Abstract
Objective
To determine whether greater childhood adversity relates to younger menarcheal age; whether younger menarcheal age relates to increased CVD risk; and whether greater childhood adversity relates to increased CVD risk, directly or indirectly (mediated by menarcheal age).
Methods
Among 650 pre-menopausal women (ages 25-45; M=34.9[5.6]), SEM was performed to estimate relations between childhood adversity, menarcheal age, and CVD risk.
Results
Results supported a covariate-adjusted model (RMSEA=0.035; CFI=0.983) in which greater childhood adversity was related to younger menarcheal age (β=−.13, p<.01) and younger menarcheal age was related to greater CVD risk (β=−.18, p<.05). Direct and indirect effects of childhood adversity on CVD risk were non-significant. Re-evaluation of the same model with additional covariate-adjustment for adulthood body composition showed the relation between menarcheal age and CVD risk attenuated (β=−.03, p=.376).
Conclusions
Cross-sectional evidence suggests family-related adversity experiences in childhood confer risk for earlier menarche which, in turn, relates to increased CVD risk in adulthood, possibly via post-pubertal body size.
Keywords: childhood adversity, puberty, pubertal timing, menarche, menarcheal age, cardiovascular risk
Associations between childhood adversity and earlier onset puberty have been reported in the developmental literature with an emphasis on the problematic psychosocial (e.g., depression, disordered eating behavior, increased substance use (Mendle et al., 2007)) and reproductive (e.g., earlier age at sexual debut, teenage pregnancy, increased risk for sexually-transmitted infections (Fisher et al., 1991; Deardorff et al., 2005; Dunbar et al., 2008)) outcomes that commonly occur among early-maturing adolescent girls. Separately, associations between earlier menarcheal age and increases in cardiovascular disease (CVD) risk factors, incident CVD-related events, CVD-specific mortality, and all-cause mortality have been reported in the epidemiological literature (Cooper et al., 1999; Frontini et al., 2003; Remsberg et al., 2005; Jacobsen et al., 2007; Feng et al., 2008; Kivimaki et al., 2008; Jacobsen et al., 2009; Lakshman et al., 2009; Lakshman et al., 2009), suggesting such early-maturing girls may go on to suffer additional health problems in adolescence and adulthood. There is a paucity of research relating these literatures, however, limiting our ability to develop more comprehensive models by which links between childhood adversity, pubertal timing, and disease risk trajectories may be investigated. This gap is particularly notable given the modifiable nature of many of the psychosocial risk factors for earlier onset puberty that have been identified (e.g., negative parenting practices) which, if ameliorated, could plausibly improve the life-course trajectories of disease risk in vulnerable girls.
The timing and rate of progression of puberty, influenced both by genetic and environmental factors (Mustanski et al., 2004), is highly variable (Marshall et al., 1969). Age at menarche, although occurring late in pubertal development, is a commonly used indicator of pubertal timing that has been shown to correlate (r = .53) with pubertal onset as measured by medical provider reports of Tanner stages (Belsky et al., 2007), a well-established system for identifying stages of sexual maturation (Marshall et al., 1969). In addition to childhood nutrition and body size (Ahmed et al., 2009), contextual factors reflecting childhood adversity experiences have also been shown to explain variability in pubertal timing. In longitudinal investigations, factors indexing problematic early environments such as marital conflict, father absence, negative parenting practices, parent-child relationship difficulties, lower socioeconomic status (SES), and fewer positive parenting/family interactions all predicted earlier onset puberty as well as younger menarcheal age (Moffitt et al., 1992; Wierson et al., 1993; Campbell et al., 1995; Graber et al., 1995; Ellis et al., 1999; Ellis et al., 2000; Belsky et al., 2007; Ellis et al., 2007; Saxbe et al., 2009). Life history models have proposed that early life (ages 0-7) is a period of increased sensitivity to environmental cues which shape an adolescent girl’s reproductive strategy, biasing her toward more accelerated reproductive development when the environment threatens her reproductive lifespan via signals that its resources (i.e., parental investment) are limited and/or unpredictably available (Belsky et al., 1991; Ellis 2004).
In addition to associations with negative psychosocial and reproductive outcomes, earlier menarcheal age has been linked prospectively to more problematic CVD risk factor profiles in adolescence and in adulthood (Frontini et al., 2003; Remsberg et al., 2005; Feng et al., 2008; Kivimaki et al., 2008; Lakshman et al., 2009) as well as a worsening of these profiles over time (Frontini et al., 2003; Remsberg et al., 2005). A similar pattern has also been shown when CVD-related non-fatal and fatal incident events were examined. In Lakshman et al. (Lakshman et al., 2009), the prospective examination of 15,807 women over a median follow-up period of 10.6 years showed earlier menarcheal age predicted higher incident CVD, incident coronary heart disease, and all-cause mortality. In two large cohort studies, earlier menarcheal age was also related to risk for all-cause mortality (Jacobsen et al., 2007; Jacobsen et al., 2009) as well as ischemic heart disease- and stroke-specific mortality (Jacobsen et al., 2009). To date, findings are mixed, however, with respect to whether obesity may account for observed relations between menarcheal age and CVD outcomes. For example, following statistical adjustment for indicators of body composition, studies have shown menarcheal age to continue to predict CVD risk (Cooper et al., 1999; Frontini et al., 2003; Remsberg et al., 2005), whereas in other studies, effects of earlier menarcheal age on CVD outcomes attenuated fully (Kivimaki et al., 2008) or partially (Jacobsen et al., 2009; Lakshman et al., 2009), leaving open questions regarding the mechanisms by which earlier menarcheal age and CVD risk are linked.
