SUMMARY
Ongoing transmission and re-infection, primarily in congregate settings, is a key factor fueling the global multidrug-resistant/extensively drug-resistant tuberculosis (MDR/XDR-TB) epidemic, especially in association with the human immunodeficiency virus. Even as efforts to broadly implement conventional TB transmission control measures begin, current strategies may be incompletely effective under the overcrowded conditions extant in high-burden, resource-limited settings. Longstanding evidence suggesting that TB patients on effective therapy rapidly become non-infectious and that unsuspected, untreated TB cases account for the most transmission makes a strong case for the implementation of rapid point-of-care diagnostics coupled with fully supervised effective treatment. Among the most important decisions affecting transmission, the choice of an MDR-TB treatment model that includes community-based treatment may offer important advantages over hospital or clinic-based care, not only in cost and effectiveness, but also in transmission control. In the community, too, rapid identification of infectious cases, especially drug-resistant cases, followed by effective, fully supervised treatment, is critical to stopping transmission. Among the conventional interventions available, we present a simple triage and separation strategy, point out that separation is intimately linked to the design and engineering of clinical space and call attention to the pros and cons of natural ventilation, simple mechanical ventilation systems, germicidal ultraviolet air disinfection, fit-tested respirators on health care workers and short-term use of masks on patients before treatment is initiated.
Keywords: nosocomial, resistance, drug, tuberculosis
In many high-burden countries, cases of multidrug-resistant tuberculosis (MDR-TB) are being generated by transmission in congregate settings (i.e., hospitals, clinics, prisons, and various crowded living situations) much faster than slowly emerging treatment programs can cure them. It is estimated that less than 10% of the estimated number of MDR-TB cases worldwide are being treated, and as many as half of MDR-TB cases occur in previously untreated cases, indicating transmission.1 Moreover, transmission is predominantly from unrecognized or inadequately treated persons with MDR and XDR-TB—a critically important spigot fueling the global drug-resistant TB epidemic, especially where the human immunodeficiency virus (HIV) infection is also prevalent. These statements are not intended to challenge the widely understood importance of erratic treatment in selecting for drug-resistant Mycobacterium tuberculosis strains. Rather, once mutant organisms are selected by poor chemotherapy, we emphasize the urgency of controlling their highly efficient airborne spread in congregate settings. By far the most important way to control transmission of MDR-TB in institutions, as well as in the community, is its prompt diagnosis and effective treatment.
In addition to other patients, health care workers and staff of other facilities, many HIV-co-infected in high-burden settings, are also at risk for infection and re-infection.2 Moreover, health care workers disabled by drug-resistant TB directly reduce the critical workforce needed to effectively treat TB and HIV patients, and fear of nosocomial infection indirectly undermines the staffing of in-patient, ambulatory, and community-based treatment programs.3 Summary reports of a series of recent Institute of Medicine meetings on the global MDR-TB crisis held in the United States, South Africa, and Russia highlight these important concerns.4
It is our contention that long-term control of drug-resistant TB will require not only an unprecedented massive scale-up of complex, effective treatment programs, but also a simultaneous seismic shift in efforts to control transmission in congregate settings as well as in communities. Current TB transmission control guidelines assume a prominent role for hospitals and clinics in the management of MDR- and XDR-TB. They also assume substantial delays in the identification of TB patients and the diagnosis of drug-resistant cases because of the limitations of currently available diagnostics. However, a report from the recent ministerial meeting in Beijing of high MDR/XDR-TB burden countries not only urges the broad implementation of transmission control measures, but also calls for the continued development and implementation of rapid point-of-care diagnostics and the selection of socially acceptable and cost-effective alternative models of care delivery.5 In this review, we make the case that the combination of rapid diagnostic testing followed by prompt effective community-based treatment and the implementation of institutional transmission control interventions could profoundly reduce institutional and community transmission and have a major impact on the MDR-/XDR-TB epidemic, especially in high HIV prevalence settings.
THE IMPORTANCE OF TRANSMISSION AND RE-INFECTION
Among the most important barriers to establishing effective treatment programs is the absence of laboratory capacity to diagnose MDR-, and especially XDR-, TB.1,6 In the absence of rapidly identifying and effectively treating the vast majority of drug-resistant cases, transmission from unsuspected cases in the community, clinics, hospitals, and other congregate settings continues.1 The revised Global Plan to Stop TB estimates that by 2015 1.3 million cases will require treatment, most of them in the former Soviet Union countries, India and China.1 Cure rates in the best MDR-TB treatment programs average about 60%, with the only prospect of improvement (using currently available regimens) being achieved through more effective delivery programs.1,7 Cure rates are lower in the presence of XDR-TB or untreated HIV co-infection.8
The 2010 World Health Organization (WHO) drug resistance surveillance data confirm that as many or more MDR-TB cases now occur among previously untreated patients, clearly indicating transmission.1 Moreover, an unknown fraction of previously treated TB cases that develop MDR-TB are misclassified as ‘acquired’, when they actually were ‘primary’, i.e., exogenously re-infected by drug-resistant strains. The proportion of transmitted MDR-TB strains is therefore systematically underestimated by the current classification. For example, in Tomsk, Siberia, a recent retrospective study of risk factors for MDR-TB found the unanticipated result that hospitalization of adherent patients during an initial course of treatment for drug-susceptible TB was the major risk factor (odds ratio [OR] > 6) for development of MDR-TB.9 Having previously been treated, these cases would be routinely classified as acquired rather than primary drug resistance.
