Abstract
Object
Hypoxia induces an aggressive phenotype in some brain tumors in part due to hypoxia inducible factor 1α (HIF-1α) and integrin expression. The importance of hypoxia in medulloblastoma is unclear and the interaction of HIF-1α and c-Myc in medulloblastoma has not been explored. The objective of this project was to determine if hypoxia induces an aggressive phenotype in human medulloblastoma cells that constitutively express high (D283 Med) or low (DAOY) levels of c-Myc and to determine if blocking αv integrins with the monoclonal antibody, intetumumab, inhibits hypoxia-induced cellular stress responses.
Methods
Cells were grown at 21% and 1% O2 and in the presence or absence of intetumumab. Measures of malignancy evaluated included cell proliferation, cell migration, and expression of vascular endothelial growth factor (VEGF), αv integrins, HIF-1α, and c-Myc.
Results
Both cell lines robustly expressed αv integrins. Hypoxic DAOY cells had significantly increased proliferation compared to normoxic controls (P < 0.05) whereas D283 Med cells did not. Both cell lines exhibited a dose-dependent decrease in proliferation when treated with intetumumab, (P < 0.05). Hypoxia did not increase DAOY migration. Regardless, intetumumab significantly inhibited migration at both oxygen conditions (P < 0.05). Intetumumab significantly decreased VEGF levels in DAOY cells at both oxygen conditions (P < 0.05) and in normoxic D283 cells (P < 0.01). Neither cell line had increased HIF-1α expression in response to hypoxia. However, hypoxic D283 Med cells grown in the presence of intetumumab demonstrated significantly decreased c-Myc expression (P < 0.05).
Conclusions
Hypoxia did not clearly induce a more aggressive phenotype in medulloblastoma cells. Despite this, intetumumab decreased medulloblastoma cell proliferation and migration and variably decreased VEGF and c-Myc expression in hypoxic conditions. Targeting αv integrins represents a promising potential adjuvant modality in the treatment of medulloblastoma, particularly subtypes that metastasize and overexpress VEGF and c-Myc.
Keywords: medulloblastoma, intetumumab, αv integrin, hypoxia, c-Myc, HIF-1α
Introduction
Hypoxia is a key component of aggressive behavior in many types of neoplasms. It upregulates the transcription factor, hypoxia-inducible factor-1α (HIF-1α), that regulates signaling pathways involved in glycolysis, growth-factor signaling, tissue invasion, proliferation,9 and expression of vascular endothelial growth factor (VEGF) in angiogenesis.27 Hypoxic conditions increase glioma cell migration,2 and increasing HIF-1α expression correlates with increasing glioma grade,18 particularly in the pseudopalisading cells in glioblastoma (GBM).2 Hypoxia-inducible factor 1β is a constitutively-expressed protein that when combined with HIF-1α, constitutes the heterodimer HIF-1.9 Irradiation also upregulates HIF-1 signaling that leads to recovery of tumor growth in stressful conditions and conveys resistance to radiation30 and chemotherapy.12
Another protein associated with regulation of cell proliferation is c-Myc.7 Overexpression of c-Myc is correlated with poor outcomes in medulloblastoma.14,24 Increased c-Myc expression occurs in up to 40% of medulloblastomas and MYC status has been proposed as a marker to direct risk stratification.14 However, the role of c-Myc is not well-characterized in the hypoxic tumor environment but may be inhibited as an adaptive cellular mechanism to survive in limited energy conditions.26 This inhibition of c-Myc may be due to increased HIF-1α production.7,10
The clinical significance of hypoxia in the pathogenesis of medulloblastoma is currently unclear but is beginning to be explored. Pistollato and colleagues determined that medulloblastoma-derived precursor cells require hypoxic conditions for in vitro expansion.28 Medulloblastoma cells grown in intermittently hypoxic conditions also demonstrated increased invasion, migration, and angiogenesis compared to those grown in chronic hypoxia or normoxia.8 HIF-1β has been found to be expressed in medulloblastoma specimens at similar concentrations as glioblastoma specimens in a small series of pediatric patients.12 Additionally, necrosis, the histological finding associated with hypoxia, was found in 48.1% of 556 pediatric medulloblastoma specimens and was associated with poor prognosis.34
Integrins are a family of cell surface proteins that have multiple roles including migration, cell-cell adhesion,11 and angiogenesis.5 Certain integrins are integral factors in cellular adhesion to the leptomeninges in medulloblastoma4 and leukemia.1 Additionally, Skuli and colleagues recently discovered that cell membrane αvβ3 and αvβ5 integrins are increased in hypoxic conditions, and blocking these integrins led to a decrease in HIF-1α and subsequent decrease in glioblastoma vessel density.32
The primary objective of this study was to determine if hypoxia induces a more aggressive phenotype in medulloblastoma cells that constitutively express high (D283 Med) and low (DAOY) amounts of c-Myc, respectively.31,35 The secondary objective was to determine if blocking cell membrane-bound αv integrins with the monoclonal antibody, intetumumab, decreases cellular stress responses to hypoxic conditions in these cell lines.