In the current study, we evaluated associations between childhood adversity, menarcheal age, and CVD risk in a multi-ethnic sample of 650 pre-menopausal women ages 25-45. First, we evaluated covariate-adjusted associations between individual markers of childhood adversity and menarcheal age as well as menarcheal age and individual markers of CVD risk. Next, we utilized a modeling framework to assess an integrated covariate-adjusted model in which associations between childhood adversity, menarcheal age, and CVD risk were estimated simultaneously. Specifically, a model was fit 1) to determine whether greater childhood adversity related to younger menarcheal age which, in turn, related to increased CVD risk; and 2) to determine whether childhood adversity related to CVD risk either directly or indirectly (mediated by menarcheal age). In addition, evaluation of the same model was repeated but included additional covariate-adjustment for waist circumference, waist-to-hip ratio (WHR), and body mass index (BMI) to determine whether the relation between menarcheal age and CVD risk (if observed) would persistent independently of statistical control for adulthood body composition. Childhood adversity was modeled as a latent construct using 5 indicators (family conflict, family expressiveness, family cohesion, family disruption events, and abuse events) derived from self-report questionnaires and CVD risk was modeled as a latent construct using 8 indicators (total cholesterol, total:high-density lipoprotein [HDL], HDL, low-density lipoprotein [LDL], triglycerides, glucose, insulin, and hypertension).
METHODS
Participants
The current sample was derived from the Ovarian Aging (OVA) Study, an investigation of reproductive aging, including women belonging to Kaiser Permanente (KP) of Northern California, a large, integrated health care delivery system that provides medical care to approximately one third of the population of Northern California. Comparisons of the KP membership with the population of Northern California indicate that the KP membership is generally representative in its socio-demographic and health-related characteristics, particularly if the comparison is limited to those with health insurance (Gordon 2006). Selection criteria for the OVA Study included that participants be between ages 25-45, have regular menses, and have their uterus and both ovaries intact. All participants self-identified as one of five race/ethnicities: white, African-American, Latina, Chinese, or Filipina and spoke/read English, Spanish, or Cantonese. Women were excluded if they reported a major medical illness, were on medications affecting the menstrual cycle within the 3 months prior to study participation, or were pregnant or breastfeeding.
The OVA Study protocol required women to participate in an in-person interview, transvaginal ultrasound, anthropometric assessment, and blood draw. Participants also completed a questionnaire packet of self-report measures that was added to the study protocol after its initiation. Of the 1019 women who completed the OVA Study, 879 women participated in the study in the timeframe in which the questionnaire packet was added to the study protocol. Of these women, 650 were retained for analysis in the current study. Of the 229 women who were excluded, 37 Filipina women were excluded due to their small numbers, 163 women were excluded because they did not return the questionnaire packet, and 29 women were excluded due to missing data on a variable of primary interest. The 29 women with missing data included 4 women who could not recall their age at menarche and 25 women missing information on a question pertaining to the educational attainment of their parents (10 did not have a mother or father-figure, 4 refused/did not know the information, and 11 left the question blank). The study protocol was approved by the University of California San Francisco Committee on Human Research as well as the KP of Northern California Institutional Review Board. Informed, written consent was obtained from all study participants.
Measures
Family Environment
The Relationship subscales of the Family Environment Scale (FES) (Moos et al., 1994)) were used to measure participants’ perceptions of their family life during childhood. On a 5-point scale, response choices indicated the level of agreement (“strongly disagree” scored 1 to “strongly agree” scored 5) with each of 27 statements (Plomin et al., 1988). Items were then summed to produce three 9-item Relationship subscale scores for dimensions of Family Conflict, Family Expressiveness, and Family Cohesion. For the current study, scores were reversed so that higher values reflected more family conflict, less family expressiveness, and less family cohesion. Internal consistency (0.61 to 0.78) and test-retest (0.52 to 0.91) reliabilities for the FES are adequate (Moos et al., 1994) and validity of the FES is supported by studies showing the FES to discriminate between distressed and non-distressed families (Moos et al., 1994). In the current sample, internal consistency reliabilities for the Relationship subscales of the FES were all high: Conflict (α=0.85), Expressiveness (α=0.73), and Cohesion (α=0.84).