Transmission and re-infection are driving the epidemic in both warm and cold climates. In rural South Africa, for example, the widely publicized report of rapidly fatal XDR-TB cases called the world’s attention to the potential for rapid spread from one or more unsuspected XDR-TB cases to HIV-infected patients in multi-bed wards common throughout resource-limited regions.10 Of the 53 cases initially reported, 55% had not been treated previously, but two thirds had been hospitalized, and 85% of cases had isolates with the same genotypes, strongly suggesting transmission and probably re-infection.
The importance of exogenous re-infection in MDR-TB propagation was also highlighted in another high HIV setting in South Africa. Using molecular finger-printing, Sonnenberg et al. examined the risk factors for recurrence among South Africa coal miners cured of an episode of TB, and found re-infection in 52% of relapses occurring within 6 months of completing treatment and HIV co-infection as an important risk factor.11 However, in Shanghai, China, in an area with little HIV co-infection, 62% of MDR/XDR-TB cases were attributable to re-infection based on molecular fingerprinting.12
Based on animal models and the epidemiology of TB in India, Balasubramanian et al. argued that reinfection is an essential alternate pathway for TB propagation in endemic areas, where individuals may have heightened immunity from previous exposure to TB, environmental mycobacteria or bacille Calmette-Guérin vaccination, or where circulating M. tuberculosis strains may be attenuated by drug resistance.13 The distinction between MDR/XDR-TB occurring as a result of transmission or re-infection instead of reactivation or treatment non-adherence alone is critically important. If transmission dominates as a cause, there is a need to reduce the ongoing pressure of transmission through prompt diagnosis, effective treatment, reduced hospitalization and implementation of institutional transmission control measures.
IMPORTANCE OF THE UNSUSPECTED OR DRUG-RESISTANT CASE
Most institutional guidelines on TB transmission control focus on the known or suspected TB case already on therapy, but it has long been known that the greater risk in hospitals is from unsuspected, untreated cases.14 The numbers of unsuspected TB patients, including drug-resistant cases, have rarely been documented. However, Willingham et al. screened 250 patients admitted to a large female medical ward in a busy general hospital in Lima, Peru, over a year for TB.15 They found 40 patients who were TB culture-positive, including 26 (65%) smear-positive and 13 (33%) unsuspected TB patients. Of the 40 culture-positive cases, eight had MDR-TB (six unsuspected, including three smear-positive cases). Without prompt identification of TB and drug resistance, followed by effective treatment, transmission from such patients continues.
IMPACT OF TREATMENT ON TB TRANSMISSION
The original studies from Madras, India, supporting ambulatory care had shown that, compared to treatment in hospital, treatment at home did not increase the risk of infection or disease for persons living in the patient’s home during treatment or over the following 5 years.16 Other epidemiologic studies have confirmed the safety of treating TB in the community and the disassociation between smear and culture positivity and infectiousness in treated patients.17–20 However, the most direct evidence of the impact of treatment on reducing TB transmission comes from several animal experiments where large numbers of sentinel guinea pigs (a well-established animal model to quantify TB transmission) breathed the air exhausted from experimental TB wards. In Riley’s first study over 60 years ago, all transmission to guinea pigs stopped when sputum smear-positive patients just started on effective therapy for drug-susceptible TB were admitted to the ward, and resumed only when sputum smear-positive drug-resistant patients on ineffective treatment were admitted.21 In Riley et al.’s second, 2-year study, he and his colleagues demonstrated the rapid effect of treatment on reducing transmission: sputum smear-positive patients with drug-susceptible TB just started on treatment were only 2% as infectious as untreated sputum smear-positive patients.22 Recently, Escombe et al. repeated those experiments in a similar facility in Peru with HIV-co-infected patients, and found that 98% of guinea pig infections were attributable to just nine unsuspected or inadequately treated MDR-TB patients among the 97 pulmonary TB patients (both drug-susceptible and MDR-TB) to which the guinea pigs were exposed.23 Just three patients with drug-susceptible TB, all of whom were not on therapy because of delays or side effects, infected a total of three guinea pigs. Globally, delays in the diagnosis of drug resistance mean that many patients may remain on ineffective therapy and continue to transmit.
WHAT CAN BE DONE?
