Abstract
Background
Ovarian cancer is commonly treated with cisplatin/paclitaxel but many tumors become resistant. Acetaminophen reduced glutathione and enhanced chemotherapy efficacy in treating hepatic cancer. The objective of this study was to examine if acetaminophen enhances the cytotoxicity of cisplatin/paclitaxel in ovarian cancer.
Materials and Methods
SKOV3 human ovarian carcinoma cells in vitro and a subcutaneous tumor nude rat model were used and treated with cisplatin/paclitaxel with or without acetaminophen.
Results
In vitro, acetaminophen enhanced apoptosis induced by cisplatin and paclitaxel with similar effects on glutathione, reactive oxygen species and mitochondrial membrane potential but different effects on nuclear factor erythroid 2-related factor 2 (NRF2) translocation. In vivo, acetaminophen was uniformly distributed in tissue and significantly reduced hepatic glutathione. Acetaminophen enhanced cisplatin chemotherapeutic effect by reducing tumor recurrence
Conclusion
Our results suggest that acetaminophen as a chemoenhancing adjuvant could improve the efficacy of cisplatin and paclitaxel in treating patients with ovarian carcinoma and other tumor types.
Keywords: Acetaminophen, glutathione, chemo-enhancement, cisplatin, ovarian cancer
Ovarian cancer is the leading cause of death from gynecological disease in the United States. Chemotherapy with a platinum agent (cisplatin, carboplatin) and/or paclitaxel remains the front-line treatment for ovarian cancer (1, 2). However, chemoresistance usually develops, and relapse remains almost inevitable in patients with advanced disease (3, 4). Cisplatin forms both intrastrand and interstrand crosslinks of DNA to induce apoptosis (5), while paclitaxel promotes the assembly of microtubules and inhibits microtubule depolymerization (6). These differing initial mechanisms cause a cascade of toxic effects in cancer cells, such as a decrease in mitochondrial membrane potential (Δψm) (7), or generation of reactive oxygen species (ROS) (8), converging to cell death. Ovarian cancer often exhibits cross-resistance to platinum agents and paclitaxel (4). Mechanisms to reduce resistance and enhance cytotoxicity should improve chemotherapy efficacy.
A major cause of chemotherapy resistance appears to be inactivation of the drug and activation of drug efflux pumps by endogenous thiols, including glutathione (GSH) and other sulfhydryls such as metallothionein (9, 10). Glutathione-S-transferase mRNA expression was found to be inversely correlated to sensitivity to platinum chemotherapy (11), and chemosensitivity may be associated with polymorphisms in the gene encoding this enzyme (2).
Acetaminophen, a commonly used analgesic and antipyretic drug, can alter glutathione levels in different cell types (12–15). The mixed function oxidase system of enzymes, most relevantly cytochrome P450 2E1 (CYP2E1), generates a reactive arylating intermediate that is normally detoxified by reduced GSH (16). Large doses of acetaminophen overwhelm GSH stores allowing the toxic metabolite, N-acetyl-p-benzoquinonimine, to bind macromolecules and induce cell death (17). High-dose acetaminophen can enhance chemotherapy activity against different cancer cells in vitro (15, 18, 19) and in vivo (20–22). Kobrinsky et al. reported a clinical case of a child with unresectable stage III hepatoblastoma who had failed frontline cisplatin-based therapies (22). This patient was disease-free seven years post surgery after receiving a treatment regimen that combined cisplatin after high-dose acetaminophen followed with N-acetylcysteine (NAC) rescue 8 h later to reduce toxicity to normal liver. Acetaminophen may suppress ovulation and affect cell proliferation, angiogenesis, and apoptosis of the ovary epithelium (23). It has also been proposed that acetaminophen inhibits ovarian carcinogenesis through the depletion of GSH leading to apoptosis and necrosis (23).
Regardless of CYP2E1 protein levels, our laboratory previously showed that acetaminophen enhanced cisplatin-induced apoptosis in human hepatocarcinoma and hepatoblastoma cells by reducing GSH concentrations (19). The objective of this study was to further investigate the potential effect of acetaminophen in enhancing cisplatin/paclitaxel-mediated cytotoxicity in ovarian carcinoma. These results may provide clinically relevant information about a potential chemotherapeutic regimen in which acetaminophen is combined with cisplatin/paclitaxel in treating of patients with ovarian cancer.
Materials and Methods
Cell culture, pharmacological agents and antibodies
The SKOV3 human ovarian adenocarcinoma cell line from the American Type Culture Collection (Rockville, MD, USA) was grown in media supplemented with 10% fetal bovine serum (FBS) and antibiotics in a humidified atmosphere of 5% CO2. Sterile solutions of cisplatin (1 mg/ml) were obtained from the Oregon Health and Sciences University (OHSU) pharmacy. Acetaminophen was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) and sterilely filtered for in vitro studies. For the in vivo animal studies, commercial baby Tylenol liquid formula containing acetaminophen (100 mg/ml) was purchased from the OHSU pharmacy. Rabbit anti-poly (ADP-ribose) polymerase (PARP) antibody was purchased from Cell Signaling (Danvers, MA, USA), mouse antibody to tubulin was from Sigma-Aldrich, and rabbit antibodies to nuclear factor erythroid 2-related factor 2 (NRF2) and β-actin were from Santa Cruz Biotech (Santa Cruz, CA, USA).
