Abstract
Transition-metal peroxos have been implicated as key intermediates in a variety of critical biological processes involving O2. Due to their highly reactive nature, very few metal-peroxos have been characterized. The dioxygen chemistry of manganese remains largely unexplored despite the proposed involvement of a binuclear Mn-peroxo, either as a precursor to O2, or derived from O2, in both photosynthetic H2O oxidation and DNA biosynthesis, arguably two of the most fundamental processes of life. Neither of these biological intermediates has been observed. Herein we describe the dioxygen chemistry of coordinatively unsaturated [MnII(SMe2N4(6-MeDPEN))] +(1), and the characterization of intermediates formed en route to a binuclear mono-oxo bridged Mn(III) product {[MnIII(SMe2N4(6-MeDPEN)]2-(μ-O)}2+ (2), the oxo atom of which is derived from 18O2. At low-temperatures, a dioxygen intermediate, [Mn(SMe2N4(6-MeDPEN))(O2)]+ (4), is observed (by stopped-flow) to rapidly and irreversibly form in this reaction (k1(−10 °C)= 3780±180M−1s−1, ΔH1‡ = 26.4±1.7 kJ mol−1, ΔS1‡ = − 75.6±6.8 J mol−1K−1), and then convert more slowly (k2(−10 °C)= 417±3.2 M−1s−1, ΔH2‡ = 47.1±1.4 kJ mol−1, ΔS2‡ = − 15.0±5.7 J mol−1K−1) to a species 3 with isotopically sensitive stretches at νo-o (Δ18O) = 819(47) cm−1, kO–O= 3.02 mdyn/Å, and νMn-O(Δ18O) = 611(25) cm−1 consistent with a peroxo. Intermediate 3 releases approximately 0.5 equiv of H2O2 per Mn ion upon protonation, and the rate of conversion of 4 to 3 is dependent on [Mn(II)] concentration, consistent with the formation of a binuclear Mn-peroxo. This was verified by X-ray crystallography, where the peroxo of {[MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)}2+ (3) is shown to be bridging between two Mn(III) ions in an end-on trans-μ-1,2-fashion. This represents the first characterized example of a binuclear Mn(III)-peroxo, and a rare case in which more than one intermediate is observed en route to a binuclear μ–oxo bridged product derived from O2. Vibrational and metrical parameters for binuclear Mn-peroxo 3 are compared with those of related binuclear Fe- and Cu-peroxo compounds.
Introduction
Transition-metal ions promote a wide variety of biochemical processes involving O2.1-6 Metal-peroxos are proposed as key intermediates in a majority of these processes.2,3,7,8 For example, binuclear peroxo-bridged Mn intermediates (Mn–μ–(O22−)–Mn) are proposed to form during two of the most fundamental processes of life, that of photosynthetic H2O splitting9-15 and DNA biosynthesis.16-18 Photosynthetic H2O splitting9-15 generates approximately 80% of the O2 on our planet. However, neither the photosynthetic Mn-peroxo, nor the Mn-peroxo implicated in DNA biosynthesis has been observed. Under extremely high (30 bar) O2 pressures a transient Mn species is observed during photosynthetic H2O splitting,19 however, the identity of this species is unknown. Although similar peroxo-bridged intermediates are implicated in both of these fundamental processes, one forms via O–O bond cleavage en route to a more reactive high-valent metal-oxo intermediate,16-18,20 while the other forms via O–O bond formation. Our understanding of the structural and electronic properties that favor O–O bond formation versus cleavage is extremely limited for Mn. Understanding how to efficiently oxidize H2O to O2 by examining the microscopic reverse process involving the reduction of dioxygen (O2) to water (H2O) is