among women who tested positive for any HPV type (N=327)
| n (%) | Ferritin (ug/L) | sTfR (ug/L) | sTfR-F Index | |
|---|---|---|---|---|
| Median (Min – Max) | Median (Min – Max) | Median (Min – Max) | ||
| Age (years)a | ||||
| ≤ 20 | 36 ( 11.0 ) | 26.6 (14.2 – 391.5) | 2.1 (1.3 – 5.0) | 1.2 (0.6 – 2.8) |
| 21 – 30 | 135 ( 41.3 ) | 30.5 (1.0 – 350.0) | 1.9 (0.1 – 4.8) | 1.0 (0.1 – 3.2) |
| 31 – 40 | 102 ( 31.2 ) | 26.4 (12.9 – 302.2) | 2.0 (1.1 – 8.2) | 1.1 (0.4 – 6.2) |
| > 40 | 54 ( 16.5 ) | 22.0 (11.5 – 315.9) | 2.0 (1.0 – 7.5) | 1.0 (0.6 – 4.6) |
| Race/Ethnicitybc | ||||
| White | 192 ( 58.9 ) | 26.4 (1.0–391.5) | 1.9 (0.1 – 8.2) | 1.0 (0.1 – 6.2) |
| Non-White | 134 ( 41.1 ) | 27.5 (14.3 – 350.0) | 2.1 (1.1 – 7.1) | 1.2 (0.4 – 4.3) |
| Marital Status | ||||
| Common Law | 113 ( 34.6 ) | 29.3 (13.1 – 338.7) | 2.0 (1.2 – 7.1) | 1.1 (0.6 – 4.3) |
| Divorced | 28 ( 8.6 ) | 22.1 (11.5 – 184.4) | 1.9 (1.0 – 7.5) | 1.0 (0.6 – 4.6) |
| Married | 109 ( 33.3 ) | 25.1 (1.0 – 302.2) | 2.0 (0.1 – 8.2) | 1.0 (0.1 – 6.2) |
| Single | 70 ( 21.4 ) | 31.7 (10.9 – 391.5) | 2.1 (1.0 – 4.5) | 1.2 (0.5 – 2.9) |
| Widow | 7 ( 2.1 ) | 21.3 (15.5 – 46.5) | 1.8 (1.4 – 2.1) | 0.9 (0.6 –1.3) |
| Education | ||||
| < Elementary | 63 ( 19.3 ) | 27.6 (12.9 – 315.9) | 2.2 (1.1 – 5.8) | 1.2 (0.4 – 3.1) |
| Elementary | 181 ( 55.5 ) | 26.4 (1.0 – 391.5) | 2.0 (0.1 – 7.5) | 1.0 (0.1 – 4.6) |
| < High School | 44 ( 13.5 ) | 36.7 (10.9 – 338.7) | 1.9 (1.0 – 4.5) | 1.0 (0.6 – 2.9) |
| ≥ High School | 38 ( 11.7 ) | 24.5 (13.2 – 209.1) | 1.8 (1.2 – 8.2) | 1.0 (0.5 – 6.2) |
| Monthly Income (US$) | ||||
| < 250 | 75 ( 23.7 ) | 26.5 (11.54 – 350.0) | 2.0 (1.0 – 7.1) | 1.1 (0.4 – 4.3) |
| 250 – 450 | 83 ( 26.2 ) | 26.6 (1.0 – 240.1) | 1.9 (0.1 – 7.5) | 1.1 (0.1 – 4.6) |
| 451 – 724 | 71 ( 22.4 ) | 27.9 (13.1 – 230.2) | 2.0 (1.2 – 5.2) | 1.1 (0.6 – 4.5) |
| ≥ 725 | 88 ( 27.8 ) | 28.8 (10.9 – 391.5) | 2.0 (1.0 – 5.4) | 1.1 (0.6 – 3.2) |
| Smoking statusb | ||||
| Never | 161 ( 49.4 ) | 27.7 (1.0 – 391.5) | 2.1 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