In vitro assays
SKOV3 ovarian carcinoma cells were treated with or without acetaminophen (10 mM) for 2 and 24 h, with or without addition of cisplatin (5 μg/ml) or paclitaxel (100 nM). Cells treated with DMSO alone were used as vehicle control. For in vitro GSH and ROS assays, each treatment group was performed in triplicate in at least three independent experiments. Cellular GSH levels were monitored and analyzed using the Quanticrom Glutathione Assay Kit from BioAssay Systems (Hayward, CA, USA) according to the manufacturer's protocol. The results were normalized to protein concentration measured using a BCA assay kit (Pierce Biotechnology, Rockford, IL, USA). For cell cycle analysis, cells were collected and fixed in 70% ethanol 24 h after treatments. Cells were stained with propidium iodine and subjected to BD Calibur cytometer at the OHSU flow cytometry core and analyzed by BD Modfit software (San Jose, CA, USA). Cellular ROS levels were analyzed by incubating cells with dihydrorhodamine 123 dye, an oxidant-sensitive fluorochrome (Sigma-Aldrich) at 2.5 μg/ml for 30 min at 37°C as previously described (24). Whole cell lysate was then subjected to FLX-800 plate reader with Gen5 software (Biotek, Winooski, VT, USA). The cellular protein level in cells under different treatments was measured by western blot analysis using a chemiluminescence substrate (Pierce Biotechnology, Rockford, IL, USA) as previously described (25). Cellular mitochondrial membrane potential (Δψm) was analyzed using the JC-1 detection reagent (eBioscience, San Diego, CA, USA) at 3 μg/ml for 30 min at 37°C based on the method described by Reers et al. (26). Stained cells were viewed using a Zeiss Observer fluorescent microscope and photographs of representative cells were taken by Zeiss AxioCam camera. The percentage of green stained cells was counted in six random fields per group (200–500 cells). For measurement of cellular platinum levels, SKOV3 cells were pre-incubated with or without 10 mM acetaminophen for 2 h prior to a 2 h treatment with or without cisplatin (10 μg/ml). Whole cell lysates of each group were digested in nitrous acid and then subjected to inductively coupled plasma mass spectrometer (ICP-MS) at the OHSU proteomic core shared resource.
In vivo animal studies
Female nude rats (rnu/rnu, 200–250 g; from the OHSU Blood Brain Barrier Program in-house colony) were pretreated with cyclophosphamide (100 mg/kg; i.p.) 24 h before tumor cell administration. We have shown that cyclophosphamide reduces innate immunity and increases vascular endothelial growth factor production to improve the consistence of xenograft tumor development in nude rats (27). SKOV3 ovarian carcinoma cells (2.5×107 in 250 μl) mixed with 250 μl Matrigel (BD Biosciences, Bedford, MA, USA) were injected in the left flank. For the chemotherapeutic study, tumor-bearing rats were untreated (control), treated with cisplatin (4 mg/kg; i.p.) alone, or acetaminophen (600 mg/kg; p.o.) 2 h before cisplatin treatment on day 7 after inoculation (500–800 mm3 tumor volume). Tumor growth was measured by dial caliper twice per week. Whole blood (1 ml) was withdrawed through the jugular vein at 7 d and 24 d after treatment when the experiment was terminated. Blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations were analyzed on a DRI-CHEM Veterinary Chemistry Analyzer (HESKA, Waukesha, WI, USA). For the pharmacological study, tumor-bearing rats received acetaminophen 0, 300, 600, and 1000 mg/kg p.o. (0, 2.1, 4.2 and 7 g/m2 respectively). Serum and tissues (liver, brain and tumor) were collected 2 h after treatment. Tissue and serum acetaminophen and GSH concentrations were determined using an acetaminophen direct enzyme-linked immunosorbent assay (ELISA) (Immunalysis Corp., Pomona, CA, USA) and Quanticrom Glutathione Assay Kit (BioAssay Systems, Hayward, CA, USA). Tissue data are presented after normalization with protein concentration.
Statistical analysis
Significant differences between the mean values of treated cells/animals and controls were determined by two-sided Student's t-test modified by the number of comparisons performed (n) according to Tukey's correction. Two-way analysis of variance was performed by comparing the different arms of treatment for the two variables with Bonferroni post-test to compare means among the different treatments. Significant difference between treatment and control (or vehicle) groups or any two other groups was determined at the 5% level.
Results
Enhancement of chemotherapy toxicity by acetaminophen in ovarian carcinoma cells
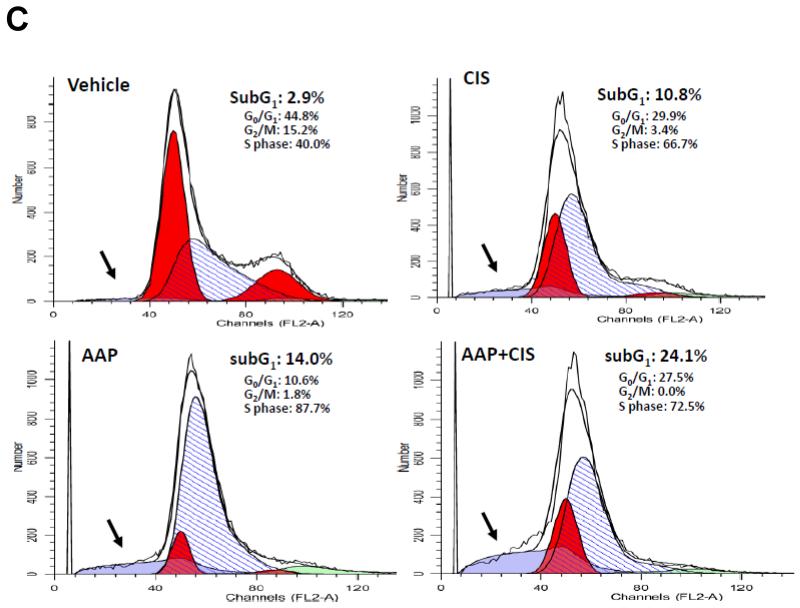
Western blotting analysis for cleaved PARP was performed to determine the level of apoptosis in SKOV3 cells. After 24 h incubation, cisplatin (5 μg/ml) in combination with acetaminophen (10 mM) induced significantly more apoptosis in SKOV3 cells than acetaminophen or cisplatin alone (Figure 1A). There was no apoptosis found among any treatment groups at the 2 h time point. Similar results were found when SKOV3 cells were treated with paclitaxel (100 nM) with and without acetaminophen (10 mM) (Figure 1B). For cell cycle analysis, the vehicle control of SKOV3 cells contained only 2.9% subG1 population (apoptotic cells). The subG1 population was elevated to 24.1% in group treated with acetaminophen and cisplatin compared to 10.8% in cisplatin and 14.0% in acetaminophen alone. Additionally, there were no cells found in G2/M phase in group treated with acetaminophen and cisplatin (Figure 1C).
Figure 1. Acetaminophen enhances cisplatin and paclitaxel cytotoxicity.
Human SKOV3 ovarian carcinoma cells were treated with acetaminophen (AAP; 10 mM) with/without cisplatin (5 μg/ml, panel A) or paclitaxel (100 nM, panel B). Whole cell lysates were collected 2 and 24 h after treatments and subjected to immunoblotting analysis. Tubulin and β actin were used as equal loading controls. C: Cell cycle analysis. Cells were collected and fixed in 70% ethanol 24 h after treated with acetaminophen (AAP; 10 mM) with/without cisplatin (CIS; 5 μg/ml), then subjected to cytometry after staining with propidium iodine. The apoptotic subG1 cell population (light blue area) among treatment groups is indicated by arrows.