critical21 to the development of renewable solar fuels.22 Given that it can promote these reactions under mild ambient conditions, Nature has much to teach us. Peroxo O–O bond cleavage has been shown with other metals (Cu and Fe) to be facilitated either by binding the peroxo between two metal ions, ideally in a side-on μ–η2:η2–bridging mode,23-26 or, by protonating one of the oxygens.27-29 The activation barrier to O2 binding and activation has been shown to be lowered by thiolate ligands (RS−).30 Thiolate ligands also provide a convenient spectroscopic handle with which to probe reactivity.31 Despite their key role in biological catalysis, very few Mn-peroxo species have been characterized,32-43 due, in part, to their instability.40,44-46 In fact, structurally characterized examples of middle-to-late first-row transition-metal peroxos are, in general, scarce.1,23,47,48 Of the seven structurally characterized metastable Mn(III)-peroxo compounds,33,36-39,43 all but one49 are mononuclear, and five contain the peroxo in a side-on (η2) binding mode. The only structural evidence for an end-on (η1) peroxo binding mode with Mn(III) was reported in 1988,49 and more recently by our group.33 There are only two examples of peroxo-bridged Mn-clusters, one binuclear Mn(IV)2 peroxo compound,40 and one trinuclear Mn(III)3 peroxo compound.49
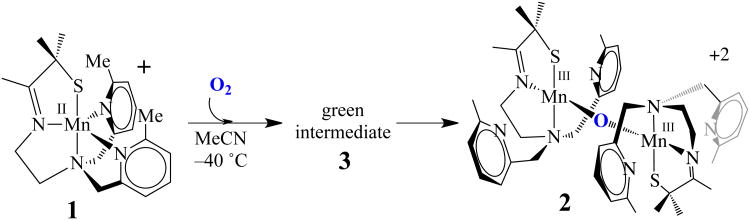
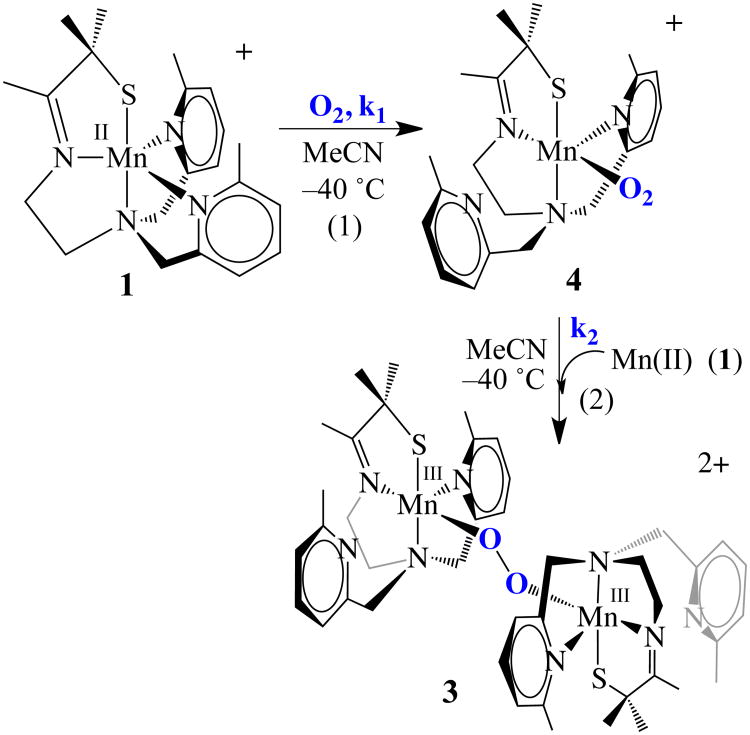
Recently we reported a series of coordinatively unsaturated thiolate-ligated Mn(II) complexes that react with dioxygen, via an observable intermediate, to afford rare examples of unsupported binuclear mono oxo bridged Mn(III) complexes.50 Isotopic labeling studies showed that the oxo atom is derived from O2.50 Herein we describe the kinetics of this O2 reaction, and provide evidence that more than one intermediate is involved, one of which is shown to be a binuclear peroxo-bridged Mn(III) complex. Crystallographic characterization of this dioxygen-derived intermediate provides the first structurally characterized example of a binuclear Mn(III)-peroxo. The significance of this structure is that it is analogous to key intermediates proposed to be involved in photosynthetic O2 evolution and DNA biosynthesis.9-18,20
Experimental Section
General Methods
All manipulations were performed using Schlenk line techniques or under an N2 atmosphere in a glovebox. Reagents and solvents were purchased from commercial vendors, were of the highest available purity, and were used without further purification unless otherwise noted. Propionitrile (CaH2) and MeCN (CaH2), were dried and distilled prior to use. Et2O was rigorously degassed and purified using solvent purification columns housed in a custom stainless steel cabinet and dispensed by a stainless steel schlenk-line (GlassContour). UV/vis spectra were recorded on a Varian Cary 50 spectrophotometer equipped with a fiber optic cable connected to a “dip” ATR probe (C-technologies). A custom-built two-neck solution sample holder equipped with a threaded glass connector was sized specifically to fit the “dip” probe. Magnetic moments (solid state) were obtained with polycrystalline samples in gel-caps from 5 to 300 K by zero-field cooling experiments using a Quantum Design MPMS S5 SQUID magnetometer. Pascal's constants were used to correct for diamagnetic contributions to the experimental magnetic moment. X-ray crystallography data was recorded on either a Bruker APEX II single crystal X-ray diffractometer with Mo-radiation or a Bruker SMART Apex CCD diffractometer with Mo Kα radiation.