Methods
Cell Lines and Agents
DAOY and D283 Med human medulloblastoma cell lines (obtained from American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics and minimum essential medium Eagle (EMEM) supplemented with 20% FBS and antibiotics, respectively. Intetumumab, a human anti-αv integrin monoclonal antibody (Int, CNTO 95) was donated by Janssen Biotech, Inc. (Horsham, PA).
Integrin-Mediated Cell Adhesion
DAOY and D283 Med cells (5,000 cells) in growth medium were added to wells of the Fluorimetric Alpha/Beta Integrin-Mediated Cell Adhesion Array Combo Kit (Chemicon International, Temecula, CA) and manufacturer's recommendations were followed regarding incubation and addition of CyQuant GR dye. Plates were then read on a BioTek (Winooski, VT) FLx800 fluorometer (485/20 nmEx)/(528/20 nmEm). Experiments were duplicated.
Cell Proliferation
DAOY and D283 Med cells were plated at a concentration of 5.0 × 103 cells/mL. Groups included control, control plus vehicle (0.5 μL 0.9% NaCl), and intetumumab at 3 different concentrations: 0.5 mg/mL, 1.0 mg/mL, and 1.5 mg/mL. Each cell line and condition was replicated in 8 wells and experiments were duplicated. Cells were grown at 37°C in 5% CO2 in either in atmospheric O2 (21%) or in 1% O2 for 24 hours in a hypoxia chamber (BioSpherix, Lacona, NY). One hundred μL CellTiter-Fluor Cell Reagent (Promega, San Luis Obispo, CA) was added to each well and the plates were incubated for an additional 2 hours at 37°C in their respective oxygen condition. Plates were then read using a BioTek FLx800 fluorometer (360/40nmEx)/(485/20nmEm).
Migration Assay
DAOY cells were grown to > 95% confluence in 6 well plates in DMEM plus 10% FBS. D283 Med cells do not grow in a consistent monolayer to culture plates and thus were not included in this experiment. Cells were starved for 2 hours in serum-free DMEM in either 21% or 1% O2 at 37°C and 5% CO2. Plates were then rinsed with phosphate buffered saline (PBS) and a 200 μL pipette tip was used to make 3 vertical scratches in the confluent cells perpendicular to a black line drawn on the underslide of the wells. The cells were then grown in DMEM plus 10% FBS with nothing (control) or 0.5 mg/mL intetumumab in their respective oxygen environment. Previous pilot studies using lower and higher concentrations of intetumumab did not show a significant difference in migration retardation (unpublished). Scratches were photographed (Zeiss, Thornwood, NY) at 50x immediately after the scratch, at 3 hours, 8 hours, 15 hours, and 24 hours. Total area of scratch closure were then calculated using ImageJ 1.42 software (National Institutes of Health, Bethesda, MD).