The case for community-based treatment
Implementing interventions to prevent TB transmission requires action from the national policy to the institution level, and all levels in between.24 The need to implement a rapid point-of-care diagnostic has been emphasized. Another crucial national policy decision, impacting on institutions, is where drug-resistant TB patients are treated: in hospitals, clinics, community, or some combination of the three.25–28 Although some hospital capacity is needed for socially and medically complicated cases, especially in rural areas, accumulating experience in sites as diverse as Peru, Lesotho, and Karachi, Pakistan, is demonstrating enhanced treatment adherence with favorable clinical and presumably transmission control consequences.27 Although the programmatic decision of where to treat drug-resistant TB patients is often complex—influenced by national, regional, and local community policy and customary practice, the need to support hospitals, and by perceptions of the relative risks of transmission in each environment, programs are making these choices without fully considering the impact of institutional transmission on the propagation of the disease, especially in high HIV prevalence areas.29 Finally, there are probably not enough hospital beds in the world for the initial 6-month treatment of the estimated 1.3 million drug-resistant cases who will require treatment by 2015. As effective out-patient follow-up is needed to complete 18–24 months treatment of those patients started in hospital, the incremental programmatic investment necessary to develop a full community-based treatment program may be cost-effective, assuming that it would not only improve treatment outcomes, but greatly reduce hospitalization and hospital-related transmission.30–32
Interventions in institutions
The widely promulgated standard approach to TB transmission control includes administrative interventions, engineering or environmental measures, and respiratory protection.33 This approach is detailed in both the new WHO TB infection control policy and the existing facility-level document, soon to be updated.24,34 The reader seeking more details on conventional approaches should consult these sources as well as the current Centers for Disease Control and Prevention (CDC) guidelines, although the latter are written primarily for low-prevalence, resource-rich settings.33
The process begins with the creation of an administratively empowered and funded multidisciplinary TB infection control team. The team then assumes responsibility for the institutional TB transmission risk assessment, policy development, implementation plan, and monitoring of both process and outcomes. In high-risk settings one of the most relevant and readily available metrics of risk and risk reduction is the annual rate of active TB among institutional workers, such as nurses, doctors, laboratory workers or prison staff.2 One can assume that if institutional workers are protected, there should also be less transmission among patients, prisoners and other institutional residents.
Administrative controls are often said to be the least expensive and most effective interventions. The interventions emphasized earlier in this review, such as rapid diagnosis and community-based treatment, are both administrative policy decisions. They are likely to be highly effective and cost-effective in terms of both treatment outcomes and transmission prevention. Conventional administrative strategies focus on identifying coughing patients for acid-fast smear testing and prompt separation of TB suspects into environments that protect workers and other patients. Depending on existing conditions, building renovations or new construction may be essential for effective airborne infection control.24 The following example from rural Haiti illustrates the practical integration of community-based treatment, building design, and the role of rapid, although basic, diagnostic tests in a simple triage and separation strategy.
A basic triage and separation strategy—an example in Haiti
Haiti is the poorest country in the Western hemisphere, and has a disproportionate burden of both TB and HIV disease.35 In the 1980s, a community-based TB and HIV treatment program was integrated with a non-governmental organization-managed ambulatory and hospital-based primary care health program in the Central Plateau, providing free care over a large, mountainous area whose population included displaced farmers. This program has pioneered the community-based treatment of patients with TB and HIV disease, using trained, affordable community workers called ‘accompagnateurs’ to visit patients once or twice daily to assure treatment adherence and monitor treatment progress and side effects. The program provides badly needed jobs in the community and has achieved remarkably good results for TB and HIV.36,37 Because most TB is treated in the community, hospitalization is reserved for patients with complications or concomitant illnesses that require in-patient care. In that rural setting, the following basic triage and separation strategy has proven logistically workable for more than 20 years.38,39
The triage scheme is based on sputum acid-fast bacilli (AFB) stain for TB and a rapid HIV test. Patients requiring hospitalization with cough and other symptoms of respiratory infection are admitted to the general medical ward if AFB sputum smear-negative, regardless of HIV status. The rationale for placing HIV-positive patients on the general medical ward is that all symptomatic patients who might have TB have been screened by sputum smear, the test that most closely correlates with infectiousness. Moreover, all TB suspects are placed on therapy. Patients with symptoms of respiratory infection who are sputum smear-positive but HIV-negative are placed on a special TB pavilion with air disinfection enhanced by natural ventilation and germicidal ultraviolet fixtures. Finally, patients with symptoms of respiratory infection who are AFB-positive and HIV-positive are assigned to one of six simple isolation rooms, with airflow into the rooms assured by a simple exhaust fan, and additional air disinfection achieved by upper room germicidal ultraviolet fixtures. These same individual isolation rooms are used for sputum smear-positive patients known or suspected of having MDR-TB, to avoid the risk of transmission to other TB patients and staff (Figure 1).
Figure 1.
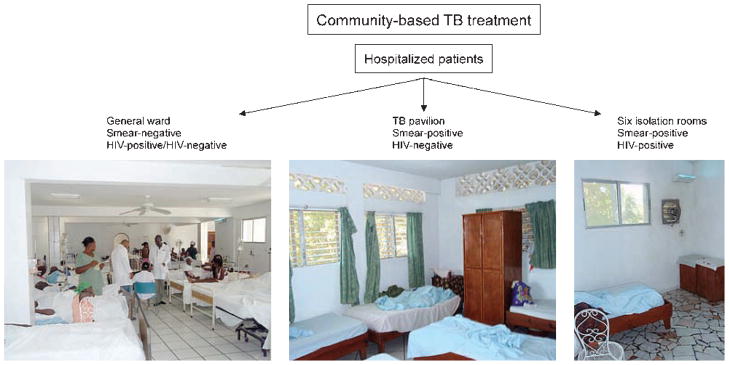
A basic triage and separation strategy, Haiti. TB = tuberculosis; HIV = human immunodeficiency virus. All images in this article can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000010/art00004
The limitations of this simple triage and separation system are several, and it would not work in all settings. The sputum smear is not a perfect predictor of TB disease or infectiousness, being positive in only about 50% of culture-positive potentially infectious cases—even less among HIV co-infected persons. Although less likely, sputum smear-negative patients can still transmit TB. However, empirically treating known and suspect TB cases in a setting of low drug resistance probably mitigates the limitations of the sputum smear. The scheme assumes availability of rapid AFB sputum stains and HIV testing. There are relatively few isolation rooms and the scheme is geared to a population where most TB cases are not HIV co-infected, and where MDR-TB is relatively uncommon. In sub-Saharan Africa, however, with much higher rates of HIV and MDR-TB, the triage challenge is much greater and a different scheme and separation facilities would be required. In that setting, programs minimizing hospitalization in favor of community-based care would make sense.