Synthesis of Peroxo-Bridged [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2– O2)(BPh4)2•2CH3CH2CN (3)
[MnII(SMe2N4(6-Me-DPEN)](BPh4) (1) was synthesized as previously described for [MnII(SMe2N4(6-Me-DPEN)](PF6), 50 by replacing the NaPF6 counterion with NaBPh4. Peroxo–bridged 3 was prepared by cooling a propionitrile solution of 1 to −80°C, opening the flask to air, and then layering the cold solution with pre-cooled Et2O.
Hydrogen Peroxide Detection Assay
A concentrated propionitrile solution of 1 (0.1 mL, 5.5 mM) was prepared under an inert atmosphere. After cooling the solution to −80 °C (with a dry ice/acetone bath), O2 was gently bubbled into the solution, directly from a cylinder, for approximately two minutes. This procedure promoted a color change from light yellow to dark green, indicating the formation of peroxo-bridged 3. A minimal amount of concentrated H2SO4 (3-4 drops) was then added to the dark green solution, causing the solution to turn clear. The resulting reaction mixture was then passed through a very small silica plug, collected, and then added in a single aliquot to a stirring aqueous solution of KMnO4 (3 mL, 6.15 × 10−5 M). The resulting changes in absorbance values were then recorded in one minute intervals until no further changes were observed. The amount of hydrogen peroxide present was calculated based on the absorbance at 550 nm (ε (M−1cm−1, 298 K) = 2455) caused by the following reaction:
In a control experiment, the same procedure was performed upon oxo-bridged 2, which failed to illicit any considerable absorbance changes to aqueous solutions of KMnO4 of known concentration.
Stopped Flow Kinetic Measurements
Acetonitrile and propionitrile solutions of the reagents were prepared in an MBraun glovebox filled with high purity argon and placed in Hamilton gastight syringes. Time-resolved spectra (400-800 nm) were acquired at low temperatures using a TgK Scientific (U.K.) SF-61DX2 Multi-Mixing CryoStopped-Flow Instrument, a J&M TIDASD-AQ diode array detector and MCS UV/NIR light source (Spectralytics, DenMark). The stopped-flow instrument was equipped with PEEK tubings fitted inside stainless steel plumbing, a 1.00 cm3 quarz mixing cell, and an anaerobic kit purged with an inert gas. The temperature in the mixing cell was maintained to 0.1 °C and the mixing time was 2-3 ms. All flow lines of the instrument were extensively washed with degassed, anhydrous acetonitrile or propionitrile before charging the driving syringes with reactant solutions. The reactions were studied by rapid scanning spectrophotometry under pseudo-first-order conditions with excess oxygen (see experimental details in Supplemental Material). Saturated solutions of O2 were prepared by bubbling dry O2 gas for 15 minutes at 25 °C into gastight syringes containing dry CH3CN or CH3CH2CN; dilutions of the O2 saturated solvent were performed anaerobically to obtain the desired [O2]. The solubility of O2 was taken as 8.1 mM in CH3CN and 8.8 mM in CH3CH2CN at 25°C.51 All of the experiments were performed in a single-mixing mode of the instrument, with a 1:1 (v/v) mixing ratio. A series of three or four measurements gave an acceptable standard deviation (within 10%). Data analysis was performed with Kinetic Studio software from Hi-Tech Scientific and IGOR Pro software from Wavemetrics, Inc.