ELISA
DAOY and D283 Med cells were grown at a concentration of 3.0 × 105 cells/mL overnight at 37°C at 5% CO2 in either 21% O2 or 1% O2 and either with 1.5 mg/mL intetumumab or vehicle (1.5 mg/mL NaCl). This dose was selected because it most greatly inhibited cell proliferation in the aforementioned cell proliferation experiment. Cells and media for both VEGF and HIF-1α ELISA were frozen at −80°C, thawed, and lyzed in PBS in a vortex in 2 iterations. The whole cell lysate was then used for HIF-1α analysis and supertnatant was used for VEGF analysis. Samples and serially diluted standards were then placed into wells of a human VEGF ELISA Kit (Thermo Scientific, Waltham, MA) and human HIF-1α ELISA kit (TSZ, Waltham, MA) and manufacturer recommendations were followed regarding incubation, washing, and addition of reagents. Plates were then read on a BioTek (Winooski, VT) EL800 plate reader at 450 nm. A 4 parameter logistic curve was then fit using Gen5 software (BioTek, Winooski, VT) and VEGF and HIF-1α concentrations were calculated.
Western Blotting
Approximately 2.0 × 106 DAOY and D283 Med cells were grown in 5% CO2 at either 21% or 1% O2 for 24 hours in either 750 μL 0.9% NaCl vehicle or 1.5 mg/mL intetumumab. Cell pellets were then resuspended in TypLE® (Life Technologies, Grand Island, NY), spun down, resuspended in ice-cold PBS, centrifuged, lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors, and frozen at −80° C until ready for Western blotting. Samples were then thawed, vortexed, and centrifuged. Supernatants of the whole-tissue lysates were analyzed and protein concentration was determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein (25–30 μg/lane) from cell lysates were separated by electrophoresis through 7.5% and 12% polyacrylamide gels and then transferred to a polyvinylidene fluoride (PVDF) membrane. Four different primary antibodies were used: monoclonal mouse anti-HIF-1α (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA), polycolonal rabbit anti-αv integrin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA), polycolonal rabbit anti c-Myc (1:300, Santa Cruz Biotechnology, Santa Cruz, CA), and monoclonal mouse anti-tubulin (1:3,000, Sigma Aldrich, St. Louis, MO). These antibodies were incubated with the transfer membrane at 4°C overnight. After 3 wash cycles, membranes were incubated with animal-specific secondary antibodies conjugated to horseradish peroxidase for 1.5 hours. The targeted antigens were visualized using standard chemical luminescence methods (Amersham ECL, GE Healthcare BioSciences, Piscataway, NJ). Equal loading of protein was confirmed and adjusted by intracellular tubulin. To semi-quantify target protein expression, blot images from 3 independent experiments were analyzed using Un-Scan-It (Silk Scientific, Orem, Utah).
Statistical Analysis
Data was analyzed using GraphPad Prism 4.0 (La Jolla, CA). One-way analysis of variance (ANOVA) with post hoc Dunnett's or Tukey's multiple comparison tests, ANOVA of repeated measures, and unpaired 2-tailed Student's t-tests were completed as appropriate. Values P ≤ 0.05 were considered significant.
Results
Medulloblastoma Cells Express αv Integrins
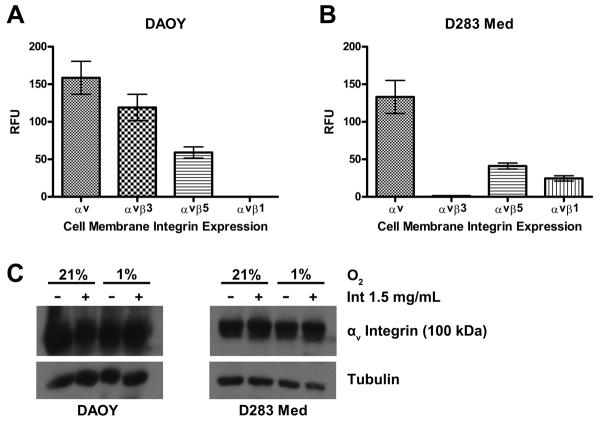
We first determined if medulloblastoma cells express cell membrane αv integrins and therefore would provide a potential therapeutic target for αv integrin blocking by intetumumab. An integrin-mediated cell adhesion assay demonstrated expression of αv, αvβ3, and αvβ5 integrins in DAOY cells and αv, αvβ5, and αvβ1 in D283 Med cells (Figure 1). Western blotting of the two cells lines demonstrated robust expression of αv (Figure 1). Baseline αv integrin expression appeared to be greater in DAOY cells, but this difference was not significant. Neither oxygen environment nor the presence of intetumumab affected αv integrin expression significantly.
Figure 1.