| Former | 55 ( 16.9 ) | 28.4 (13.1 – 315.9) | 1.9 (1.1 – 5.0) | 1.0 (0.4 – 3.1) |
| Current | 110 ( 33.7 ) | 26.0 (12.9 – 350.0) | 1.9 (1.2 – 7.5) | 1.0 (0.5 – 4.6) |
| Alcohol usebc | ||||
| Yes | 237 ( 72.5 ) | 26.6 (1.0 – 391.5) | 2.0 (0.1 – 7.1) | 1.0 (0.1 – 4.3) |
| No | 90 ( 27.5 ) | 27.5 (11.5 – 315.9) | 2.1 (1.0 – 8.2) | 1.2 (0.5 – 6.2) |
| Oral contraceptive usec | ||||
| Never | 61 ( 18.7 ) | 26.5 (10.9 – 391.5) | 2.0 (1.0 – 7.5) | 1.2 (0.5 – 4.6) |
| < 6 years | 185 ( 56.8 ) | 29.4 (11.5 – 247.2) | 2.1 (1.0 – 8.2) | 1.1 (0.6 – 6.2) |
| ≥ 6 years | 80 ( 24.5 ) | 22.1 (1.0 – 338.7) | 1.9 (0.1 – 5.2) | 0.9 (0.1 – 4.5) |
| Total no. of pregnancies | ||||
| 0 – 1 | 67 ( 20.7 ) | 26.3 (10.9 – 391.5) | 2.0 (1.0 – 4.5) | 1.1 (0.6 – 2.9) |
| 2 – 3 | 133 ( 41.2 ) | 28.9 (1.0 – 240.1) | 1.9 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
| 4 – 6 | 94 ( 29.1 ) | 25.7 (11.5 – 315.9) | 2.0 (1.0 – 5.4) | 1.0 (0.4 – 3.1) |
| 7+ | 29 ( 9.0 ) | 33.8 (15.2 – 104.4) | 2.2 (1.4 – 7.1) | 1.2 (0.6 – 4.3) |
| Age at first intercourse | ||||
| ≤ 15 | 101 ( 31.0 ) | 27.2 (1.0 – 391.5) | 2.1 (0.1 – 5.4) | 1.1 (0.1 – 3.2) |
| 16 – 17 | 88 ( 27.0 ) | 26.4 (11.5 – 338.7) | 1.9 (1.0 – 7.1) | 0.9 (0.6 – 4.3) |
| 18 – 19 | 73 ( 22.4 ) | 28.0 (10.9 – 184.4) | 2.0 (1.0 – 5.0) | 1.1 (0.6 – 3.8) |
| ≥ 20 | 64 ( 19.6 ) | 26.7 (15.5 – 350.0) | 2.0 (1.1 – 8.2) | 1.2 (0.4 – 6.2) |
| Lifetime no. of sexual partnersbc | ||||
| 0 – 1 | 112 ( 34.4 ) | 25.9 (1.0 – 338.7) | 2.0 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
| 2 – 3 | 125 ( 38.3 ) | 29.1 (10.9 – 391.5) | 2.1 (1.0 – 7.1) | 1.2 (0.6 – 4.3) |
| ≥ 4 | 89 ( 27.3 ) | 25.5 (11.5 – 209.1) | 1.8 (1.0 – 5.0) | 1.0 (0.5 – 3.8) |
| Total no. of sexual partners in the last five years | ||||
| 0 – 1 | 208 ( 63.8 ) | 28.5 (1.0 – 338.7) | 2.0 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
| ≥ 2 | 118 ( 36.2 ) | 26.0 (10.9 – 391.5) | 2.0 (1.0 – 5.4) | 1.1 (0.6 – 3.2) |
| Total no. of sexual partners during the last year | ||||
| 0 – 1 | 291 ( 89.8 ) | 27.5 (1.0 – 391.5) | 2.0 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