HOSPITAL AND CLINIC DESIGN FOR MDR-TB TRANSMISSION CONTROL IN RESOURCE-LIMITED SETTINGS
It is generally believed, but unproven, that most TB transmission takes place indoors because of the protection afforded by the infinite dilution available outdoors. Overcrowded hospital wards increase the risk of TB transmission for two reasons. More patients on the ward increase the chance that some will be infectious and increases the number of other patients exposed. In an unpublished study of medical student skin test conversion in Lima, Peru, Accinelli et al. reported a 25.5% conversion risk among students doing their clinical training in a hospital with a room volume of 16.2 m3/bed, compared to 12.7% for students training in a hospital with 41.4 m3/bed.40 Both hospitals serve urban poor populations at high risk for TB. The hospital with large room volumes also had very high ceilings to accommodate tall windows that permitted copious natural ventilation (Figure 2). The hospital with the smaller room volumes had lower ceilings, fewer and smaller windows, and a generally ineffective mechanical ventilation system.
Figure 2.
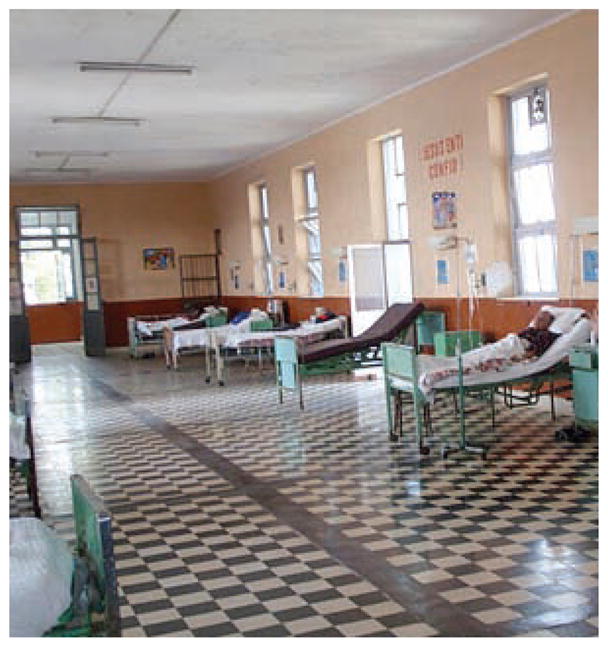
Typical hospital room with high ceilings and tall windows in Lima, Peru.
There is a growing need for architects and engineers trained in airborne infection control. The design process begins with an in-depth study of work practices, patient volume and flow, an understanding of high-risk and lower risk areas, and an appreciation of local climate and resource limitations. Next, a design ‘brief’ conceptualizing the proposed reconfigurations, renovations, or new construction is produced. The ‘brief’ is not a detailed plan from which a contractor can work, but a framework for additional input from all those who will work within the space. Once there is agreement on the brief, detailed construction plans can be drawn (Figure 3). The following are two examples of recent renovations and new constructions that the authors feel capture the state of the art in resource-limited settings.
Figure 3.

New general hospital, Haiti, with improved design for airborne infection control.
In Lesotho, another community-based treatment program for MDR-TB and HIV has renovated a small hospital for in-patient and out-patient care. Because Lesotho is mountainous, cold temperatures prohibit complete reliance on natural ventilation. A simple mechanical ventilation system, maintained under a service contract, assures an adequate number of air changes. Germicidal ultraviolet air disinfection is also in use (Figure 4).
Figure 4.

Lesotho, with simple mechanical ventilation system, maintained by a service contract, and separation and isolation capacity.
In Karachi, Pakistan, a new clinic and community-based MDR-TB program based on the Peru model has required the design and construction of a new ambulatory treatment center and laboratory. The design includes covered outdoor waiting areas, a novel patient flow scheme and a building that fully takes advantage of natural ventilation, including metal stacks which heat up in the sun to generate additional air-flow through the building (Figure 5).
Figure 5.
Design and drawings of a new multidrug-resistant tuberculosis clinic and waiting area, Indus Hospital, Karachi, Pakistan.