Mechanisms for chemo-enhancement by acetaminophen in ovarian carcinoma cells
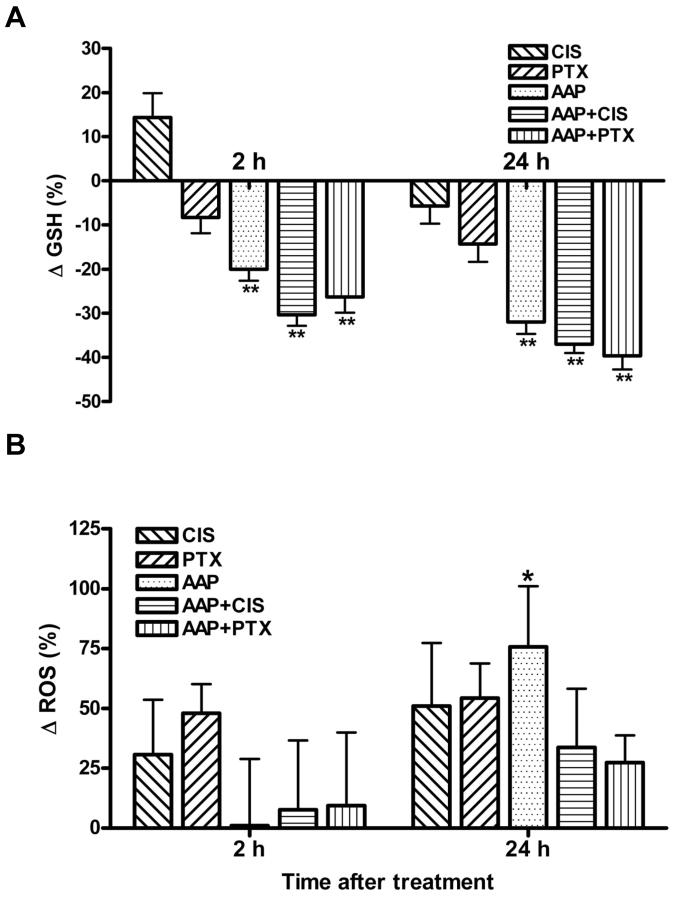
The effects of acetaminophen, cisplatin and paclitaxel on cellular GSH and ROS levels were determined at both 2 and 24 h. Acetaminophen treatment reduced GSH levels in SKOV3 cells at both the 2- and 24-h time points (p<0.01, Figure 2A) and increased ROS level significantly only after 24 h (p<0.05) not 2 h incubation (Figure 2B). Neither cisplatin nor paclitaxel treatment alone affected cellular GSH or ROS levels. There was no further reduction of GSH or increase in ROS when cells were treated with the combination of acetaminophen and cisplatin or paclitaxel (Figure 2A and B).
Figure 2. In vitro mechanism of acetaminophen chemoenhancement.
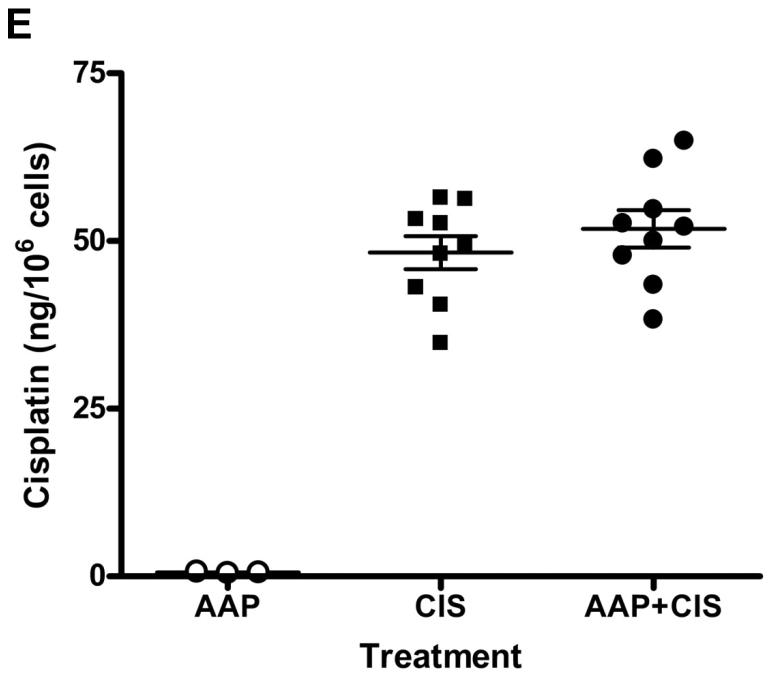
Human SKOV3 ovarian carcinoma cells were treated with acetaminophen (AAP,10 mM) with/without cisplatin (CIS, 5 μg/ml) or paclitaxel (PTX, 100 nM) for 2 and 24 h. Cells treated with dimethyl sulfoxide (DMSO) alone were used as control. Whole cell lysates were collected 2 and 24 hr after treatments and subjected to analysis for glutathione (GSH; A), reactive oxygen species (ROS; B) and mitochondrial membrane potential (Δψm; C, D). A, B) Data are presented as the mean±SD of percentage change in GSH and ROS compared with the control (n=3 per treatment group). C: Representative micrographs of each treatment group at 2 h. Healthy cells are orange and cells with reduced Δψm are green under fluorescence microscopy. D: Quantification and analysis of the percentage of green cells in six random fields. E: SKOV3 ovarian carcinoma cells were treated with or without acetaminophen (10 mM) for 2 h, cisplatin (10 μg/ml) was then added and cells incubated for an additional 2 h. Whole cell lysates were subjected to inductively coupled plasma mass spectrometry for platinum measurement (* p<0.05; ** p<0.01; *** p<0.001).