| ≥ 2 | 33 ( 10.2 ) | 27.0 (13.2 – 350.0) | 2.1 (1.2 – 4.7) | 1.1 (0.7 – 2.6) |
| Age at Menarche | ||||
| 0–11 years | 74 ( 22.6 ) | 26.3 (13.1 – 209.1) | 2.0 (1.2 – 8.2) | 1.1 (0.6 – 6.2) |
| 12–19 years | 253 ( 77.4 ) | 27.5 (1.0 – 391.5) | 2.0 (0.1 – 7.5) | 1.1 (0.1 – 4.6) |
| Condom use | ||||
| Always | 14 ( 4.3 ) | 33.4 (18.8 – 153.9) | 1.8 (1.3 – 3.6) | 1.0 (0.6 – 2.8) |
| Never/Occasionally | 313 ( 95.7 ) | 26.6 (1.0 – 391.5) | 2.0 (0.1 – 8.2) | 1.1 (0.1 – 6.2) |
Kruskal-Wallis test was used to compare means,
Ferritin vary significantly,
sTfR vary significantly,
sTfR per Ferritin vary significantly,
sTfR= Soluble Transferrin Receptor
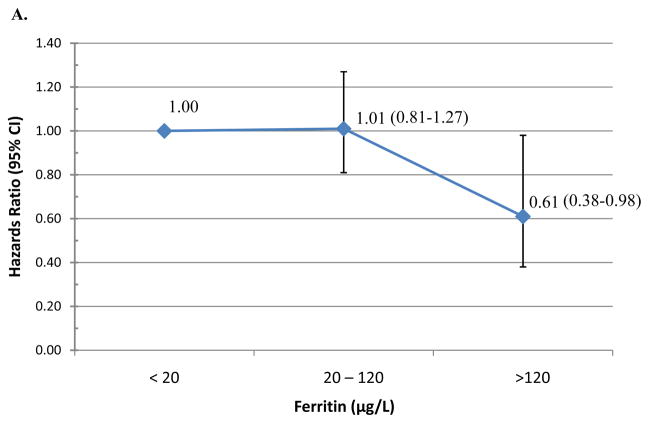
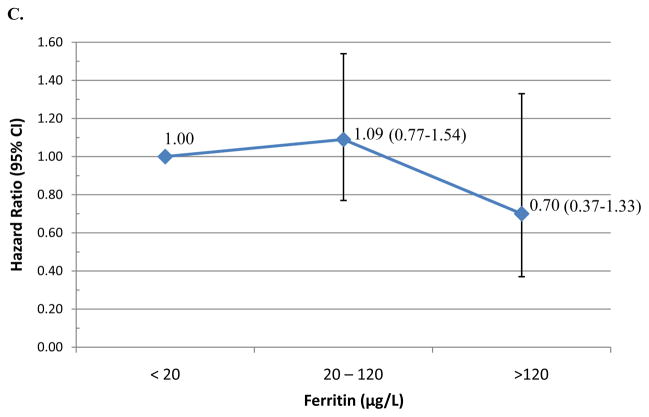
Table 2 presents the median time-to-clearance of type-specific incident HPV infections and the adjusted hazard ratios (AHR) of type-specific HPV clearance by iron status. Median duration of HPV infections did not significantly differ by iron status (Table 2). However, women with ferritin levels above the median were less likely to clear an incident oncogenic HPV infection (AHR=0.73; 95% CI 0.55–0.96). Using physiological cut-points, women with enriched iron stores (≥120 μg/L) were less likely to clear incident any type HPV (AHR=0.61, 95% CI 0.26–1.41; Figure 1A) or oncogenic HPV infections (AHR=0.34; 95% CI 0.15–0.81; Figure 1B) compared to those with low-levels of iron (<20μg/L). There was no significant association between ferritin at adequate or enriched levels and clearance of incident non-oncogenic HPV infections (Figure 1C). A total of 57 incident HPV-16 infections were detected during the first three years of follow-up. Overall, the median duration of an HPV-16 infection was 6.9 months (95% CI 6.0–12.1) and 9.6 months (95% CI 6.0–12.2) for women with ferritin below or above the 26.6 μg/L (p-value=0.86) cut-point. Women with elevated ferritin were less likely to clear HPV-16 infections (AHR=0.29, 95%CI 0.11–0.73). There was no significant difference in HPV-16 clearance by sTfR level or sTfR-F index.
Table 2.