DAOY and D283 Med cells express αv integrins. A. DAOY cells express αv, αvβ3 and αvβ5 integrins on the cell surface in vitro. B. D283 Med cells express αv, αvβ5 and αvβ1 integrins on the cell surface in vitro. Data represent the mean of 2 different experiments. Values are adjusted to the background level of fluorescence. C. Representative Western blot demonstrates robust expression of αv integrins in both DAOY and D283 Med cell lines. There was no significant difference in the amount of αv integrin expression between the two cell lines, between cells grown at 1% and 21% O2, or cells treated with intetumumab or vehicle. Error bars are standard error. RFU= relative fluorescent unit, Int = intetumumab.
Cell Proliferation may be Dependent on c-Myc Expression
Both DAOY and D283 Med cells were cultured in 1% O2 and 21% O2 conditions and in the presence of vehicle or increasing doses of intetumumab. DAOY cell proliferation was increased when grown under hypoxic conditions (Figure 2) (P < 0.04). In contrast, there was decreased D283 Med cell proliferation when grown under hypoxic conditions that did not reach statistical significance (P = 0.07 to 0.22).
Figure 2.
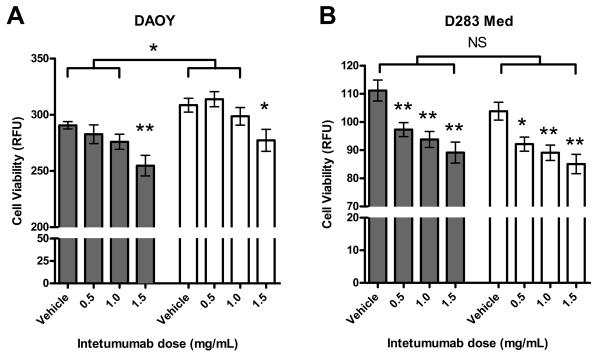
Proliferative response to hypoxia differs in DAOY and D283 Med cells and is inhibited by intetumumab. A. DAOY cells grown in 1% (white bars) or 21% O2 (grey bars) in the presence of 3 escalating doses of intetumumab. Cells grown in the presence of 1.5 mg/mL intetumumab had significantly less proliferation compared to cells grown in the presence of vehicle in their respective oxygen condition. Cells grown at 1% O2 had significantly more proliferation compared to their counterparts grown at 21% O2 with the exception of cells grown in the presence of 1.5 mg/mL intetumumab. B. D283 Med cells grown under 1% or 21% O2 conditions at 3 escalating doses of intetumumab. Cells grown in the presence of all doses of intetumumab had significantly less proliferation compared to cells grown in the presence of vehicle within their respective oxygen condition. There was no difference in proliferation between cells grown at 1% and 21% O2. Figures are means of 3 different experiments. Error bars are standard error. RFU= relative fluorescent unit, * = P < 0.05, ** = P < 0.01, NS = not significant.
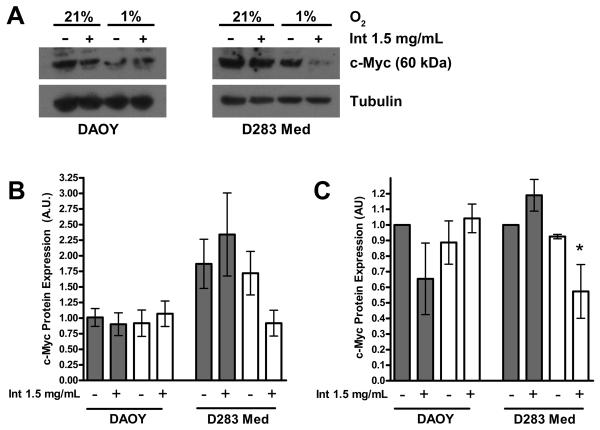
To determine if the decrease in D283 Med cell proliferation in hypoxic conditions was a stress-induced protective mechanism due to its relatively high constitutive expression of c-Myc, we first confirmed that D283 Med cells constitutively express c-Myc at higher levels than DAOY cells (Figure 3). There was significantly decreased c-Myc expression in D283 Med cells grown at 1% O2 in the presence of intetumumab (P < 0.05). There was no significant increase in HIF-1α production in cells grown at 1% O2 as demonstrated by both ELISA and Western blot (Figure 4). These findings suggest that reduction of c-Myc expression is not necessarily dependent on increased HIF-1α expression and is in part due to blocking cell surface αv integrins.