Resonance Raman Experiments
The Raman system consists of a Coherent I90C-K Kr+ laser, a SPEX model 1877 CP triple monochromator with a filter and blazed holographic gratings of 2400 grooves/mm and a Newton DU940N-BU 2048 × 512 pixel back-illuminated, thermoelectric-cooled CCD array. Crystalline samples of 16O and 18O-labelled peroxo-bridged 3 were prepared as described above. Spectra were taken at 77K with 5 - 40 mw laser power, and the sample was continuously rotated to minimize photo-decay.
X-Ray Crystallographic Structure Determination
A green needle of 3 measuring 0.20 × 0.15 × 0.05 mm3, was mounted on a glass capillary with oil. Data was collected at −173°C on a Bruker APEX II single crystal X-ray diffractometer, using Mo-radiation. The crystal-to-detector distance was set to 40 mm, and the exposure time was 30 s deg−1 for all sets of exposure. The scan width was 0.5°. Data collection was 89.4% complete to 25.0° in θ. A total of 27,190 merged reflections were collected covering the indices, h = −15 to 15, k = −22 to 22, l = −22 to 22. 7,909 reflections were symmetry independent and the Rint = 0.0964 indicated that the data was of less than average (0.07) quality. Indexing and unit cell refinement indicated a monoclinic P lattice with the space group be P 21/c (No.14).
The data for 3 was integrated and scaled using SAINT, and SADABS within the APEX2 software package by Bruker. Solution by direct methods (SHELXS, SIR97) produced a complete heavy atom phasing model consistent with the proposed structure. The structure was completed by difference Fourier synthesis with SHELXL97.52,53 Scattering factors are from Waasmair and Kirfel.54 Hydrogen atoms were placed in geometrically idealised positions and constrained to ride on their parent atoms with C---H distances in the range 0.95-1.00 Angstrom. Isotropic thermal parameters (Ueq) for the hydrogens were fixed such that they were 1.2Ueq of their parent atom Ueq for methylenes and CH's, and 1.5Ueq of their parent atom Ueq in case of methyl groups. All non-hydrogen atoms were refined anisotropically by full-matrix least-squares. Crystal data for 3 are summarized in Table 1, and metrical parameters are provided in Table 2.
Table 1.
Crystal data for [MnIII(SMe2N4(6-Me-DPEN)]2 (trans–μ–1,2–O)(BPh4)2 •2CH3CH2CN (3).
| 3 | |
|---|---|
|
| |
| Formula | C108 H128B2Mn2N14O2S2 |
| MW | 1849.86 |
| T, K | 100(2) K |
| Unit cellA | Monoclinic |
| a, Å | 14.2799(10) |
| b, Å | 18.3957(14) |
| c, Å | 18.5282(13) |
| α, deg | 90 |
| β, deg | 94.541(2) |
| γ, deg | 90 |
| V, Å3 | 4851.9(6) |
| Z | 2 |
| d(calc), g/cm3 | 1.266 |
| Space group | P 21/c |
| R | 0.0613b |
| Rw | 0.1614c |
| GOF | 1.002 |
In all cases: Mo Kα(λ = 0.71070 Å) radiation.
R = Σ ‖Fo| - |Fc‖/Σ |Fo|; Rw = [Σw(|Fo| = |Fc|)2/ΣwFo2]1/2, where w−1 = [σcount2 + (0.05F2)2]/4F2.
Table 2.