Stressful Life Events
The original Life Events Checklist (Tennant et al., 1977) was adapted to include 26 items pertaining both to conventional life events (e.g., parental divorce) as well as traumatic life events (e.g., sexual abuse). For each item, participants indicated whether they experienced the event and their age(s) at the time the event occurred. For each of the 10 items relevant to early childhood, participants were assigned one point if they endorsed experiencing the event between the ages of birth to 7 years old. This timeframe was chosen to be consistent with life history theories suggesting this age range to be a time of increased sensitivity to environmental influences on pubertal timing (Belsky et al., 1991). Two subscale scores were computed reflecting dimensions of family disruption and abuse history. The family disruption subscale (score range=0-7) consisted of 7 items pertaining to death of a parent/caregiver; separation or divorce of parents/caregivers; serious marital/relationship problems of parents/caregivers; witnessing physical fights between parents/caregivers; witnessing frequent arguments between parents/caregivers; living with a relative who has a serious drinking or drug problem; and living with a relative who has a psychiatric illness. The abuse history subscale (score range=0-3) consisted of 3 items pertaining to physical abuse; sexual abuse; and severe neglect.
Menarcheal Age
In a structured medical history interview, women were asked to report the age of their first menstrual period. The reliability of retrospective reports of menarcheal age is well-established (Bergsten-Brucefors 1976; Koprowski et al., 2001); for example, in one study, later adulthood retrospective reports and original adolescent reports of menarcheal age were shown to correlate highly (r=0.79) up to 33 years after the initial assessment (Must et al., 2002).
Cardiovascular Risk Factors
CVD risk factors related to lipid profiles (total cholesterol, total:HDL, HDL, LDL, and triglycerides), fasting glucose/insulin, measures of body composition, and hypertension were examined. Assays for lipids, glucose, and insulin were performed by Quest Diagnostics (San Jose, CA). Lipids were assayed using enzymatic methods; fasting glucose was assayed by the glucose oxidase method; and insulin was assayed using the Siemens Immulite (Tarrytown, NY) immunochemiluminometric assay. Body composition measures, including waist circumference, WHR, and BMI were derived from a standardized anthropometric assessment performed by a study nurse. Lastly, previously diagnosed hypertension (yes/no) and use of anti-hypertensive medications (yes/no) was derived from an in-person medical history interview; endorsement of one or both items was used as a surrogate for elevated systolic/diastolic blood pressure which was not assessed in the study protocol.
Statistical Analyses
A two-stage analytical strategy was used in which regression analyses were first performed to assess covariate-adjusted associations between individual markers of childhood adversity and menarcheal age as well as menarcheal age and individual markers of CVD risk. Next, structural equation modeling (SEM) analyses were performed to assess an integrated covariate-adjusted model in which associations between childhood adversity, menarcheal age, and CVD risk were evaluated simultaneously.
In the first set of regression analyses, covariates were entered on step 1 and individual markers of childhood adversity were entered on step 2 in predicting menarcheal age. Five separate linear regression equations were performed for the examination of individual markers of childhood adversity: family conflict, family expressiveness, family cohesion, disruption events, and abuse events. Covariates included age, race/ethnicity, and parental education. Race/ethnicity was represented by three dummy coded variables with white as the reference group. Parental education was computed by summing the standardized distributions of the number of years each participant’s primary maternal caregiver (i.e., mother, step-mother, or female guardian) and primary paternal caregiver (i.e., father, step-father, or male guardian) attended school before the participant reached 18 years of age. For 33 participants who did not have a paternal caregiver, only mother/mother-figure’s education contributed to the parental education composite; for 2 participants who did not have a maternal caregiver, only father/father-figure’s education contributed to the parental education composite.
In the second set of regression analyses, covariates were entered on step 1 and menarcheal age was entered on step 2 in predicting individual markers of CVD risk. Eleven separate regression equations were performed for the examination individual markers of CVD risk: total cholesterol, total:HDL, HDL, LDL, triglycerides, glucose, insulin, waist circumference, WHR, BMI, and hypertension. Linear regression was used when examining all outcomes except for hypertension in which logistic regression was used. Covariates included age, race/ethnicity, individual-level education (1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school [PhD, MS]; 6=professional degree [MD, JD, DDS, MBA]), smoking (0=never smoked; 1=current/past smoking), use of hormone-containing medication for birth control (0=no history of use; 1=positive history of use), and parity (0=no live births; 1=1+ live births). The total:HDL, triglycerides, waist circumference, and BMI distributions were normalized using a logarithmic transformation.
SEM was then used to evaluate two models. In the first model, associations between childhood adversity, menarcheal age, and CVD risk were evaluated simultaneously, modeling childhood adversity as a latent construct using 5 indicators (family conflict, family expressiveness, family cohesion, disruption events, abuse events) and CVD risk as a latent construct using 8 indicators (total cholesterol, total:HDL, HDL, LDL, triglerides, glucose, insulin, hypertension). The relation between childhood adversity and menarcheal age was adjusted for covariates (age, race/ethnicity [0=white; 1=non-white], and parental education) and the relation between menarcheal age and CVD risk was adjusted for covariates (age, race/ethnicity, individual-level education, smoking, use of hormone-containing medication for birth control, and parity). In the second model, the same associations were evaluated except that the relation between menarcheal age and CVD risk was additionally adjusted for waist circumference, WHR, and BMI to determine whether the relation between menarcheal age and CVD risk (if observed) persisted independently of body composition. The selection of numerous correlated indicators to model the childhood adversity and CVD risk latent constructs is supported by the SEM methodology which was employed here because of its advantages over conventional statistical approaches, including its ability to model error variance, incorporate measured and unmeasured (latent) variables, and test complex relationships among multiple inter-related variables simultaneously (Hershberger 2003). Covariance matrices of models were analyzed using the maximum likelihood method. RMSEA values ≤0.05 and CFI values >0.95 were used to determine adequate model fit (Hu et al., 1998; Hu et al., 1999; Kline 2005).