The pros and cons of natural ventilation in resource-limited settings
Facilities in warm climates can take advantage of outdoor waiting areas, covered open walkways and open windows much of the year. Studies using carbon dioxide as a tracer gas have shown very high indoor exchange rates in some settings when windows are open compared to when they are closed.41 While facility planners are encouraged to take full advantage of natural ventilation, several limitations should be understood. First, effective natural ventilation is rarely as simple as opening a window. Studies of the volume and direction of airflow under various climatic conditions and at different times of day are essential. The effects of opening and closing interior doors should also be considered. Comprehensive, evidence-based guidelines on natural ventilation to control airborne infections in health care settings have recently been issued by the WHO.42 A number of minimal hourly average ventilation rates is suggested, taking into account fluctuations in conditions: 160 l/s/patient for new airborne infection isolation rooms or major renovations; 60 l/s/patient for general wards and out-patient departments; and 2.5 l/s/m3 for corridors and other transient spaces without a fixed number of patients. Direction of airflow should be designed to go from source patient to the outside. When these rates and airflow direction cannot be reliably achieved by natural ventilation alone, mechanical ventilation or mixed-mode systems are recommended. We would also recommend consideration of upper room germicidal air disinfection as a low-cost complementary system to natural ventilation, for example at night or during cold seasons when windows may be closed.43
The pros and cons of upper room germicidal ultraviolet air disinfection in resource-limited settings
Apart from natural ventilation, where it is applicable, no other engineering intervention offers as much potential benefit for as little cost as properly designed and installed upper room ultraviolet germicidal irradiation (UVGI).44 This is especially true in cold climates not suitable for natural ventilation or high-volume mechanical ventilation systems. However, upper room UVGI is poorly understood and frequently poorly applied. Germicidal irradiation is used in three different ways: 1) direct, unshielded room disinfection, 2) disinfection in ventilation duct or room air cleaners, and 3) upper room air disinfection. In Eastern Europe, unshielded germicidal UV (UV-C, 254 nm UV) is commonly used to disinfect entire unoccupied rooms, but this is an ineffective application of UVGI for TB transmission control, as 1) there is no evidence that M. tuberculosis, once settled on surfaces, can be resuspended as particles small enough to reach the alveoli of the lung where infection must begin, 2) UV is not an ideal surface decontaminant, missing any shadowed surfaces, and 3) air disinfection is most useful for protecting room occupants when the infectious source is present.45 UVGI in ventilation ductwork can reduce recirculated contagion, but is of little benefit in reducing transmission within a room or hospital ward, which is the main goal. Likewise, UVGI in a properly designed room air cleaner will effectively disinfect the air going through it, but the overall effect in a room is limited to the number of germ-free equivalent room air changes added per hour, or ‘clean air delivery rate’.46 Small air cleaning units with very low clean air delivery rates are often mounted to the walls of corridors or placed in patient rooms, but these are often of little or no benefit unless the room is very small. These units are often sold as a quick and easy solution for TB transmission control, giving a false sense of security and no meaningful risk reduction. The same limitations apply to room air cleaners using filters with or without germicidal lamps.
In contrast, upper room UVGI fixtures disinfect a large volume of room air at once.47,48 Vertical air mixing, optimally aided by slow paddle fans, efficiently disinfects air in the lower room at rates difficult to achieve by mechanical ventilation alone (Figure 6).49
Figure 6.

Upper room ultraviolet germicidal irradiation fixture.
Recent controlled studies in hospitals in South America and South Africa have demonstrated air disinfection efficacy of 70–80%, disactivating patient-generated TB aerosols at rates equivalent to an added 10–20 room air changes/h.50–52 These results are highly dependent on the technical details of the installation: specifically, the average upper room UV fluence rate (irradiation dose from multiple sources) and the amount of vertical room air mixing.49
There are currently two major technical barriers to the wider use of upper room UVGI. First, UVGI technology does not belong to any one professional discipline. Engineers are not taught about its use, in part because the field is not fully developed. Architects and lighting designers are equally unfamiliar with its applications. An international cadre of engineers and architects fully trained in applying this intervention is needed. To accomplish this, UVGI experts must develop international standards based on the best available evidence. A first attempt at such a document, but with a domestic focus, was recently published by the US National Institute of Occupational Safety and Health (NIOSH), but its dosing guidelines are not easily applied.53 It recommends a target upper room average UV fluence rate of 30–50 uW/cm2, but predicting UV fluence rates before installation is difficult, and there are no standard methods to measure average UV fluence after installation. Another rule of thumb is to provide 1.87 W UV-C irradiance per m2 floor space. For those interested, the Medical Research Council of South Africa has developed guidelines for the use of upper room UVGI (http://www.sahealthinfo.org/tb/guidelines.pdf). For a safe and effective upper room installation, a knowledgeable consultant and a good quality UV meter with a detector specifically designed to measure UV-C are recommended. The free website, Global Health Delivery On Line (GHDonline.org) is also a good source of TB infection control advice, including identifying knowledgeable international consultants on all air disinfection modalities.
A second barrier is the lack of good quality, low-cost UVGI fixtures for use in resource-limited settings.54 Once performance specifications are available, local manufacturers should be encouraged to produce compliant fixtures for standardized testing by universities or health and safety agencies. Fixtures that meet standards should be recommended for local or regional use.
254 nm wavelength (UV-C)
Finally, there is the barrier of UV safety. Modern germicidal lamps generate predominantly 254 nm UV irradiation (UV-C) and produce very little ozone. While unprotected airborne microbes are readily inactivated at even low exposure levels to 254 nm UV, most human exposure is absorbed by the outer, dead layer of skin, with very little irradiation penetrating to reach the viable skin layers or the lens of the eye.55 Therefore, skin cancer and cataracts, two major complications of the longer, more penetrating wavelength UV found in sunlight, are unlikely to be caused by germicidal UV.56 Two recent publications address the low risks to room occupants of properly applied upper room UVGI.57,58 Data on UV maintenance have also been published.22 Maintenance requirements are limited to keeping lamps clean of dust with a periodic alcohol wipe and changing lamps on a regular schedule. As with ventilation, finding a knowledgeable company to regularly service a UVGI system will assure its continued effectiveness.