Comparison of Vibrational and Metrical Parameters for Selected Metal-Peroxo Complexes.
| Complex | vO-O, cm-1 | O–O, Å | Peroxo binding mode | Ref |
|---|---|---|---|---|
|
| ||||
| [Mn2(SMe2N4(6-Me-DPEN))2(O2)]2+ (3) | 819 | 1.452(5) | trans μ-1,2 | This work |
| [Mn(3,5-iPr2pzH){(HB(3,5-iPr2pz)3}(O2)] | 892 | 1.428(7) | side-on η2 | (37) |
| [Mn(H3bupa)(O2)]− | 885 | N/A | side-on η2 | (35) |
| [Mn(tmc)(O2)]+ | N/A | 1.403(4) | side-on η2 | (43) |
| [Fe2(O2)(O2CCH2Ph) {(HB(pz′)3}2] | 888 | 1.408(9) | trans μ-1,2 | (7), (76) |
| [Fe2 (O)2(N-Et-hptb)(Ph3PO)2)2]3+ | 900 | 1.416(7) | cis μ-1,2 | (63), (61) |
| [Fe2 (6Me2-BPP)2(OH)(O2)]+ | 908 | 1.396(5) | cis μ-1,2 | (59) |
| [Cu2(tmpa)2(O2)]+ | 825 | 1.432(6) | trans μ-1,2 | (26), (64), (63) |
| [Cu2(Me6tren)2(O2)]2+ | 814 | 1.368(9) | trans μ-1,2 | (92) |
| [Cu2{(HB(3,5-iPr2pz)3}2(O2)] | 741 | 1.412(12) | μ-η2: η2 | (25), (56) |
| [Cu2(MeAN)2(O2)]2+ | 721 | 1.540(5) | μ-η2: η2 | (57) |
Two highly disordered propionitriles were found to co-crystallize with peroxo-bridged [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)(BPh4)2•2CH3CH2CN (3), one modeled over two positions (83/17 ratio), and the second modeled over three positions (66/17/17 ratio). The occupancies of each disorder component were refined freely while restraining the total occupancy sum to one. Disorder in one of the phenyl rings of the tetraphenylborate anion was modeled over two positions (66/34 ratio) without any additional restraints.
Results and Discussion
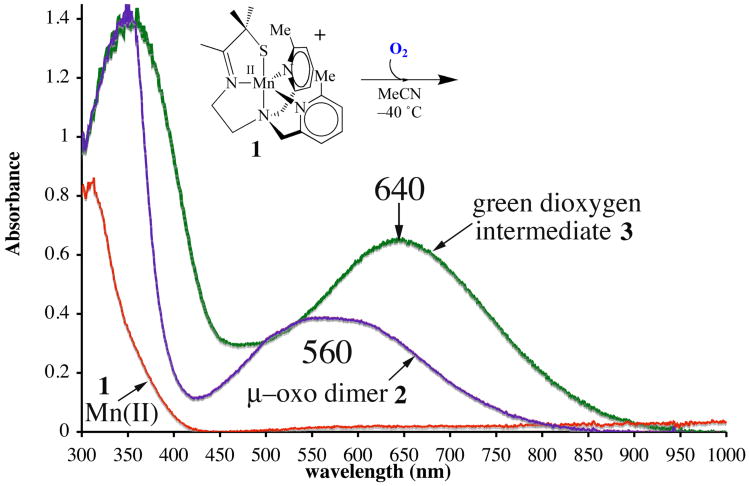
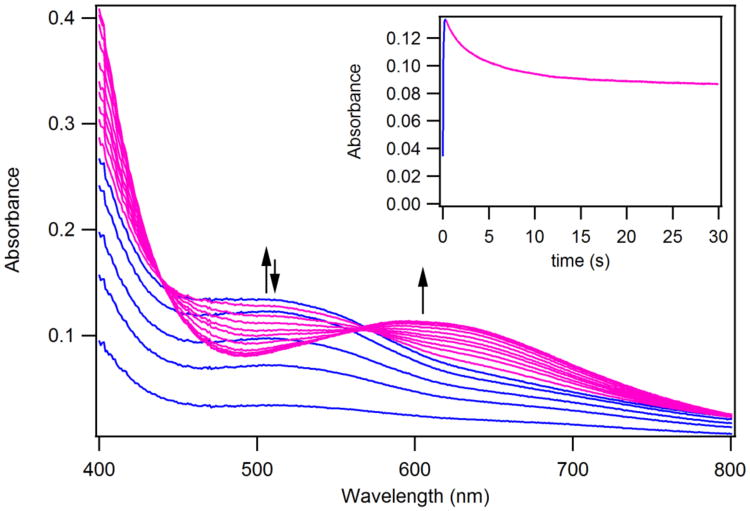
Coordinatively unsaturated [MnII(SMe2N4(6-MeDPEN))](BF4) (1) was synthesized and structurally characterized as previously described.50 Dioxygen addition to 1 at ambient temperature causes an immediate color change, from colorless to purple, which is accompanied by the growth of a peak at 560(ε= 520 M−1cm−1) nm in the electronic absorption spectrum (Figure 1). Crystallization of the product and characterization by X–ray crystallography showed the final O2–derived product to be a rare example of a stable unsupported mono-oxo-bridged binuclear Mn(III) compound, [MnIII(SMe2N4(6-MeDPEN)]2-(μ-O)(BF4)2(2) (Scheme 1).50 18O-labelling studies50 showed that the bridging oxo atom (blue, Scheme 1) of 2 is derived from dioxygen. When this reaction is monitored at low temperatures (−40 °C, in MeCN) via electronic absorption spectroscopy, a green metastable intermediate, 