RESULTS
Sample Characteristics
Information pertaining to socio-demographics, reproductive health, childhood adversity and CVD risk factors among all participants (N=650) is reported in Table 1. Women were ethnically diverse (26.8% white, 24.6% African-American, 20.9% Latina, and 27.7% Chinese) and ranged in age from 25 to 45, M=34.9 [5.6]). On average, women received some education / vocational training beyond high school and the mean number of years their parents attended school was 12.
Table 1.
Factors related to sociodemographics, reproductive health, childhood adversity, and CVD risk.
| Total (N = 650) | |
|---|---|
| Mean (SD) or % | |
| Socio-demographics: | |
| Age | 34.9 (5.6) |
| White | 26.8% |
| African-American | 24.6% |
| Latina | 20.9% |
| Chinese | 27.7% |
| Individual educationa | 3.6 (1.2) |
| Parental educationb (years) | 12.2 (4.5) |
| Smoking (% current/past smoking) | 21.1% |
| Reproductive Factors: | |
| Menarcheal age | 12.6 (1.6) |
| Birth control (% with history of use) | 67.1% |
| Parity (% with 1+ live birth) | 41.1% |
| Childhood Adversity: | |
| Family Conflictc | 23.8 (6.6) |
| Family Expressivenessc | 26.9 (5.4) |
| Family Cohesionc | 21.5 (6.1) |
| Disruption Events (% with 1+ event) | 37.8% |
| Abuse Events (% with 1+ event) | 14.9% |
| Cardiovascular Risk Factors: | |
| Total Cholesterol | 173.3 (31.3) |
| Total:HDL | 3.1 (1.0) |
| HDL | 60.0 (15.0) |
| LDL | 95.4 (27.6) |
| Triglycerides | 89.8 (58.4) |
| Glucose | 86.6 (8.6) |
| Insulin | 5.0 (5.1) |
| Waist Circumference | 83.8 (14.9) |
| WHR | 0.80 (0.07) |
| BMI | 26.7 (6.8) |
| Hypertension (% with diagnosis) | 6.2% |
Education was coded 1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school (PhD, MS); 6=professional degree (MD, JD, DDS, MBA).
Parental education was derived by taking the mean of mother and father education in years.
Higher scores reflect more family conflict, less family expressiveness, and less family cohesion.
Regression Analyses: Childhood Adversity and Menarcheal Age
Results of regression analyses performed to examine the association between childhood adversity and menarcheal age are reported in Table 2. Reported from the final models of separate regression equations, results showed that women who had families who were less expressive (β= −.09, p=.03), less cohesive (β= −.08, p=.03), and who experienced more family disruption events (β= −.11, p=.01) were younger at their first menses. These associations were present independently of covariate-adjustment for age, race/ethnicity, and parental education. In contrast, family conflict and abuse events were unrelated to menarcheal age (p’s>.05).
Table 2.
Results from the final models of five separate regression equations in which markers of childhood adversity were examined in relation to menarcheal agea.
| Linear Regression | Beta | P | Bb | 95% CI for Bb | R2 | ΔR2 |
|---|---|---|---|---|---|---|
|
DV: Menarcheal Age
IV’s: | ||||||
| 1. Family Conflict | −.060 | .128 | −.014 | (−0.033 – 0.004) | .034 | .003 |
| 2. Family Expressiveness | −.085 | .031 | −.025 | (−0.048 – −0.002) | .038 | .007 |
| 3. Family Cohesion | −.084 | .033 | −.022 | (0.042 – −0.002) | .038 | .007 |
| 4. Disruption Events | −.105 | .009 | −.140 | (−0.244 – −0.036) | .041 | .010 |
| 5. Abuse Events | −.046 | .247 | −.145 | (−0.390 – 0.101) | .033 | .002 |
Covariates were entered on the first step of each regression equation: age, race, parental education
Unstandardized regression coefficient
Regression Analyses: Menarcheal Age and CVD risk
Results of regression analyses performed to examine the association between menarcheal age and CVD risk are reported in Table 3. Reported from the final models of separate regression equations, results showed that women who were younger at their first menses exhibited more problematic cardiovascular risk factor profiles in adulthood. That is, younger menarcheal age was related to higher total:HDL ratio (β= −.10, p=.01), lower HDL (β= .08, p=.04), higher glucose (β= −.08, p=.03), higher insulin (β= −.15, p=.000), higher waist circumference (β= −.15, p=.000), and higher BMI (β= −.20, p=.000). These associations were present independently of covariate-adjustment for age, race/ethnicity, individual-level education, smoking, use of hormone-containing medication for birth control, and parity. In contrast, menarcheal age was unrelated to total cholesterol (p>.05) and was related only marginally to LDL (β= −.07, p=.09), triglycerides (β= −.07, p=.06), and hypertension (β= −.18, p=.10, OR=0.84, 95% confidence interval (CI): 0.68-1.04).
Table 3.