The pros and cons of respiratory protection in resource-limited settings
By convention, respiratory protection refers to the use of respirators (not masks) designed to protect the wearer from airborne hazards, in this case airborne infectious droplet nuclei. Surgical masks, in contrast, refer to simpler, less expensive mouth and nose covers not intended to protect the wearer, but to protect the surgical field from the expulsion of relatively larger respiratory droplets. They have also been used to reduce the generation of respiratory droplets by TB and influenza patients, although their efficacy is unknown. The most widely used respirators are certified in the United States as N95 and in Europe as FFP2. The disposable models consist of a filtering face piece in various configurations (cup, duckbill) and sizes, generally two elastic bands to achieve a tight face seal, and a malleable nose clip to prevent leaks around the nose. The US CDC provides guidance on all aspects of an effective respirator program at http://www.cdc.gov/niosh/docs/99-143/, although this site is geared toward resource-rich, low-prevalence settings.
There are many barriers to the effective use of respirators in resource-limited settings. Respirators are uncomfortable and cannot be worn continuously, but the risk from known or unsuspected infectious patients may be ever-present. For optimal protection, each worker should be fit-tested using a commercial fit-testing kit—a process that is easily learned and implemented in the field, but not often done. For proper fitting, several respirator models and sizes should be available since no single model or size fits everyone. All of this entails cost and training. Because of the cost, ranging from US$1 to $2 each in high-burden settings, the most frequently asked question often is, ‘how long can a disposable respirator be used?’ The response of one expert is ‘as long as it is structurally intact,’ pointing out that the biggest limitation is the integrity of the elastic bands that are intended to maintain the critical face seal. These tend to become flaccid fairly quickly, depending on quality and how often the respirator is doffed and donned.
The MDR-TB and HIV treatment program in Lesotho has piloted the use of a variety of non-disposable rubberized respirators, primarily to cut costs. They also tend to fit better. One non-disposable respirator costs about the same as 10–20 disposable respirators, but can be used indefinitely by replacing the disposable filter cartridges once every 6–12 months under clean conditions. There was concern initially that these brightly colored industrial respirators (see Figure 7) would frighten patients and impair communication. The impression of the staff, however, is that patients quickly adapted to their strange appearance. While communication is more difficult, many workers have chosen to continue to wear them because they are generally easier to fit and they feel better protected working with MDR/XDR-TB cases. It is also possible to wipe clean the surface of these respirators if models are chosen with partially enclosed filter cartridges. Ultimately, better non-disposable respirator designs are needed for medical use in resource-limited settings. Ideally they would have a more clinical appearance and allow better verbal communication.
Figure 7.
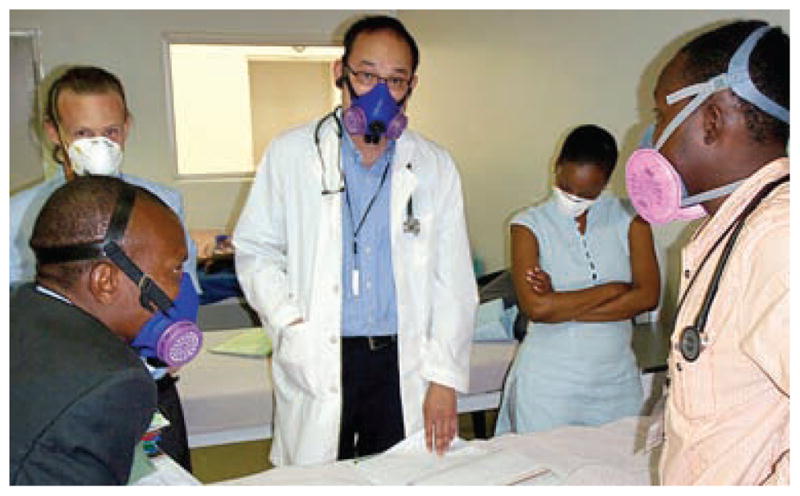
Routine use of disposable and reusable respirators on rounds at the PIH MDR-TB (Partners in Health Multidrug-Resistant Tuberculosis) Hospital, Lesotho.
CONCLUSION
We have emphasized the role of ongoing infection and reinfection in the propagation of drug-resistant tuberculosis globally and the critical need to turn off that spigot. For patients with drug-resistant isolates for whom effective treatment is available, early recognition through symptom screening, triage and rapid diagnostics leading to prompt supervised treatment is the single most important strategy for reducing transmission in hospitals, clinics and communities. Moving treatment from hospitals and clinics to communities, with appropriate infrastructure development, is another promising strategy to reduce institutional transmission. Risk in the community is minimized if effective treatment is assured by trained workers. For unsuspected tuberculosis cases in waiting rooms and general wards, surveillance, triage, rapid diagnosis and presumptive treatment are essential, but so are buildings that are thoughtfully designed to prevent airborne transmission among patients and to health care workers. Triage, rapid diagnosis and separation are especially important for XDR-TB patients, where the rapid effect of treatment on transmission cannot be assured. Depending on local conditions, natural ventilation, mechanical ventilation and germicidal ultraviolet air disinfection all have important roles in reducing transmission risk from unsuspected and inadequately treated drug-resistant tuberculosis cases in institutions. Respiratory protection using properly fitted respirators remains the final level of protection for health care workers. Although incompletely effective alone, respiratory protection complements all of the other strategies discussed.