Mitochondria play an important role in regulating oxidative stress and apoptosis. Cellular mitochondrial membrane potential (Δψm) was evaluated using the JC-1 stain. Healthy cells are orange/reddish and apoptotic cells with reduced Δψm are green under fluorescence microscopy. Acetaminophen alone increased the percentage of cells exhibiting mitochondrial toxicity from 1.7±0.3% to 7.9±0.4% (Figure 2C and D). Acetaminophen significantly (p<0.001) enhanced the mitochondrial toxic effects of both cisplatin (25.3±1.5%) and paclitaxel (21.6±1.2%). Acetaminophen pretreatment did not alter cellular cisplatin accumulation (51.8±2.8 vs. 48.3±2.5 ng/106 cells; n=9; Figure 2E).
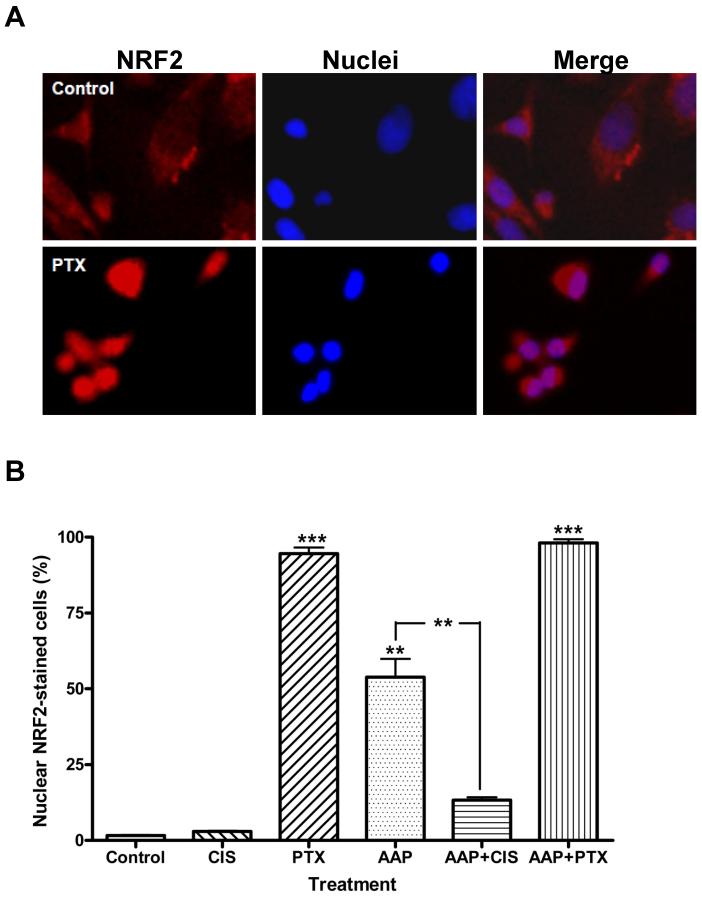
NRF2 is a nuclear transcription factor that protects against oxidative stress and inflammation by regulating several detoxification and xenobiotic transporter genes. Acetaminophen alone induced nuclear NRF2 translocation in 53.9±6.0% of cells. Cisplatin alone had no effect on NRF2 translocation (3.0±0.2%), while the combination of acetaminophen and cisplatin (13.3±1.0%) significantly (p<0.01) reduced nuclear NRF2 localization compared to acetaminophen alone. In contrast, paclitaxel alone induced nuclear translocation of NRF2 (94.6±1.9%), which was not affected by addition of acetaminophen (98.1±1.2%; Figure 3). Our results suggest that activation of the NRF2-mediated pathway is independent of ROS production and acetaminophen-enhanced cisplatin- and paclitaxel-induced cytotoxicity is independent of nuclear NRF2 translocation pathway.
Figure 3. Effect of acetaminophen and chemotherapy on nuclear translocation of nuclear factor erythroid 2-related factor 2 (NRF2).
Human SKOV3 ovarian carcinoma cells were treated with acetaminophen (AAP, 10 mM) with/without cisplatin (CIS, 5 μg/ml) or paclitaxel (PTX, 100 nM) for 2 h. Cells treated with dimethyl sulfoxide (DMSO) alone were used as control. Cells were fixed and stained for immunofluorescence with rabbit anti-NRF2 with 4', 6-diamidino-2-phenylindole (DAPI) nuclear counterstain. A: Representative micrographs of nuclear NRF2-negative (vehicle control; top panel) and -positive (100 nM paclitaxel; bottom panel) stained SKOV3 cells. B: Quantification and analysis of the percentage of nuclear NRF2-positively stained cells in four random fields (** p<0.01; *** p<0.001).
In vivo acetaminophen pharmacology and toxicology studies
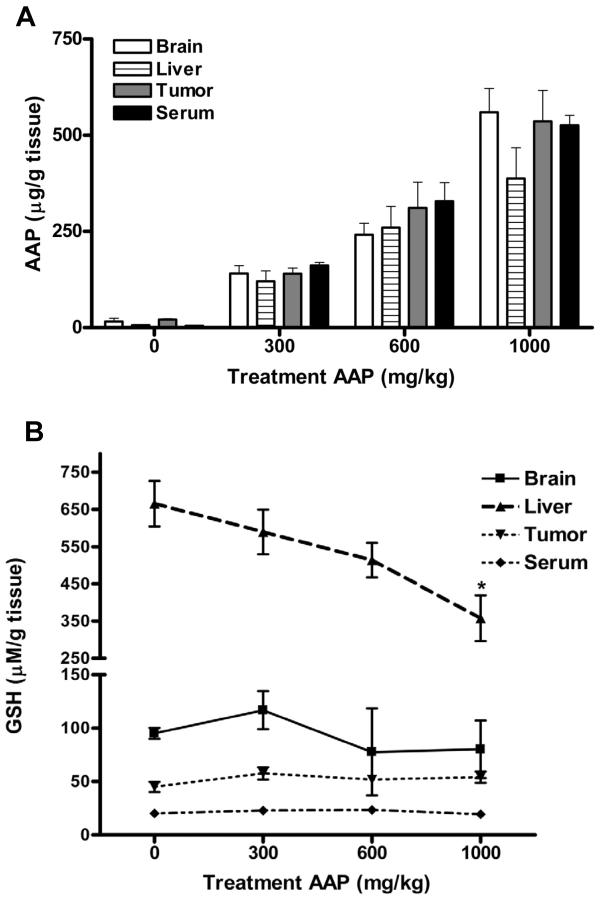
To investigate the effect of acetaminophen on tissue distribution of acetaminophen and GSH level, subcutaneous tumor-bearing nude rats received 0, 300, 600 or 1000 mg/kg (~0, 2, 4 and 7 g/m2) acetaminophen orally (n=3 per treatment). At 2 h after administration, acetaminophen levels in tissues and serum were elevated in a linear correlated dose-dependent manner (Figure 4A). The acetaminophen doses tested in this study did not alter acetaminophen tissue distribution of acetaminophen among the samples (brain, liver, serum and subcutaneous tumor). Rats given 600 mg/kg acetaminophen had a mean acetaminophen tissue level above 250 μg/ml (1.65 mM) in brain, liver, tumor and serum at 2 h. Compared to the control, GSH levels were reduced in the liver of acetaminophen-treated rats in a dose-dependent manner, with significant (p<0.05) difference only being detected at 1000 mg/kg dosage (665.5±61.1 vs. 357.9±61.3 μM/g tissue). GSH levels in subcutaneous tumor, serum and brain did not significantly differ between acetaminophen-treated and untreated animals (Figure 4B).