Results from the final models of eleven separate regression equations in which menarcheal age was examined in relation to cardiovascular risk factorsa.
| Linear Regression | Beta | P | Bb | 95% CI for Bb | R2 | ΔR2 |
|---|---|---|---|---|---|---|
|
IV: Menarcheal Age
DV’s: | ||||||
| 1. Total Cholesterol | −.038 | .325 | −.757 | −2.268 – 0.754 | .060 | .001 |
| 2. Total:HDL | −.104 | .006 | −.019 | −0.032 – −0.006 | .140 | .010 |
| 3. HDL | .080 | .035 | .749 | 0.052 – 1.445 | .126 | .006 |
| 4. LDL | −.066 | .092 | −1.144 | −2.473 – 0.185 | .064 | .004 |
| 5. Triglycerides | −.072 | .057 | −0.021 | −0.042 – 0.001 | .041 | .010 |
| 6. Glucose | −.084 | .031 | −0.455 | −0.869 – −0.041 | .068 | .007 |
| 7. Insulin | −.154 | .000 | −0.494 | −0.733 – −0.255 | .121 | .023 |
| 8. Waist Circumference | −.154 | .000 | −0.016 | −0.023 – −0.009 | .341 | .023 |
| 9. WHR | −.045 | .195 | −0.002 | −0.005 – 0.001 | .254 | .002 |
| 10. BMI | −.203 | .000 | −0.029 | −0.039 – −0.020 | .365 | .039 |
| Logistic Regression | Beta | p | OR | 95% CI for OR | ||
| 11. Hypertension | −.177 | .102 | .838 | 0.677 – 1.036 | - | - |
Covariates were entered on the first step of each regression equation: age, race, education, birth control, parity, smoking
Unstandardized regression coefficient
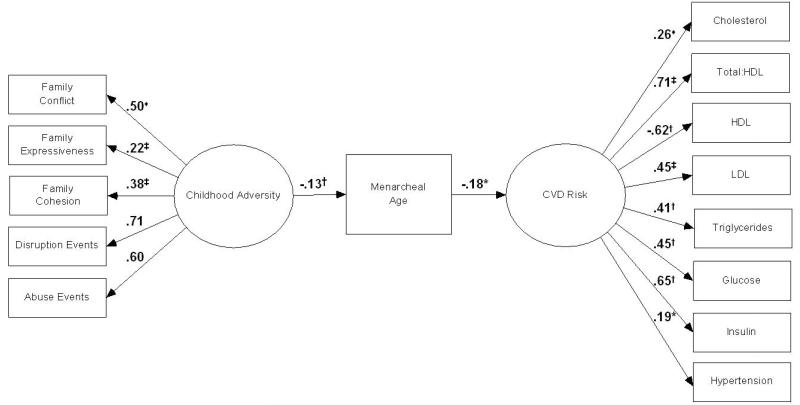
SEM Analyses: Model 1 (Figure 1)
Figure 1.
Covariate-adjusted model depicting SEM standardized estimates of associations between childhood adversity, menarcheal age, and CVD risk.
HDL: High-density lipids; LDL: Low-density lipids
♦ Regression path constrained to equal 1; * p <.05; † p < .01; ‡ p <.001
Results from SEM analyses performed to evaluate the proposed model depicting associations between childhood adversity, menarcheal age, and CVD risk are reported in Figure 1. Model fit indices showed good model fit (χ2=243.1, df=135, p<.001; RMSEA=0.035, 90% CI: 0.03-0.04; CFI=.983). Examination of the path between childhood adversity and menarcheal age showed greater childhood adversity was related significantly to younger menarcheal age (p<.01), independently of covariates (age, race/ethnicity, and parental education). The standardized regression coefficient for this path indicated that with each 1 standard deviation (SD) increase in childhood adversity, menarcheal age decreased by 0.130 SD. That is, every 1 SD increase in childhood adversity was related to a 2.5 month decrease in menarcheal age. Squared multiple correlations (SMCs) showed childhood adversity and covariates to account for 3.9% of the variance in menarcheal age. Examination of the path between menarcheal age and CVD risk showed younger menarcheal age related significantly to higher CVD risk (p<.05), independently of covariates (age, race/ethnicity, individual-level education, smoking, use of hormone-containing medication for birth control, and parity). The standardized regression coefficient for this path indicated that with each 1 SD decrease in menarcheal age, CVD risk was increased by 0.181 SD. SMCs showed menarcheal age and covariates to account for 20.3% of the variance in CVD risk.
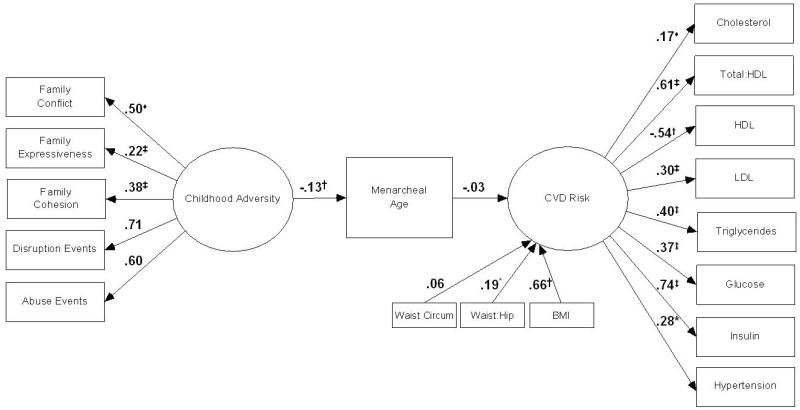
SEM Analyses: Model 2 (Figure 2)
Figure 2.