3, is detected, which displays an absorption band at 640(ε= 830 M−1cm−1) nm (Figure 1). The BF4− salt of this intermediate converts to oxo-bridged 2 within minutes at −40 °C. If the BF4− or PF6− counterion is replaced with BPh4−, then the low-temperature (−40 °C) lifetime of 3 can be extended (to ∼45min) in MeCN. The conversion of 3 to oxo-bridged 2 is nearly quantitative (96-98%) with either counterion, based on the amount of Mn(II) starting material, implying that metastable 3 is well-behaved, and cleanly converts to 2. Low temperature addition of H2SO4 to in situ generated solutions of 3, reproducibly affords 0.43(4) equiv of H2O2 per equiv of Mn2+, as detected and quantified via a MnO4− titration using the method described in the experimental section (Figure S-1). The reproducible observation of hydrogen peroxide in a 2:1 (Mn: H2O2) stoichiometry suggested that metastable 3 is a bimetallic peroxo, Mn-(μ-O22−)-Mn.
Figure 1.
Electronic absorption spectrum of thiolate-ligated [MnII(SMe2N4(6-MeDPEN))] +(1), and the reaction product 2, and intermediate 3, formed in its low-temperature reaction with dioxygen (O2) in MeCN.
Scheme 1.
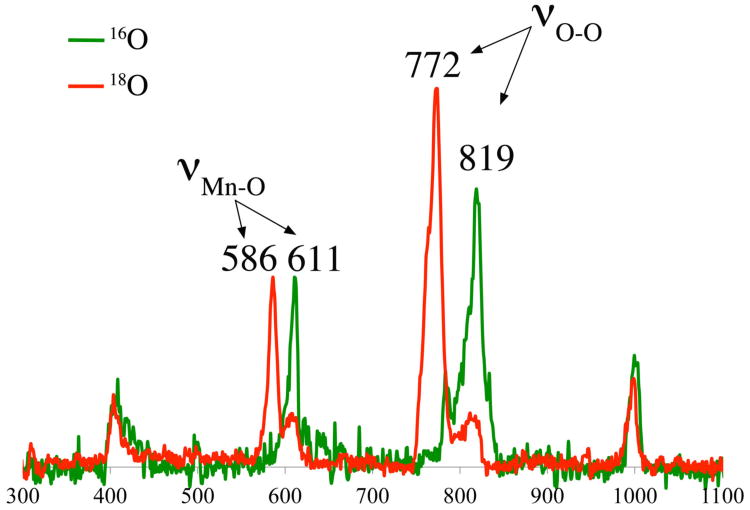
Although there is only one reported example of a resonance Raman–characterized Mn-peroxo species,34,49 we were able to successfully perform these experiments using crystalline samples of 3. Resonance Raman spectra were collected using a series of excitation wavelengths, and resonance enhanced νO-O and νMn-O vibrations are only observed when an excitation frequency of 413 nm near the high energy absorption band of 3 (at 385 nm) is used. Excitation wavelengths near the lower energy visible band (at 640 nm; Figure S–2) did not, on the other hand, result in resonance enhanced peroxo vibrations, indicating that the higher energy, as opposed to lower energy band, corresponds to a peroxo-to-metal charge transfer band. This is in contrast to iron-peroxo compounds, which display a peroxo-to-metal charge transfer band in the visible region (at ∼700 nm),20,55 but is in agreement with the TD-DFT calculated peroxo-to-metal charge transfer transition (πop*(O-O) → Mn(dyz)) energies (∼450 nm) previously reported for Mn(III)–peroxo complexes [MnIII(O2)(L7py2R)]2+.34 As shown in Figure 2, isotopically sensitive resonance-enhanced vibrations for 3, not seen with 1 and 2, are observed at νO-O(Δ18O) = 819(47) cm−1 and νMn-O(Δ18O) = 611(25) cm−1, consistent with a