Figure 4. In vivo acetaminophen distribution and glutathione levels.
Female nude rats with subcutaneous SKOV3 xenografts were treated with acetaminophen (AAP; 0, 300, 600 and 1000 mg/kg; n=3 per treatment) on d 7 after tumor inoculation. Serum and tissues were collected and analyzed for levels of acetaminophen (A) and glutathione (B) 2 h after treatment. Tissue data (except serum) are presented after normalization with protein concentration (* p<0.05).
Acetaminophen potentiated the chemotherapeutic effect of cisplatin in a SKOV3 subcutaneous xenograft model
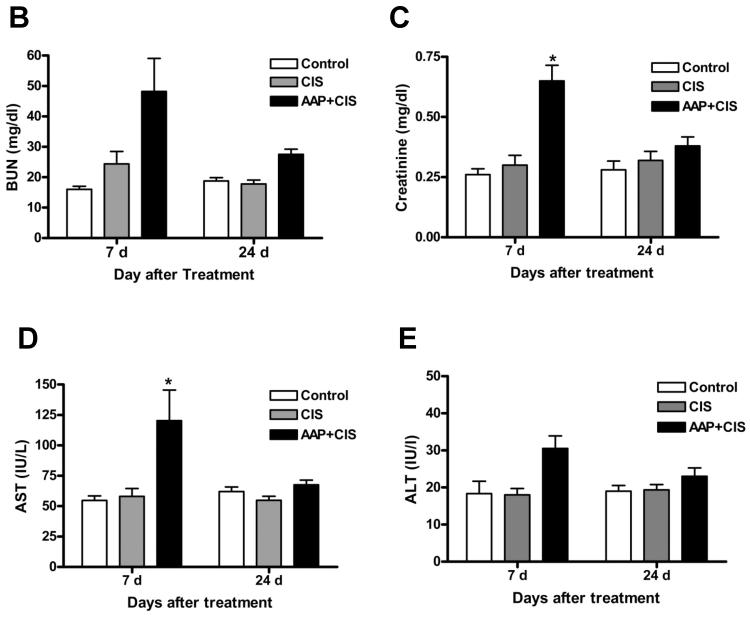
To test if acetaminophen treatment enhances the chemotherapeutic effect of cisplatin in SKOV3 tumor-bearing animals, acetaminophen (600 mg/kg) was given orally 2 h before cisplatin (4 mg/kg) treatment on day 7 after tumor inoculation (500–800 mm3 tumor volume; n=5 per treatment). Rats given 1000 mg/kg acetaminophen exhibited hypothermia during treatment; therefore we set the maximum dose at 600 mg/kg. Compared to control animals, cisplatin both alone and with acetaminophen significantly (p<0.001) reduced tumor volume starting from 3 d until the end of the study (Figure 5A). There is no significant difference between groups treated with cisplatin alone and those treated with acetaminophen and cisplatin at 3, 7, 10 and 14 d after treatment (Figure 5A). Tumor recurrence was found in animals in the cisplatin-treated group at 17 d after treatment. In contrast, the group treated with acetaminophen and cisplatin retained low tumor volumes of between 35–55% of baseline during this period of time. This resulted in significant differences (p<0.01) between the cisplatin and combined treatment groups. In these animals, serum BUN, creatinine, AST and ALT levels were elevated by the combination of acetaminophen with cisplatin treatment when compared to cisplatin alone (48.2±10.9 vs. 24.4±4.1 mg/dl; 0.65±0.06 vs. 0.30±0.04 mg/dl; 115.8±26.1 vs. 58.0±6.6 IU/l; 30.5±3.4 vs. 18.0±1.7 IU/l) at 7 d and then returned back to normal at 24 d after treatment (Figure 5 B–E). Our data demonstrated that acetaminophen combined with cisplatin prevented subcutaneous tumor recurrence in SKOV3 tumor-bearing rats with minimal and managable side-effects.
Figure 5. Acetaminophen enhances cisplatin chemotherapeutic efficacy in ovarian subcutaneous xenografts.
Female nude rats with subcutaneous SKOV3 xenografts were treated with acetaminophen (AAP; 600 mg/kg) or saline orally 2 h before cisplatin treatment (4 mg/kg; i.p.) on d 7 after inoculation. A: Subcutaneous tumor growth and tumor volume among the treatment groups was measured by dial caliper twice per week. Tumor volume at each time point was normalized to that at pretreatment baseline. The statistical significance between cisplatin and combination groups is indicated by ** p<0.01 and *** p<0.001. Blood urea nitrogen (BUN; B), creatinine (C), aspartate aminotransferase (AST; D) and alanine aminotransferase (ALT; E) levels in serum 7 and 24 d after treatment. The statistical significance between control and treatment group is indicated by * p<0.05.
Discussion
Ovarian carcinoma often exhibits resistance to chemotherapy, limiting the efficacy of chemotherapeutics such as cisplatin or paclitaxel. Circumvention of cancer cell resistance against platinum-based anticancer chemotherapy is of great interest in clinical chemotherapy (28). One of the strategies to overcome resistance to anticancer agents is the application of chemosensitizers or drug-resistance modulators, including multidrug-resistant protein inhibitors and intracellular GSH-depleting agents (29). Buthionine sulfoximine (BSO) and acetaminophen may work to chemosensitize tumor cells by depleting intracellular GSH levels (30, 31). Previous studies showed that acetaminophen could be used as a cancer preventative but not chemo-enhancement agent in ovarian cancer (23). In this study, we have demonstrated that a combined regimen of acetaminophen and cisplatin or paclitaxel enhances cytotoxicity towards SKOV3 cells relative to the use of cisplatin or paclitaxel alone in vitro and cisplatin alone in vivo.