Figure 3.
c-Myc expression in D283 Med cells is inhibited by the combination of hypoxia and intetumumab. A. Representative Western blot demonstrates decreased expression of c-Myc in DAOY cells compared to D283 Med cells and D283 Med cells grown at 1% in the presence of intetumumab. B. Expression of c-Myc in 3 separate experiments normalized to tubulin appear greater in D283 Med cells than DAOY cells although this difference was not significant due to large variance. C. Expression of c-Myc in 3 separate experiments, normalized to tubulin and to the control (21% O2 vehicle). Neither hypoxic conditions nor the presence of intetumumab significantly affected the expression of c-Myc in DAOY cells. D283 Med cells grown in 1% O2 in the presence of intetumumab had significantly less c-Myc expression compared to controls. Error bars are standard error. Clear bars = 1% O2, grey bars = 21% O2, * = P < 0.05, Int = intetumumab.
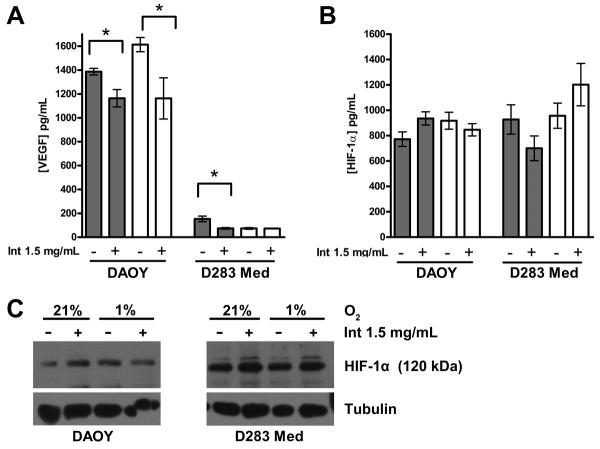
Figure 4.
Intetumumab inhibits VEGF but not HIF-1α expression in DAOY and D283 Med cells. A. Intetumumab significantly decreased VEGF production in DAOY cells grown at both oxygen conditions and D283 Med cells grown at 21% O2 in 2 separate experiments. B. There was no significant difference in the production of HIF-1α in cells grown in the presence of intetumumab or hypoxic conditions in both DAOY and D283 Med cell lines. C. Representative Western blot demonstrates no significant difference in HIF-1α expression in both cell lines grown in various conditions. Error bars are standard error. Grey bars = 21% O2, clear bars = 1% O2,* = P < 0.05, Int = intetumumab.
Intetumumab Inhibits Medulloblastoma Proliferation, Migration, and VEGF Production
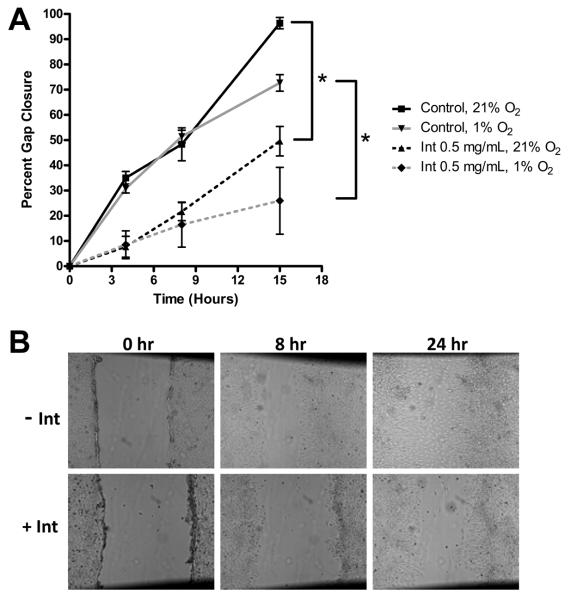
DAOY and D283 Med cells were cultured in the presence of increasing doses of intetumumab at normoxic and hypoxic conditions. Both cell lines demonstrated a dose-dependent decrease in proliferation (Figure 2). In DAOY cells, only cells grown in the presence of 1.5 mg/mL intetumumab demonstrated a significant reduction in proliferation (P < 0.05) while all doses of intetumumab decreased proliferation in D283 Med cells. Oxygen concentration did not affect inhibition of proliferation by intetumumab. Intetumumab also decreased VEGF production in DAOY grown in both oxygen conditions and D283 Med cells grown in normoxic conditions (Figure 4). Confluent DAOY cells cultured in the presence or absence of 0.5 mg/mL intetumumab manifested significantly decreased DAOY cell migration in both normoxic and hypoxic conditions (Figure 5) compared to controls.