Covariate-adjusted model depicting SEM standardized estimates of associations between childhood adversity, menarcheal age, and CVD risk, additionally controlling for adulthood body composition.
HDL: High-density lipids; LDL: Low-density lipids
♦ Regression path constrained to equal 1; * p <.05; † p < .01; ‡ p <.001
Results from SEM analyses performed to evaluate the proposed model depicting associations between childhood adversity, menarcheal age, and CVD risk while additionally controlling for body composition, marked by waist circumference, WHR, and BMI, are reported in Figure 2. Model fit indices showed good model fit (χ2=395.0, df=174, p<.001; RMSEA=0.044, 90% CI: 0.04-0.05; CFI=.976). Examination of the path between menarcheal age and CVD risk showed the previously significant relation between menarcheal age and CVD risk attenuated to a non-significant level (p=.38).
In addition, the direct and indirect (mediated) paths between childhood adversity and CVD risk were estimated in Models 1 and 2; however, no significant associations were observed (p’s>.05).
DISCUSSION
In separate literatures, childhood adversity experiences have been linked prospectively to earlier onset puberty (Moffitt et al., 1992; Wierson et al., 1993; Campbell et al., 1995; Graber et al., 1995; Ellis et al., 1999; Ellis et al., 2000; Belsky et al., 2007; Ellis et al., 2007; Saxbe et al., 2009) and earlier onset puberty has been linked prospectively to CVD risk (Cooper et al., 1999; Frontini et al., 2003; Remsberg et al., 2005; Jacobsen et al., 2007; Feng et al., 2008; Kivimaki et al., 2008; Jacobsen et al., 2009; Lakshman et al., 2009). The current study aimed to integrate these literatures by utilizing a statistical modeling framework to assess simultaneously associations between childhood adversity, menarcheal age, and CVD risk. Results showed that greater childhood adversity was related to younger menarcheal age which, in turn, was related to greater CVD risk. Associations were independent of covariate-adjustment for age, race/ethnicity, parental education, individual education, smoking, use of hormone-containing medication for birth control, and parity. Re-evaluation of the same model with additional covariate-adjustment for adulthood body composition, marked by waist circumference, WHR, and BMI, showed the previously significant relation between younger menarcheal age and increased CVD risk attenuated to a non-significant level. Effects of childhood adversity on CVD risk, both direct and indirect (mediated by menarcheal age), were non-significant.
The observed association between greater childhood adversity and younger menarcheal age is consistent with results from previous longitudinal investigations which have suggested that the quality of the family environment is a primary source of variation in explaining why some girls experience puberty earlier than their same-age peers (Moffitt et al., 1992; Wierson et al., 1993; Campbell et al., 1995; Graber et al., 1995; Ellis et al., 1999; Ellis et al., 2000; Belsky et al., 2007; Ellis et al., 2007; Saxbe et al., 2009). Findings from the current study also showed there to be an association between younger menarcheal age and increased CVD risk that attenuated when markers of adulthood body composition were modeled as covariates, suggesting links between the timing of menarche and CVD risk factor development may depend on adulthood body size. This finding contributes to a mixed literature in which effects of menarcheal age on CVD outcomes have been shown to persist independently of body size in some studies (Cooper et al., 1999; Frontini et al., 2003; Remsberg et al., 2005), whereas in others effects have been shown to attenuate fully (Kivimaki et al., 2008) or partially (Jacobsen et al., 2009; Lakshman et al., 2009). While further clarification of the role of obesity is necessary, it is not unexpected that body composition would at least partially drive puberty-CVD risk relations in so far as earlier onset puberty has been related to an acceleration of weight gain post-pubertally (Wellens et al., 1992; Frontini et al., 2003; Remsberg et al., 2005) and that obesity plays a primary role in promoting CVD risk factors.
The current finding that childhood adversity was unrelated to CVD risk was unexpected and generally inconsistent with previous studies which have found that adversities experienced early in life are related prospectively to cardiovascular outcomes in adulthood, including both cardiovascular risk factors and cardiovascular clinical events (Golding 1994; Dong et al., 2004; Goodwin et al., 2004; Korkeila et al., 2010; Rich-Edwards et al., 2012). It is not clear why results from the current study do not replicate this association in particular because our conceptualization of childhood adversity is quite similar to other studies in which adversity has been defined to include indicators of negative family relations, stressful life events occurring within the family, as well as experiences of childhood abuse. We can only speculate that our selected measures, methodologies, or sample differs in some way that this relation was not observed. Nevertheless, based on a robust literature supporting there to be important psychosocial and health-related correlates of pubertal timing, we believe consideration of how pubertal development may transmit effects of early life experiences on adulthood health remains a worthwhile endeavor.