Acetaminophen alone has demonstrated toxicity in hepatocellular carcinoma (12, 19, 32, 33) and glioma cell lines (34, 35), but had mixed results in breast cancer cell lines (36, 37). In a human endometrioid ovarian carcinoma cell line, acetaminophen significantly increased the percentage of cells at S-phase and reduced cell growth synergistically with carboplatin, but did not affect paclitaxel growth-inhibitory acitivity (18). In contrast, paclitaxel increased the acetaminophen-induced cytotoxicity in rat primary hepatocytes by reducing protein synthesis (38). Regardless of CYP2E1 protein levels, we found that acetaminophen enhanced cisplatin-induced apoptosis in human hepatocarcinoma and hepatoblastoma cells (19). Results of acetaminophen chemoenhancement for other chemotherapeutics are mixed, with increased staurosporine-induced cell death in human neuroblastoma cells (15) but reduced doxorubicin toxicity in hepatocarcinoma cells (12).
Our studies demonstrated that acetaminophen reduces GSH levels and increased ROS, reduced Δψm and increased nuclear NRF2 translocation to enhance the cytotoxicity by cisplatin or paclitaxel of SKOV3 cancer cells in vitro. Acetaminophen enhanced apoptosis in both cisplatin- and paclitaxel-treated SKOV3 cells regardless of whether the same (GSH, ROS and mitochondrial pathways) or different (nuclear translocation of NRF2) mechanisms involved. Our results confirm previous studies showing that paclitaxel reduced Δψm without affecting ROS in SKOV3 cells (7) and a human T-cell lymphoblastic leukemia cell line (39). GSH depletion with BSO or acetaminophen increased nuclear NRF2 translocation in mouse hepatocytes (40). A high level of NRF2 in cancer is associated with sensitivity to oxidative stress and chemotherapy (41). Jiang et al. (41) found that stable knockdown of NRF2 significantly sensitized human type II endometrial cancer cells to cisplatin and paclitaxel. Our results suggest that activation of the NRF2-mediated pathway is independent of ROS production and that acetaminophen-enhanced cisplatin- and paclitaxel-induced cytotoxicity is independent of nuclear NRF2 translocation pathway. Copple et al. (42) demonstrated that NRF2 can be activated either through GSH depletion alone or by direct modification of NRF2 suppressor protein (KEAP1). NRF2 translocation to the nucleus has been shown to be insufficient for the up-regulation of expression of multiple drug resistance ATP-binding cassette transporter proteins (43). Lack of enhanced transporter activity might explain why acetaminophen did not alter cellular cisplatin accumulation in vitro. Similarly, BSO treatment reduced cellular GSH but did not affect the intracellular melphalan accumulation in human colorectal HT29 cancer cells (44).
Two phase I dose-escalation studies have examined the potential of acetaminophen as a chemotherapeutic adjuvant (20, 21). Due to the significant toxicities experienced at the 20 g/m2 dose level in these clinical trials, the maximum tolerated dose of acetaminophen was set at 15 g/m2, yielding a plasma concentration of 250 μg/ml (20). In the current study, we found that oral acetaminophen at 600 mg/kg (~4 g/m2) gave a mean tissue AAP level >250 μg/ml at 2 h, equivalent to the concentrations achievable in patients (22). This dose level moderately prevented subcutaneous tumor progression when combined with cisplatin in tumor-bearing rats because of the slow growing characteristic of SKOV3 cells. In this study, cisplatin treatment (4 mg/kg; i.p.) alone significantly exhibited antitumor efficacy, which might explain why there was no difference between cisplatin and acetaminophen with cisplatin at the early time points after treatment. The in vivo results would be more promising and convincing if we could lower the cisplatin dosage and extend the in vivo animal study longer. Shaw et al. (45) also found that SKOV3 cells were less aggressive and formed tumors more slowly (two to three months) in a xenograft model. In addition, the potential side-effects (increase of serum BUN, creatinine, AST and ALT) by acetaminophen plus cisplatin treatment are minimal and managable. Previous studies from our laboratory have shown that delayed i.v. NAC or sodium thiosulfate administration (4–8 h) protected from the side effects without affecting the chemotherapeutic efficacy (46, 47).
There was a dose-dependent reduction of GSH in the liver, the organ most active in acetaminophen metabolism. However, we found no GSH reduction in serum and subcutaneous tumors in rats even at 1000 mg/kg (~ 7 g/m2) dose. The mechanisms involved in the acetaminophen-enhanced cisplatin chemotherapeutic efficacy of ovarian carcinoma in vivo remain unclear. Patients given up to 20 g/m2 of acetaminophen have similarly shown no reduction in serum GSH levels (20). Rat hepatocytes are found to be far more sensitive to acetaminophen treatment than human hepatocytes (48). In addition, the activity of glutathione-S-transferase in rodents is 10 to 20 times higher than in humans (49). These factors exemplify that the major limitations of this study are: a single ovarian cancer cell line was used, although similar studies had been carried out on hepatic tumors (19) and atypical teratoid rhabdoid tumors (50) in our laboratory; the SKOV3 subcutaneous xenograft model with relatively slow growth rate was not ideal or adequately close to the clinical setting; the treatment regimen for maximal efficacy (dosage, timing, route and frequency of acetaminophen and chemotherapy administration) needs to be further defined; and due to the difference in acetaminophen sensitivity among different species, using a rodent model is not ideal for studying the potential of acetaminophen incorporation into a pre-clinical trial.
In conclusion, we found that acetaminophen enhanced the cytotoxic activity of cisplatin/palcitaxel in human ovarian carcinoma in vitro and cisplatin treatment in vivo. In addition to the potential preventative role of acetaminophen in ovarian cancer incidence (23), incorporation of high-dose acetaminophen, an inexpensive, relatively safe, and widely available drug, into cisplatin- or palcitaxel-containing chemotherapeutic regimens may enhance chemotherapeutic efficacy towards ovarian carcinomas and other malignancy. Potential side-effects could be abrogated by delayed administration of antioxidant chemoprotective agents such as NAC, sodium thiosulfate or other GSH precursors. Therefore, this is a promising approach that merits further clinical trials as the potential treatment benefits could impact on different tumor types.