Figure 5.
Cell migration is inhibited by intetumumab. A. DAOY cell migration in both hypoxic and normoxic conditions was significantly inhibited by intetumumab. There was no difference in rate of migration between the two oxygen environments. B. Representative photos (50 ×) of intetumumab inhibiting cell migration (gap closure) at 0, 8, and 24 hour time points. * = P < 0.05, Int = intetumumab.
Discussion
In this study, we investigated the relationship of hypoxia and inhibiting αv integrins in medulloblastoma cells that do and do not constitutively express c-Myc to determine if HIF-1α interacts with c-Myc to make these cells behave more or less aggressively. We found that both DAOY and D283 Med cells express a variety of integrins that contain the αv subunit. Heterogeneous αvβ3 expression has also been noted in 5 human medulloblastoma specimens.17 Fiorilli and colleagues found that D283 Med cells preferentially expressed α9 and β1 integrin subunits and that selectively inhibiting these subunits lead to decreased cellular adhesion.4 Similarly, we found that inhibiting αv integrins inhibited cell migration in DAOY cells. Cell migration in D283 Med cells could not be assessed because they do not grow as an adherent monolayer in vitro.
Leptomeningeal spread of medulloblastoma is a key factor in prognosis.14 αv integrins recognize the ligands vitronectin, fibrinogen, von Willebrand Factor, firbronectin, thrombospondin, osteopontin, and collagen.11 Leptomeninges uniformly contain fibronectin and collagen types IV and I29, hence offering potential targets for integrin-mediated tumor cell adhesion. αv integrins also play a role in angiogenesis,5 production of VEGF,15 and attachment of cells to vascular endothelium.22 It is therefore conceivable that medulloblastoma cell surface integrins facilitate tumor proliferation, invasion, and leptomeningeal metastases. Accordingly, we found that inhibiting αv integrins with intetumumab decreased cellular proliferation and expression of VEGF. Intetumumab is a fully monoclonal antibody that has been shown to be safe in humans with a variety of solid tumors,3,21 melanoma,25 and a primitive neuroectodermal tumor.21 A Phase I trial using the intravenous αvβ3/5 integrin antagonist, EMD121974 (cilengitide) in three patients with medulloblastoma found no dose-limiting toxicities and one patient with stable disease.20 However, given the small number of patients in that trial and because it was not designed to evaluate outcomes, the clinically efficacy of αv integrin inhibition in patients with medulloblastoma remains unclear. Administration of intrathecal intetumumab, an inhibitor of not only αvβ3,5 integrins but also αvβ1, αvβ6,33 and αvβ8 integrins, has the potential to prevent the attachment and migration of medulloblastoma cells while avoiding delivery issues of monoclonal antibodies across the blood brain barrier.