References
- 1.World Health Organization. Multidrug and extensively drug-resistant tuberculosis (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 2.Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa-Munoz J, Palmer K, Poz MR, Blanc L, Bergstrom K, Raviglione M. The health workforce crisis in TB control: a report from high-burden countries. Hum Resour Health. 2005;3:2. doi: 10.1186/1478-4491-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine of the National Academies. Addressing the threat of drug-resistant tuberculosis: a realistic assessment of the challenge. Washington DC, USA: National Academies Press; 2009. [PubMed] [Google Scholar]
- 5.World Health Organization. A ministerial meeting of high M/ XDR-TB burden countries: meeting report. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 6.Stop TB Partnership. WHO/HTM/STB/2006.35. Geneva, Switzerland: World Health Organization; 2006. The global plan to stop TB 2006–2015. [Google Scholar]
- 7.Farmer P, Furin J, Bayona J, et al. Management of MDR-TB in resource-poor countries. Int J Tuberc Lung Dis. 1999;3:643–645. [PubMed] [Google Scholar]
- 8.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelmanova IY, Keshavejee S, Golubchikova VT, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–711. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. HIV and pulmonary tuberculosis: the impact goes beyond those infected with HIV. AIDS. 2004;18:657–662. doi: 10.1097/00002030-200403050-00010. [DOI] [PubMed] [Google Scholar]
- 12.Shen G, Xue Z, Shen X, et al. The study of recurrent tuberculosis and exogenous re-infection, Shanghai, China. Emerg Infect Dis. 2006;12:1776–1778. doi: 10.3201/eid1211.051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: pathway to apical localization. Tubercle Lung Dis. 1994;75:168–178. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 14.Kantor HS, Poblete R, Pusateria SL. Nosocomial transmission of tuberculosis from unsuspected disease. Am J Med. 1988;84:833–838. doi: 10.1016/0002-9343(88)90060-5. [DOI] [PubMed] [Google Scholar]
- 15.Willingham FF, Schmitz TL, Contreras M, et al. Hospital control and multidrug-resistant pulmonary tuberculosis in female patients, Lima, Peru. Working Group on TB in Peru Emerg. Infect Dis. 2001;7:123–127. doi: 10.3201/eid0701.010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamat SR, Dawson JJ, Devadatta S, et al. A controlled study of the influence of segregation of tuberculous patients for one year on the attack rate of tuberculosis in a 5-year period in close family contacts in South India. Bull World Health Organ. 1966;34:517–532. [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks SM, Lassiter NL, Young EC. A pilot study concerning the infection risk of sputum positive tuberculosis patients on chemotherapy. Am Rev Respir Dis. 1973;108:799–804. doi: 10.1164/arrd.1973.108.4.799. [DOI] [PubMed] [Google Scholar]
- 18.Gunnels JJ, Bates JH. Shifting tuberculosis care to the general hospital. Hospitals. 1977;51:133–134. 136, 138. [PubMed] [Google Scholar]
- 19.Riley RL, Moodie AS. Infectivity of patients with pulmonary tuberculosis in inner city homes. Am Rev Respir Dis. 1974;110:810–812. doi: 10.1164/arrd.1974.110.6P1.810. [DOI] [PubMed] [Google Scholar]
- 20.Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: the effects of chemotherapy. Tubercle. 1976;57:275–299. doi: 10.1016/s0041-3879(76)80006-2. [DOI] [PubMed] [Google Scholar]
- 21.Riley RL, Mills CC, Nyka W, et al. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995;142:3–14. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 22.Riley RL, Mills CC, O’Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 23.Escombe AR, Moore DA, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO/HTM/TB/2009.419. Geneva, Switzerland: WHO; 2009. WHO policy on TB infection control in health care facilities, congregate settings and households. [PubMed] [Google Scholar]
- 25.Kangovi S, Mukherjee J, Bohmer R, Fitzmaurice G. A classification and meta-analysis of community-based directly observed therapy programs for tuberculosis treatment in developing countries. J Community Health. 2009;34:506–513. doi: 10.1007/s10900-009-9174-4. [DOI] [PubMed] [Google Scholar]
- 26.Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59:1529–1539. doi: 10.1016/j.socscimed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 28.Farmer P, Kim JY. Community based approaches to the control of multidrug-resistant tuberculosis: introducing ‘DOTS-plus’ [see comments] BMJ. 1998;317:671–674. doi: 10.1136/bmj.317.7159.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wandwalo E, Makundi E, Hasler T, Morkve O. Acceptability of community and health facility-based directly observed treatment of tuberculosis in Tanzanian urban setting. Health Policy. 2006;78:284–294. doi: 10.1016/j.healthpol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Wandwalo E, Robberstad B, Morkve O. Cost and cost-effectiveness of community based and health facility based directly observed treatment of tuberculosis in Dar es Salaam, Tanzania. Cost Eff Resour Alloc. 2005;3:6. doi: 10.1186/1478-7547-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wandwalo E, Kapalata N, Egwaga S, Morkve O. Effectiveness of community-based directly observed treatment for tuberculosis in an urban setting in Tanzania: a randomised controlled trial. Int J Tuberc Lung Dis. 2004;8:1248–1254. [PubMed] [Google Scholar]