Acknowledgements
This study was supported by National Instute of Health (grants NS053468, CA137488, and NS44687). The authors would like to thank Sheila Taylor and Seth Lewin for their technical assistance.
Footnotes
Authorship contributions Participated in research design: Wu, A.J. Neuwelt, Muldoon, and E.A. Neuwelt.
Conducted experiments: Wu and A.J. Neuwelt.
Performed data analysis: Wu and Muldoon.
Wrote or contributed to the writing of the manuscript: Wu, Muldoon, and E.A. Neuwelt.
References
- 1.Bookman MA. First-line chemotherapy in epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:96–113. doi: 10.1097/GRF.0b013e31824b45da. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros R, Pereira D, Afonso N, Palmeira C, Faleiro C, Afonso-Lopes C, Freitas-Silva M, Vasconcelos A, Costa S, Osorio T, Lopes C. Platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma: glutathione-S-transferase genetic polymorphisms as predictive biomarkers of disease outcome. Int J Clin Oncol. 2003;8:156–161. doi: 10.1007/s10147-003-0318-8. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Kaye SB. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 4.Fracasso PM. Overcoming drug resistance in ovarian carcinoma. Curr Oncol Rep. 2001;3:19–26. doi: 10.1007/s11912-001-0038-z. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Chew M, Yang EB, Wong KP, Mack P. Modulation of cisplatin cytotoxicity and cisplatin-induced DNA cross-links in HepG2 cells by regulation of glutathione-related mechanisms. Mol Pharmacol. 2001;59:837–843. doi: 10.1124/mol.59.4.837. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz SB. Taxol (paclitaxel): Mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–6. [PubMed] [Google Scholar]
- 7.Ahn HJ, Kim YS, Kim JU, Han SM, Shin JW, Yang HO. Mechanism of taxol-induced apoptosis in human SKOV3 ovarian carcinoma cells. J Cell Biochem. 2004;91:1043–1052. doi: 10.1002/jcb.20006. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Zheng XL, Yang L, Shi F, Gao LB, Zhong YJ, Sun H, He F, Lin Y, Wang X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J Exp Clin Cancer Res. 2010;29:159–166. doi: 10.1186/1756-9966-29-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson SW, Ozols RF, Hamilton TC. Mechanisms of drug resistance in ovarian cancer. Cancer. 1993;71:644–649. doi: 10.1002/cncr.2820710224. [DOI] [PubMed] [Google Scholar]
- 10.Sprem M, Babic D, Abramic M, Vrhovec I, Skrk J, Milicic D, Ambriovic Ristov A, Kalafatic D, Osmak M. Glutathione and glutathione-S-transferases as early markers for ovarian carcinomas: Case series. Croat Med J. 2001;42:624–629. [PubMed] [Google Scholar]
- 11.Nakagawa K, Yokota J, Wada M, Sasaki Y, Fujiwara Y, Sakai M, Muramatsu M, Terasaki T, Tsunokawa Y, Terada M, Saijo N. Levels of glutathione-S-transferase π mRNA in human lung cancer cell lines correlate with the resistance to cisplatin and carboplatin. Jpn J Cancer Res. 1988;79:301–304. doi: 10.1111/j.1349-7006.1988.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manov I, Hirsh M, Iancu TC. N-Acetylcysteine does not protect HepG2 cells against acetaminophen-induced apoptosis. Basic Clin Pharmacol Toxicol. 2004;94:213–225. doi: 10.1111/j.1742-7843.2004.pto940504.x. [DOI] [PubMed] [Google Scholar]
- 13.Wan J, Bae MA, Song BJ. Acetoaminophen-induced accumulation of 8-oxodeoxyguanosine through reduction of OGG1 DNA repair enzyme in C6 glioma cells. Exp Mol Med. 2004;36:71–77. doi: 10.1038/emm.2004.10. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 15.Posadas I, Vellecco V, Santos P, Prieto-Lloret J, Cena V. Acetaminophen potentiates staurosporine-induced death in a human neuroblastoma cell line. Br J Pharmacol. 2007;150:577–585. doi: 10.1038/sj.bjp.0706993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estabrook RW. A passion for P450s (rememberances of the early history of research on cytochrome P450) Drug Metab Dispos. 2003;31:1461–1473. doi: 10.1124/dmd.31.12.1461. [DOI] [PubMed] [Google Scholar]
- 17.Kon K, Ikejima K, Okumura K, Aoyama T, Arai K, Takei Y, Lemasters JJ, Sato N. Role of apoptosis in acetaminophen hepatotoxicity. J Gastroenterol Hepatol. 2007;22(Suppl 1):S49–52. doi: 10.1111/j.1440-1746.2007.04962.x. [DOI] [PubMed] [Google Scholar]
- 18.Bilir A, Altinoz MA, Attar E, Erkan M, Aydiner A. Acetaminophen modulations of chemotherapy efficacy in MDAH 2774 human endometrioid ovarian cancer cells in vitro. Neoplasma. 2002;49:38–42. [PubMed] [Google Scholar]
- 19.Neuwelt AJ, Wu YJ, Knap N, Losin M, Neuwelt EA, Pagel MA, Warmann S, Fuchs J, Czauderna P, Wozniak M. Using acetaminophen's toxicity mechanism to enhance cisplatin efficacy in hepatocarcinoma and hepatoblastoma cell lines. Neoplasia. 2009;11:1003–1011. doi: 10.1593/neo.09688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Williams L, Pinto JT, Fleisher M, Krown SE, Hwu WJ, Livingston PO, Chang C, Chapman PB. Phase I trial of high dose paracetamol and carmustine in patients with metastatic melanoma. Melanoma Res. 2003;13:189–196. doi: 10.1097/00008390-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kobrinsky NL, Hartfield D, Horner H, Maksymiuk A, Minuk GY, White DF, Feldstein TJ. Treatment of advanced malignancies with high-dose acetaminophen and N-acetylcysteine rescue. Cancer Invest. 1996;14:202–210. doi: 10.3109/07357909609012140. [DOI] [PubMed] [Google Scholar]
- 22.Kobrinsky NL, Sjolander DE, Goldenberg JA, Ortmeier TC. Successful treatment of doxorubicin and cisplatin resistant hepatoblastoma in a child with Beckwith-Wiedemann syndrome with high dose acetaminophen and N-acetylcysteine rescue. Pediatr Blood Cancer. 2005;45:222–225. doi: 10.1002/pbc.20330. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Burford C, Barnes MN, Oelschlager DK, Myers RB, Talley LI, Partridge EE, Grizzle WE. Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on ovarian carcinoma cell lines: Preclinical evaluation of NSAIDs as chemopreventive agents. Clin Cancer Res. 2002;8:202–209. [PubMed] [Google Scholar]