Contrary to work in gliomas in which inhibiting αv integrins decreased downstream HIF-1α expression in hypoxic conditions,32 we did not find this phenomenon in medulloblastoma probably because neither cell line significantly increased HIF-1α expression in hypoxic conditions. In fact, aside from mildly increasing DAOY proliferation, hypoxia failed to induce any markers of an aggressive phenotype such as increased cellular migration or production of VEGF. In D283 Med cells, we found mildly decreased cellular proliferation in response to hypoxia that may be due to hypoxia response elements “functionally counteracting” c-Myc thereby inducing cell cycle arrest.13
The impact of hypoxia and HIF-1α expression on c-Myc is profoundly complex and remains largely nebulous. c-Myc is a transcription factor that plays a key role in regulating cellular metabolism, growth, and differentiation.10 HIF-1α displaces c-Myc from target gene promoters thereby decreasing the expression of c-Myc activated genes.7,10 HIF-1α also contributes to c-Myc proteolysis in hypoxic conditions.10 In contrast, HIF-1α has been found to cooperate with c-Myc to activate genes for glycolysis and production of VEGF.10 In this study, we did not find a clear correlation between HIF-1α and c-Myc expression despite decreased c-Myc expression in the context of hypoxia. This phenomenon has also been found in carcinoma cells that had a HIF-1α independent decrease in c-Myc expression under hypoxic conditions.26
The clinical importance of different medulloblastoma genotypes continues to be elucidated. Northcott and colleagues recently published a report in which they describe 4 distinct variants of medulloblastomas.24 c-Myc is overexpressed in the group 3 subtype of medulloblastoma which is the most likely to metastasize and has the worst prognosis of any medulloblastoma subtype.14,23,24 We found a synergistic relationship between hypoxia and intetumumab to decrease expression of c-Myc in D283 Med cells. This suggests a novel mechanism for inhibiting c-Myc and presents a new therapeutic modality for treating subtypes of medulloblastoma that overexpress c-Myc. It is plausible that blocking αv integrins transiently facilitates or even increases the expression HIF-1α or another hypoxia response element that inhibits c-Myc in these cells. Future studies will be directed at inhibiting c-Myc with hypoxia and agents that mimic hypoxia such as nickel and deferoxamine16 to elucidate the complex relationships between hypoxia, integrins, and c-Myc expression.
The two cell types used in this study were selected based on their contrasting c-Myc constitutive expression. Similar to many medulloblastoma cell lines used in preclinical studies, it should be noted that neither DAOY nor D283 Med cell lines perfectly genetically fits into one of the four distinct molecular subtypes (WNT, SHH, Group 3, Group 4)23,24 of medulloblastoma. However, because D283 Med cells overexpress c-Myc and were isolated from peritoneal metastases of a 6-year-old boy6, this cell line appears to be most phenotypically and clinically similar to the Group 3 subtype.23 The DAOY cell line appears to be most similar to the SHH group given their low constitutive expression of c-Myc and their dependence on platelet-derived growth factor receptor α to metastasize.19,24
Conclusions
Hypoxia modestly increased cell proliferation in medulloblastoma cells that do not express large amounts of c-Myc and modestly decreased cell proliferation in those that do. Aside from this, hypoxia did not clearly induce a more aggressive phenotype in the two medulloblastoma cell lines studied here. The combination of hypoxia and intetumumab decreased c-Myc expression in cells that constitutively express c-Myc and may be HIF-1α independent. This suggests a novel pathway of αv integrins in the upstream pathway of c-Myc expression. We also demonstrated that inhibiting αv integrins with intetumumab decreased cell proliferation, migration, and VEGF production in medulloblastoma cells. Taken together, targeting αv integrins represents a promising potential adjuvant modality in the treatment of medulloblastoma, particularly subtypes that metastasize and overexpress VEGF and c-Myc.
Acknowledgements
The authors wish to thank Andy Rekito, M.S., for assistance with the figures.
Funding Support: This study was supported by NIH grants NS053468, CA137488, and NS44687 to E.A.N. and a Medical Research Foundation of Oregon Early Clinical Investigator Grant to E.M.T.