Incident type specific median clearance time in months and risk of clearance by biomarkers of iron status.
|
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any HPVa | Oncogenic HPV | Non-oncogenic HPV | HPV 16 | ||||||||||||
| n | Median (95% CI)b | Adjusted HR (95% CI)c | n | Median (95% CI) | Adjusted HR (95% CI) | n | Median (95% CI) | Adjusted HR (95% CI) | n | Median (95% CI) | Adjusted HR (95% CI) | ||||
|
|
|||||||||||||||
| Ferritind | |||||||||||||||
| Continuous | 453 | - | 0.94 (0.82, 1.06) | 226 | - | 0.89 (0.73, 1.10) | 227 | - | 0.96 (0.80, 1.14) | 57 | - | 0.60 (0.26, 1.41) | |||
| Dichotomize | |||||||||||||||
| <26.6 | 228 | 6.67 (6.01, 8.54) | Ref | 116 | 8.05 (6.08, 11.73) | Ref | 112 | 6.01 (5.88, 8.05) | Ref | 28 | 6.90 (5.95, 12.01) | Ref | |||
| ≥ 26.6 | 233 | 7.89 (6.01, 9.89) | 0.88 (0.72, 1.07) | 116 | 9.79 (6.28, 11.99) | 0.73 (0.55, 0.96) | 117 | 6.01 (5.68, 8.05) | 0.99 (0.76, 1.29) | 29 | 9.63 (6.01, 12.19) | 0.29 (0.11, 0.73) | |||
| sTfRd | |||||||||||||||
| Continuous | 453 | - | 1.17 (0.94, 1.45) | 226 | - | 1.14 (0.86, 1.51) | 227 | - | 1.14 (0.81, 1.60) | 57 | - | 0.45 (0.12, 1.71) | |||
| Dichotomize | |||||||||||||||
| < 1.97 | 232 | 6.54 (6.01, 8.54) | Ref | 117 | 7.95 (6.08, 11.73) | Ref | 115 | 6.01 (5.85, 8.05) | Ref | 33 | 7.95 (6.01, 12.52) | Ref | |||
| ≥ 1.97 | 229 | 7.95 (6.01, 11.01) | 0.94 (0.77, 1.16) | 115 | 10.81 (6.54, 11.96) | 0.80 (0.58, 1.10) | 114 | 6.01 (5.62, 9.03) | 1.00 (0.75, 1.33) | 24 | 6.70 (5.91, 11.99) | 0.54 (0.19, 1.52) | |||
| sTfR-F Indexd | |||||||||||||||
| Continuous | 453 | - | 1.12 (0.92, 1.36) | 226 | - | 1.07 (0.79, 1.44) | 227 | - | 1.14 (0.86, 1.51) | 57 | - | 0.56 (0.22, 1.48) | |||
| Dichotomize | |||||||||||||||
| <1.08 | 229 | 7.36 (6.01, 9.79) | Ref | 117 | 8.05 (6.08, 11.70) | Ref | 112 | 6.05 (5.85, 9.63) | Ref | 32 | 7.06 (5.95, 12.19) | Ref | |||
| ≥ 1.08 | 232 | 6.77 (6.01, 10.81) | 1.01 (0.82, 1.25) | 115 | 11.01 (6.44, 11.96) | 0.83 (0.60, 1.16) | 117 | 6.01 (5.68, 7.92) | 1.14 (0.86, 1.52) | 25 | 6.77 (6.01, 12.02) | 0.65 (0.33, 1.29) | |||
Infections, not women, were used as the unit of analyses for each outcome.
Log-Rank test for differences in median time to clearance were not significant for all comparisons.
All Cox models were adjusted for age, condom use, education, monthly income, menarche, lifetime number of sexual partners, oral contraceptive use, race, and smoking status.
Continuous measures of Iron Status were log transformed.
sTfR=Soluble transferrin receptor, CI=Confidence Interval, Ref=Reference group
Figure 1. Hazard of clearing incident HPV infection.
by clinical Ferritin cutpoints with the reference <20 value: (A) Any-type HPV clearance; (B) Oncogenic HPV clearance; and (C) non-oncogenic HPV clearance.