Strengths of the current study were its interdisciplinary approach to integrating previously disparate literatures and its utilization of a statistical modeling framework which enabled the analysis of complex relations between multiple variables simultaneously (Hershberger 2003). Additionally, the sample was well-characterized in terms of its reproductive and medical history and relatively large in size in particular when considering its representation of women from racial/ethnic minorities which have commonly been under-represented both in previous studies of the antecedents of pubertal timing (Wierson et al., 1993; Graber et al., 1995; Ellis et al., 1999; Ellis et al., 2000; Belsky et al., 2007; Ellis et al., 2007; Saxbe et al., 2009) as well as in studies examining pubertal timing in relation to CVD risk (Cooper et al., 1999; Frontini et al., 2003; Remsberg et al., 2005; Jacobsen et al., 2007; Feng et al., 2008; Kivimaki et al., 2008; Jacobsen et al., 2009; Lakshman et al., 2009).
There were several notable weaknesses of the current study. First, its cross-sectional design limits conclusions regarding the direction of association among the variables of interest. Secondly, the measures of childhood adversity were self-reported and retrospective in nature requiring women to recall information over periods as long as 40 years. Additionally, two indicators of the childhood adversity latent factor were derived from a life events inventory, a methodology that has been criticized for lacking important details especially related to the severity and chronicity of events (Monroe 2008). Menarcheal age was also self-reported and retrospective in nature; however, studies have shown that retrospective reports of menarcheal age are highly reliable (Bergsten-Brucefors 1976; Koprowski et al., 2001) even over extended periods of time (Must et al., 2002). Thirdly, the current study used previous hypertension diagnosis and/or use of anti-hypertensive medications as a surrogate for blood pressure which was not assessed in the study protocol. Lastly, the current study lacked characterization of several key variables that could be important explanatory factors in accounting for the observed associations. Most notably, the current study did not include measures of childhood body composition which would have enabled the assessment of the role of pre-pubertal obesity in the proposed model. However, although greater childhood obesity has been related to earlier onset puberty (HermanGiddens et al., 1997; Freedman et al., 2002), it has not been shown to account for links between childhood adversity and pubertal timing (Moffitt et al., 1992; Graber et al., 1995; Ellis et al., 2007). Additional variables that were not available in the current study included relevant genetic markers (e.g., mother’s age at menarche), health behaviors related to diet and physical activity over the life course, as well as specific environmental exposures that might be relevant to pubertal onset (e.g., endocrine-disrupting chemicals (Diamanti-Kandarakis et al., 2009)).
In conclusion, results from the current study provide preliminary support for a model suggesting the origins of adulthood cardiovascular risk begin in childhood. Family-related adversity experiences in early life appear to confer risk for earlier menarche which in turn promotes the development of more negative CVD risk factor profiles, possibly via post-pubertal weight gain. Implications for this work are that the determinants of life course trajectories of disease risk may occur early in life and that primary prevention efforts may be most effective if targeted to such developmental periods. The current findings highlight that both reductions in negative experiences within a family as well as increases in positive dimensions such as greater expressiveness and cohesion among family members may be viable intervention strategies. Future investigations should be designed to be longitudinal and to include measures characterizing body size in childhood as well as relevant genetic markers, health behaviors, and environmental exposures. Additionally, future studies should attempt to identify the specific mechanisms explaining the observed associations, especially concerning the complex interplay between hormonal and metabolic factors that appears to adversely affect the adulthood health of earlier-maturing girls. Future investigations should also include the examination of a broader array of CVD risk factors (e.g., inflammatory markers) as well as preclinical markers of CVD (e.g., carotid artery intima-medial thickening) and CVD clinical events.
Highlights.
Greater childhood adversity is related to younger menarcheal age.
Younger menarcheal age, in turn, is related to increased cardiovascular disease (CVD) risk.
Childhood adversity is unrelated to CVD risk, either directly or indirectly (mediated by menarcheal age).
The relation between younger menarcheal age and increased CVD risk may be attributable to post-pubertal body size.
Acknowledgments
Sources of support: Preparation of this manuscript and the research described here were supported by NICHD / NIA (R01HD044876), NIH/UCSF-CTSI (UL1 RR024131), NIA (K08AG035375), NIMH (T32MH019391), and the Robert Wood Johnson Foundation (045820).
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- FES
Family Environment Scale
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- SEM
structural equation modeling
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
The authors have no conflict of interest to declare.