- 24.Bishop GM, Dringen R, Robinson SR. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic Biol Med. 2007;42:1222–1230. doi: 10.1016/j.freeradbiomed.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther. 2005;312:424–431. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- 26.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 27.Wu YJ, Muldoon LL, Dickey DT, Lewin SJ, Varallyay CG, Neuwelt EA. Cyclophosphamide enhances human tumor growth in nude rat xenografted tumor models. Neoplasia. 2009;11:187–195. doi: 10.1593/neo.81352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosland M, Lum B, Schimmelpfennig J, Baker J, Doukas M. Insights into mechanisms of cisplatin resistance and potential for its clinical reversal. Pharmacotherapy. 1996;16:16–39. [PubMed] [Google Scholar]
- 29.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 30.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res. 2011;17:6206–6217. doi: 10.1158/1078-0432.CCR-11-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Runowicz CD, Fields AL, Goldberg GL. Promising new therapies in the treatment of advanced ovarian cancer. Cancer. 1995;76:2028–2033. doi: 10.1002/1097-0142(19951115)76:10+<2028::aid-cncr2820761320>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Macanas-Pirard P, Yaacob NS, Lee PC, Holder JC, Hinton RH, Kass GE. Glycogen synthase kinase-3 mediates acetaminophen-induced apoptosis in human hepatoma cells. J Pharmacol Exp Ther. 2005;313:780–789. doi: 10.1124/jpet.104.081364. [DOI] [PubMed] [Google Scholar]
- 33.Manov I, Bashenko Y, Hirsh M, Iancu TC. Involvement of the multidrug resistance P-glycoprotein in acetaminophen-induced toxicity in hepatoma-derived HepG2 and Hep3B cells. Basic Clin Pharmacol Toxicol. 2006;99:213–224. doi: 10.1111/j.1742-7843.2006.pto_443.x. [DOI] [PubMed] [Google Scholar]
- 34.Casper D, Lekhraj R, Yaparpalvi US, Pidel A, Jaggernauth WA, Werner P, Tribius S, Rowe JD, LaSala PA. Acetaminophen selectively reduces glioma cell growth and increases radiosensitivity in culture. J Neurooncol. 2000;46:215–229. doi: 10.1023/a:1006492423666. [DOI] [PubMed] [Google Scholar]
- 35.Bae MA, Pie JE, Song BJ. Acetaminophen induces apoptosis of C6 glioma cells by activating the c-Jun NH(2)-terminal protein kinase-related cell death pathway. Mol Pharmacol. 2001;60:847–856. [PubMed] [Google Scholar]
- 36.Harnagea-Theophilus E, Gadd SL, Knight-Trent AH, DeGeorge GL, Miller MR. Acetaminophen-induced proliferation of breast cancer cells involves estrogen receptors. Toxicol Appl Pharmacol. 1999;155:273–279. doi: 10.1006/taap.1998.8619. [DOI] [PubMed] [Google Scholar]
- 37.Gadd SL, Hobbs G, Miller MR. Acetaminophen-induced proliferation of estrogen-responsive breast cancer cells is associated with increases in c-MYC RNA expression and NF-ΚB activity. Toxicol Sci. 2002;66:233–243. doi: 10.1093/toxsci/66.2.233. [DOI] [PubMed] [Google Scholar]
- 38.Kostrubsky VE, Lewis LD, Wood SG, Sinclair PR, Wrighton SA, Sinclair JF. Effect of Taxol on cytochrome P450 3A and acetaminophen toxicity in cultured rat hepatocytes: comparison to dexamethasone. Toxicol Appl Pharmacol. 1997;142:79–86. doi: 10.1006/taap.1996.8023. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-dependent apoptosis. J Biol Chem. 2004;279:51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- 40.Chia AJ, Goldring CE, Kitteringham NR, Wong SQ, Morgan P, Park BK. Differential effect of covalent protein modification and glutathione depletion on the transcriptional response of NRF2 and NF-ΚB. Biochem Pharmacol. 2010;80:410–421. doi: 10.1016/j.bcp.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of NRF2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, Kitteringham NR, Park BK. The hepatotoxic metabolite of acetaminophen directly activates the KEAP1-NRF2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 43.Perez MJ, Gonzalez-Sanchez E, Gonzalez-Loyola A, Gonzalez-Buitrago JM, Marin JJ. Mitochondrial genome depletion dysregulates bile acid- and paracetamol-induced expression of the transporters Mdr1, Mrp1 and Mrp4 in liver cells. Br J Pharmacol. 2011;162:1686–1699. doi: 10.1111/j.1476-5381.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vahrmeijer AL, van Dierendonck JH, Schutrups J, van de Velde CJ, Mulder GJ. Effect of glutathione depletion on inhibition of cell cycle progression and induction of apoptosis by melphalan (L-phenylalanine mustard) in human colorectal cancer cells. Biochem Pharmacol. 1999;58:655–664. doi: 10.1016/s0006-2952(99)00130-6. [DOI] [PubMed] [Google Scholar]
- 45.Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10:1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 47.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 48.Jemnitz K, Veres Z, Monostory K, Kobori L, Vereczkey L. Interspecies differences in acetaminophen sensitivity of human, rat, and mouse primary hepatocytes. Toxicol In Vitro. 2008;22:961–967. doi: 10.1016/j.tiv.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Akai S, Hosomi H, Minami K, Tsuneyama K, Katoh M, Nakajima M, Yokoi T. Knock down of gamma-glutamylcysteine synthetase in rat causes acetaminophen-induced hepatotoxicity. J Biol Chem. 2007;282:23996–24003. doi: 10.1074/jbc.M702819200. [DOI] [PubMed] [Google Scholar]
- 50.Neuwelt AJ, Nguyen T, Wu YJ, Donson AM, Vibhakar R, Venkatamaran S, Amani V, Neuwelt EA, Rapkin LB, Foreman NK. Preclinical high-dose acetaminophen with N-acetylcysteine rescue enhances the efficacy of cisplatin chemotherapy in atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24602. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]