Overall, we found that women with ferritin levels above the median were less likely to clear an incident oncogenic and HPV-16 infection. Our findings are consistent with our hypothesis that rising iron stores may increase risk of persistent HPV infection (reduced clearance) by promoting viral activity and contributing to oxidative DNA damage. Iron is a growth nutrient for humans and is required for DNA replication (2); however, it is also essential for pathogens to survive and shown essential for some viral replication.(20) Iron metabolism has been shown to be altered by several viral infections, including HIV and CMV(20); however, relatively little is known about how HPV utilizes cellular iron. In vitro, elevated iron concentrations promoted cell growth of HPV-16 SiHa cells, increased E6/E7 expression (21), and treatment with iron chelators induced growth arrest and apoptosis.(22) Furthermore, HPV is dependent on iron sensitive host transcription factors, such as NFk-B (23), for viral gene expression. Thus, elevated iron stores, (e.g. elevated ferritin), may promote viral activity and persistence by increasing the activity of cellular transcription. As viruses require iron for replication and transcription, it is biologically plausible that rising iron stores may increase risk for persistent HPV infection by promoting viral activity.
Iron also contributes to oxidative DNA damage which is an additional mechanism by which elevated iron stores may be associated with decreased clearance. Due to its ability to interact with oxygen and hydrogen peroxide, iron is an active metal species responsible for generating reactive oxygen species (ROS) through Fenton, Haber-Weiss, or iron auto-oxidation reactions.(3) ROS contribute to carcinogenesis by oxidizing cellular proteins and DNA that could result in (1) lethal mutations, (2) down regulatation of host immunity, and/or (3) altering cellular activity by activating AP-1 and NF-κB (transcription factors), cell proliferation, and apoptosis.(24, 25) Thus, it is biologically plausible that excess iron stores leads to ROS which can promote HPV viral replication and transcriptional activity (expression of HPV 16 E6 and E7 proteins), as well as cell proliferation and apoptosis; all pivotal events in cervical carcinogenesis.
HPV clearance was not associated with sTfR or sTfR-F index. Serum concentrations of sTfR are relatively stable and not influenced by infection or inflammation, unlike ferritin levels.(8) While sTfR is a good biomarker for iron deficiency(9), it may not be the most ideal biomarker when investigating the association between iron and viral infection. It is unclear why ferritin was the only iron marker that was associated with longer duration of HPV infection.
As with any observational study, there are strengths and limitations that need to be considered when interpreting the findings. In view of this being a cohort of mostly young women, we utilized a comprehensive approach in evaluating the associations between iron biomarkers and HPV clearance by examining iron across the range of values (continuous measures), dichotomized at the median, and clinically defined cutpoints as deficient, adequate, and relatively enriched iron status.(14) Furthermore, we analyzed only incident HPV outcomes (any type infection, oncogenic, non-oncogenic and HPV-16 infections). This study was nested within the Ludwig-McGill Cohort study, which had a relatively large sample size, providing sufficient power to adequately test our a priori hypothesis. As in any observational study, there is a possibility our finding were due to chance. Similar to other biological markers, the iron biomarker values may not reflect the absolute value due to possible loss during specimen processing, storage and/or extraction; however, this loss would be similar across all samples and not differ by HPV status. Therefore, the associations observed within this study should be valid with potentially a lower magnitude of the associations due to methodological errors. The study population was primarily pre-menopausal, with only 2.8% of women reported as post-menopausal at enrollment and was adequately reflected with the lower ferritin levels observed among premenopausal women.
In conclusion, we observed that women in the highest category of ferritin levels were less likely to clear incident oncogenic and HPV-16 infections compared to women with the low-levels of ferritin. This association was strongest among women with enriched iron stores (≥120 μg/L). Rising iron stores may increase risk of persistent HPV infection (reduced clearance) by promoting viral replication and transcription as well as contributing to oxidative DNA damage. Further examination of the association between iron status and HPV natural history is warranted.
Acknowledgments
Financial Support: Financial support noted for each author by initials: NCI CA70269: LLV, ELF; NCI CA81310: EMS, NP, BL, JHL, XI, LLV, ELF, ARG; NCI R25CA078447: EMS; Canadian Institutes of Health Research CIHR MA-13647, MOP-49396: ELF; Ludwig Institute for Cancer Research Intramural Grant: LLV
We are indebted to Ms. Maria L. Baggio and Ms. Lenice Galan for management of the patients and specimen collections and to Ms. Silvaneide Ferreira and Ms. Raquel Hessel for data entry, sample retrieval and shipment, as well as laboratory analysis.