- 32.Fine PE. Regarding antileprosy vaccine—an apprehension by Dr Prakash. Int J Lepr Other Mycobact Dis. 1996;64:448–449. [PubMed] [Google Scholar]
- 33.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR. 2005;54:1–141. [PubMed] [Google Scholar]
- 34.World Health Organization. WHO/TB/99.269. Geneva, Switzerland: WHO; 1999. Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. [Google Scholar]
- 35.Farmer P. Social scientists and the new tuberculosis. Soc Sci Med. 1997;44:347–358. doi: 10.1016/s0277-9536(96)00143-8. [DOI] [PubMed] [Google Scholar]
- 36.Floyd S, Ponnighaus JM, Bliss L, et al. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int J Tuberc Lung Dis. 2000;4:1133–1142. [PubMed] [Google Scholar]
- 37.Desvarieux M, Hyppolite PR, Johnson WD, Jr, Pape JW. A novel approach to directly observed therapy for tuberculosis in an HIV-endemic area. Am J Public Health. 2001;91:138–141. doi: 10.2105/ajph.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenig SP, Leandre F, Farmer PE. Scaling-up HIV treatment programmes in resource-limited settings: the rural Haiti experience. AIDS. 2004;18 (Suppl 3):S21–S25. doi: 10.1097/00002030-200406003-00005. [DOI] [PubMed] [Google Scholar]
- 39.Walton DA, Farmer PE, Lambert W, Leandre F, Koenig SP, Mukherjee JS. Integrated HIV prevention and care strengthens primary health care: lessons from rural Haiti. J Public Health Policy. 2004;25:137–158. doi: 10.1057/palgrave.jphp.3190013. [DOI] [PubMed] [Google Scholar]
- 40.Accinelli R, Alvarez L, Valles P. Annual risk of tuberculosis infection among medical students of Universidad Peruana Cayetano Heredia. Am J Respir Crit Care Med. 2002;165:A439. [Google Scholar]
- 41.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Natural ventilation for infection control in healthcare settings. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 43.Nardell EA. Use and misuse of germicidal UV air disinfection for TB in high-prevalence settings. Int J Tuberc Lung Dis. 2002;6:647–648. [PubMed] [Google Scholar]
- 44.Nardell EA. Environmental infection control of tuberculosis. Semin Respir Infect. 2003;18:307–319. doi: 10.1053/s0882-0546(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 45.Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16:26–35. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 46.Nardell E. Fans, filters, or rays? Pros and cons of the current environmental tuberculosis control technologies. Infect Control Hosp Epidemiol. 1993;14:681–685. doi: 10.1086/646669. [DOI] [PubMed] [Google Scholar]
- 47.First M, Nardell E, Chaisson W, Riley R. Guidelines for the application of upper-room ultraviolet germicidal irradiation for preventing transmission of airborne contagion—part I: basic principles. ASHRAE Transactions. 1999;105:869–876. [Google Scholar]
- 48.First M, Nardell E, Chaisson W, Riley R. Guidelines for the application of upper-room ultraviolet germicidal irradiation for preventing transmission of airborne contagion—part II: design and operations guidance. ASHRAE Transactions. 1999;105:877–887. [Google Scholar]
- 49.Rudnick SN, First MW. Fundamental factors affecting upper-room ultraviolet germicidal irradiation—part II. Predicting effectiveness. J Occup Environ Hyg. 2007;4:352–362. doi: 10.1080/15459620701298167. [DOI] [PubMed] [Google Scholar]
- 50.Escombe AR, Moore DA, Gilman RH, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dharmadhikari A, Mphahlele M, Venterer K, et al. Efficacy of upper room UV air disinfection on MDR-TB transmission in an African hospital. Preliminary results. Late breaker presentation. Cancun, Mexico: 40th Conference on World Lung Health of the International Union Against Tuberculosis and Lung Disease, 2009. Int J Tuberc Lung Dis. 2009;13 (Suppl 1):S364. [Google Scholar]
- 52.Fine PE. Vaccines, genes and trials. Novartis Found Symp. 1998;217:57–69. doi: 10.1002/0470846526.ch5. discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 53.National Institutes of Occupational Safety and Health. Environmental control of tuberculosis: basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings. Atlanta, GA, USA: NIOSH; 2009. [Google Scholar]
- 54.Dumyahn T, First M. Characterization of ultraviolet upper room air disinfection devices. Am Indust Hyg Assoc J. 1999;60:219–227. [Google Scholar]
- 55.Bruls W. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984;40:485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 56.International Commission on Illumination (CIE) UV-C Photocarcinogenesis risks from germicidal lamps. CIE 187. Vienna, Austria: CIE; 2010. CIE Technical Report; p. 2010. [Google Scholar]
- 57.Nardell EA, Bucher SJ, Brickner PW, et al. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008;123:52–60. doi: 10.1177/003335490812300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.First MW, Weker RA, Yasui S, Nardell EA. Monitoring human exposures to upper-room germicidal ultraviolet irradiation. J Occup Environ Hyg. 2005;2:285–292. doi: 10.1080/15459620590952224. [DOI] [PubMed] [Google Scholar]