REFERENCES
- Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends in Endocrinology and Metabolism. 2009;20(5):237–242. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy - an evolutionary theory of socialization. Child Development. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Roisman GI, Halpern-Felsher BL, Susman E. Family rearing antecedents of pubertal timing. Child Development. 2007;78(4):1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Bergsten-Brucefors A. Accuracy of recalled age at menarche. Annals of Human Biology. 1976;3(1):71–73. doi: 10.1080/03014467600001151. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Udry JR. Stress and age at menarche of mothers and daughters. Journal of Biosocial Science. 1995;27(2):127–134. doi: 10.1017/s0021932000022641. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology. 1999;10(3):255–259. [PubMed] [Google Scholar]
- Deardorff J, Gonzales NA, Christopher FS. Early puberty and adolescent pregnancy: The influence of alcohol use. Pediatrics. 2005;116(6):1451–1456. doi: 10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong MX, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease - Adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Dunbar J, Sheeder J, Lezotte D, Dabelea D, Stevens-Simon C. Age at menarche and first pregnancy among psychosocially at-risk adolescents. American Journal of Public Health. 2008;98(10):1822–1824. doi: 10.2105/AJPH.2007.120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130(6):920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development. 2007;78(6):1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: Maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71(2):485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, McFadyen-Ketchum S, Dodge KA, Pettit GS, Bates JE. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: A longitudinal test of an evolutionary model. Journal of Personality and Social Psychology. 1999;77(2):387–401. doi: 10.1037//0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Hong XM, Wilker E, Li ZP, Zhang WB, Jin DL, Liu X, Zang TH, Xu XP, Xu X. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–597. doi: 10.1016/j.atherosclerosis.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Rosenfeld WD, Burk RD. Cervicovaginal human papillomavirus infection in suburban adolescents and young adults. Journal of Pediatrics. 1991;119(5):821–825. doi: 10.1016/s0022-3476(05)80311-7. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: The Bogalusa Heart Study. Pediatrics. 2002;110(4) doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome × from childhood to young adulthood in female subjects with a history of early menarche: The Bogalusa Heart Study. International Journal of Obesity. 2003;27(11):1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- Golding JM. Sexual assault history and physical health in randomly selected Los Angeles women. Health Psychology. 1994;13(2):130–138. doi: 10.1037//0278-6133.13.2.130. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychological Medicine. 2004;34(3):509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Gordon N. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? 2006. [Google Scholar]
- Graber JA, Brooksgunn J, Warren MP. The antecendents of menarcheal age - heredity, family environment, and stressful life events. Child Development. 1995;66(2):346–359. doi: 10.1111/j.1467-8624.1995.tb00875.x. [DOI] [PubMed] [Google Scholar]
- HermanGiddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Hershberger SL. The growth of structural equation modeling: 1994-2001. Structural Equation Modeling. 2003;10(1):35–46. [Google Scholar]
- Hershberger SL. The growth of structural equation modeling: 1994-2001. Structural Equation Modeling. 2003 Jan;10(1):35–46. 2003. [Google Scholar]
- Hu LT, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- Hu LT, Bentler PM. Structural Equation Modeling. 1999. Cutoff criteria for fit indexes in covariance structure analysis: conventional versus new alternatives; pp. 61–55. [Google Scholar]
- Jacobsen BK, Heuch I, Kvale G. Association of low age at menarche with increased all-cause mortality: A 37-year follow-up of 61,319 Norwegian women. American Journal of Epidemiology. 2007;166(12):1431–1437. doi: 10.1093/aje/kwm237. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976-88. International Journal of Epidemiology. 2009;38(1):245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Jarvinen L, Vahtera J, Taittonen L, Juonala M, Viikari JS, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns study. American Journal of Clinical Nutrition. 2008;87(6):1876–1882. doi: 10.1093/ajcn/87.6.1876. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. 2nd edition 2005. [Google Scholar]
- Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obesity Research. 2001;9(8):478–485. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96(4):298–303. doi: 10.1136/hrt.2009.188250. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche is associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44(235):291–&. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Belsky J, Silva PA. Childhood experience and the onset of menarche - a test of a sociobiological model. Child Development. 1992;63(1):47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. Palo Alto, Annual Reviews. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Moos R, Moos B. Family Environment Scale Manual: Development, Applications, Research. 3rd Edition Consulting Psychologist Press; Palo Alto: 1994. [Google Scholar]
- Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: After 30 years, how well do women remember? American Journal of Epidemiology. 2002;155(7):672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: Longitudinal data from Finnish twins at ages 11 and 14. Developmental Psychology. 2004;40(6):1188–1198. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Pedersen NL, Nesselroade JR, Bergeman CS. Genetic influence on childhood family environment perceived retrospectively from the last half of the life-span. Developmental Psychology. 1988;24(5):738–745. [Google Scholar]
- Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SMS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: The Fels Longitudinal Study. Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2718–2724. doi: 10.1210/jc.2004-1991. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ. Physical and Sexual Abuse in Childhood as Predictors of Early-Onset Cardiovascular Events in Women. Circulation. 2012;126(8):920–U958. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL. Brief report: Fathers’ and mothers’ marital relationship predicts daughters’ pubertal development two years later. Journal of Adolescence. 2009;32(2):415–423. doi: 10.1016/j.adolescence.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Tennant C, Andrews G. Scale to measure cause of life events. Australian and New Zealand Journal of Psychiatry. 1977;11(3):163–167. doi: 10.3109/00048677709159555. [DOI] [PubMed] [Google Scholar]
- Wellens R, Malina RM, Roche AF, Chumlea WC, Guo S, Siervogel RM. Body size and fatness in young-adults in relation to age at menarche. American Journal of Human Biology. 1992;4(6):783–787. doi: 10.1002/ajhb.1310040610. [DOI] [PubMed] [Google Scholar]
- Wierson M, Long PJ, Forehand RL. Toward a new understanding of early menarche - the role of environmental stress in pubertal timing. Adolescence. 1993;28(112):913–924. [PubMed] [Google Scholar]