GRANT SUPPORT
Primary Funding: National Cancer Institute (CA70269, CA81310), NCI Cancer Prevention and Control Pre-Doctoral Fellowship (R25CA078447)
Funding for Ludwig-McGill Cohort Study: National Cancer Institute (CA70269), Canadian Institutes of Health Research (CIHR) (MA-13647, MOP-49396), and by an intramural grant by the Ludwig Institute for Cancer Research.
Footnotes
Conflicts of Interest: None of the contributing authors has a conflict of interest.
References
- 1.Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047–52. doi: 10.1056/NEJM198810203191603. [DOI] [PubMed] [Google Scholar]
- 2.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutation research. 2003;533:153–71. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Dai J, Fournier J, Ali AM, Zhang Q, Frenkel K. Ferrous ion autoxidation and its chelation in iron-loaded human liver HepG2 cells. Free radical biology & medicine. 2002;32:84–92. doi: 10.1016/s0891-5849(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 4.Palmer HJ, Paulson KE. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr Rev. 1997;55:353–61. doi: 10.1111/j.1753-4887.1997.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 5.Siegel EM, Salemi JL, Villa LL, Ferenczy A, Franco EL, Giuliano AR. Dietary consumption of antioxidant nutrients and risk of incident cervical intraepithelial neoplasia. Gynecologic oncology. 2010;118:289–94. doi: 10.1016/j.ygyno.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano A. Cervical carcinogenesis: the role of co-factors and generation of reactive oxygen species. Salud publica de Mexico. 2003;45 (Suppl 3):S354–60. doi: 10.1590/s0036-36342003000900009. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Closas R, Castellsague X, Bosch X, Gonzalez CA. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. International journal of cancer. 2005;117:629–37. doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 8.Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345–52. [PubMed] [Google Scholar]
- 9.Zeleniuch-Jacquotte A, Zhang Q, Dai J, Shore RE, Arslan AA, Koenig KL, et al. Reliability of serum assays of iron status in postmenopausal women. Annals of epidemiology. 2007;17:354–8. doi: 10.1016/j.annepidem.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MA, Akhmedkhanov A, Zeleniuch-Jaquotte A, Toniolo P, Frenkel K, Huang X. Reliability of serum iron, ferritin, nitrite, and association with risk of renal cancer in women. Cancer detection and prevention. 2003;27:116–21. doi: 10.1016/s0361-090x(03)00027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco E, Villa L, Rohan T, Ferenczy A, Petzl-Erler M, Matlashewski G. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Ludwig-McGill Study Group. Rev Panam Salud Publica. 1999;6:223–33. doi: 10.1590/s1020-49891999000900001. [DOI] [PubMed] [Google Scholar]
- 12.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. Journal of clinical microbiology. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ. The Robust Inference for the Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074– 8. [Google Scholar]
- 16.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 17.Mendes JF, Arruda SF, Siqueira EM, Ito MK, Silva EF. Iron status and oxidative stress biomarkers in adults: a preliminary study. Nutrition. 2009;25:379–84. doi: 10.1016/j.nut.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Pynaert I, De Bacquer D, Matthys C, Delanghe J, Temmerman M, De Backer G, et al. Determinants of ferritin and soluble transferrin receptors as iron status parameters in young adult women. Public health nutrition. 2009;12:1775–82. doi: 10.1017/S1368980008004369. [DOI] [PubMed] [Google Scholar]
- 19.Casabellata G, Di Santolo M, Banfi G, Stel G, Gonano F, Cauci S. Evaluation of iron deficiency in young women in relation to oral contraceptive use. Contraception. 2007;76:200–7. doi: 10.1016/j.contraception.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–52. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 21.Poljak-Blazi M, Jaganjac M, Sabol I, Mihaljevic B, Matovina M, Grce M. Effect of ferric ions on reactive oxygen species formation, cervical cancer cell lines growth and E6/E7 oncogene expression. Toxicol In Vitro. 2011;25:160–6. doi: 10.1016/j.tiv.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Simonart T, Boelaert JR, Mosselmans R, Andrei G, Noel JC, De Clercq E, et al. Antiproliferative and apoptotic effects of iron chelators on human cervical carcinoma cells. Gynecologic oncology. 2002;85:95–102. doi: 10.1006/gyno.2001.6570. [DOI] [PubMed] [Google Scholar]
- 23.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. Journal of cellular biochemistry. 2008;104:657–67. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 25.Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer letters. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]