Footnotes
Portions of this work were presented as an oral presentation at the 2012 AANS Annual Meeting in Miami, Florida.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Brandsma D, Ulfman L, Reijneveld JC, Bracke M, Taphoorn MJ, Zwaginga JJ, et al. Constitutive integrin activation on tumor cells contributes to progression of leptomeningeal metastases. Neuro Oncol. 2006;8:127–136. doi: 10.1215/15228517-2005-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 3.Chu FM, Picus J, Fracasso PM, Dreicer R, Lang Z, Foster B. A phase 1, multicenter, open-label study of the safety of two dose levels of a human monoclonal antibody to human alpha(v) integrins, intetumumab, in combination with docetaxel and prednisone in patients with castrate-resistant metastatic prostate cancer. Invest New Drugs. 2011;29:674–679. doi: 10.1007/s10637-010-9388-4. [DOI] [PubMed] [Google Scholar]
- 4.Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, et al. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest. 2008;88:1143–1156. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 6.Friedman HS, Burger PC, Bigner SH, Trojanowski JQ, Wikstrand CJ, Halperin EC, et al. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985;44:592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R, Chetty C, Bhoopathi P, Lakka S, Mohanam S, Rao JS, et al. Downregulation of uPA/uPAR inhibits intermittent hypoxia-induced epithelial-mesenchymal transition (EMT) in DAOY and D283 medulloblastoma cells. Int J Oncol. 2011;38:733–744. doi: 10.3892/ijo.2010.883. [DOI] [PubMed] [Google Scholar]
- 9.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 10.Huang LE. Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ. 2008;15:672–677. doi: 10.1038/sj.cdd.4402302. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 12.Jennings MT, Cmelak A, Johnson MD, Moots PL, Pais R, Shyr Y. Differential responsiveness among “high risk” pediatric brain tumors in a pilot study of dose-intensive induction chemotherapy. Pediatr Blood Cancer. 2004;43:46–54. doi: 10.1002/pbc.20043. [DOI] [PubMed] [Google Scholar]
- 13.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leary SE, Olson JM. The molecular classification of medulloblastoma: driving the next generation clinical trials. Curr Opin Pediatr. 2012;24:33–39. doi: 10.1097/MOP.0b013e32834ec106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Welser JV, Milner R. Absence of the alpha v beta 3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in alpha 5 beta 1 integrin expression. J Cereb Blood Flow Metab. 30:1031–1043. doi: 10.1038/jcbfm.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Kluz T, Sun H, Costa M. Mechanisms of c-myc degradation by nickel compounds and hypoxia. PLoS One. 2009;4:e8531. doi: 10.1371/journal.pone.0008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim M, Guccione S, Haddix T, Sims L, Cheshier S, Chu P, et al. alpha(v)beta(3) Integrin in central nervous system tumors. Hum Pathol. 2005;36:665–669. doi: 10.1016/j.humpath.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Li Y, Shu M, Tang J, Huang Y, Zhou Y, et al. Hypoxia-inducible factor-1alpha blocks differentiation of malignant gliomas. FEBS J. 2009;276:7291–7304. doi: 10.1111/j.1742-4658.2009.07441.x. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29:143–152. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 21.Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 22.Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- 23.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- 24.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Day S, Pavlick A, Loquai C, Lawson D, Gutzmer R, Richards J, et al. A randomised, phase II study of intetumumab, an anti-alphav-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer. 2011;105:346–352. doi: 10.1038/bjc.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuyama H, Endo H, Akashika T, Kato K, Inoue M. Downregulation of c-MYC protein levels contributes to cancer cell survival under dual deficiency of oxygen and glucose. Cancer Res. 2010;70:10213–10223. doi: 10.1158/0008-5472.CAN-10-2720. [DOI] [PubMed] [Google Scholar]
- 27.Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One. 2010;5:e12521. doi: 10.1371/journal.pone.0012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, et al. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 28:1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutka JT, Giblin J, Dougherty DV, McCulloch JR, DeArmond SJ, Rosenblum ML. An ultrastructural and immunocytochemical analysis of leptomeningeal and meningioma cultures. J Neuropathol Exp Neurol. 1986;45:285–303. doi: 10.1097/00005072-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz DL, Bankson J, Bidaut L, He Y, Williams R, Lemos R, et al. HIF-1-dependent stromal adaptation to ischemia mediates in vivo tumor radiation resistance. Mol Cancer Res. 9:259–270. doi: 10.1158/1541-7786.MCR-10-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siu IM, Lal A, Blankenship JR, Aldosari N, Riggins GJ. c-Myc promoter activation in medulloblastoma. Cancer Res. 2003;63:4773–4776. [PubMed] [Google Scholar]
- 32.Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 33.Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, et al. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 34.Verma S, Tavare CJ, Gilles FH. Histologic features and prognosis in pediatric medulloblastoma. Pediatr Dev Pathol. 2008;11:337–343. doi: 10.2350/07-09-0353.1. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Macaulay RJ. Cell-cycle gene expression in lovastatin-induced medulloblastoma apoptosis. Can J Neurol Sci. 2003;30:349–357. doi: 10.1017/s0317167100003061. [DOI] [PubMed] [Google Scholar]