metal– peroxo species. This indicates that two electron reduction of O2 has occurred, and the dioxygen O-O bond has been activated. The νO-O stretching frequency of 3 is significantly lower than the few reported νO-O frequencies (IR) for monomeric side-on Mn(III)-peroxo compounds such as [Mn(3,5-iPr2pzH){(HB(3,5-iPr2pz)3}(O2)], and [Mn(H3bupa)(O2)]− (Table 2).35,37 A comparison of force constants (vide infra) and an analysis of the coupling between O–O and Mn–O stretches would be necessary in order to comment on the relative extent of O–O bond activation in 3 versus [Mn(3,5-iPr2pzH){(HB(3,5-iPr2pz)3}(O2)], and [Mn(H3bupa)(O2)]−, however this data is not available for the side-on Mn(III)-peroxo compounds. Given the known mechanisms for O–O bond activation,27-29 this would suggest that the peroxo of 3 is either protonated or coordinated in a bridging mode. The reproducibly observed 2:1 ratio of H2O2: Mn would be more consistent with the latter, and the one example of a vibrationally characterized multinuclear Mn-peroxo compound,49 [MnIII3(dien)3(μ-OAc)2(μ3-O)(cis μ-1,2-O2)]3+, also displays a low νO-O frequency (814 cm−1) consistent with this. Although they are very different in terms of electronic structure, side-on binuclear μ-η2: η2 Cu peroxo compounds display νO-O frequencies noticeably lower than that of 3 (Table 2),25,56,57 whereas end-on binuclear Cu peroxo complexes display νO-O frequencies comparable to that of 3 (Table 2). Binuclear end-on Fe(III)-peroxos species,55,58-60 including intermediates formed in RNR and MMO,20 however, show significantly higher νO-O stretching frequencies (843 – 908 cm−1) relative to 3 (Table 2). This has been attributed to the large Fe-O-O angles associated with these binuclear Fe(III)-peroxo compounds which induces Fe-O mixing into the O-O bond.55 The νO-O stretching frequency for 3 (819 cm−1) does fall in the range (781-844 cm−1) of mononuclear Fe(III) hydroperoxo compounds,29 where there is no Fe-O mixing into the O-O vibration due to the lack of mechanical coupling.29,55 As far as vibrations involving the Mn-peroxo bond are concerned, experimentally determined νMn-O data is not available for comparison.
Figure 2.
Resonance Raman spectrum of the metastable intermediate 3, formed in the low-temperature reaction between coordinatively unsaturated [MnII(SMe2N4(6-MeDPEN))](BPh4) (1), and 16O- (green) and 18O-labled (red) dioxygen (O2). Counterion (BPh4−) stretch seen at 996 cm-1.
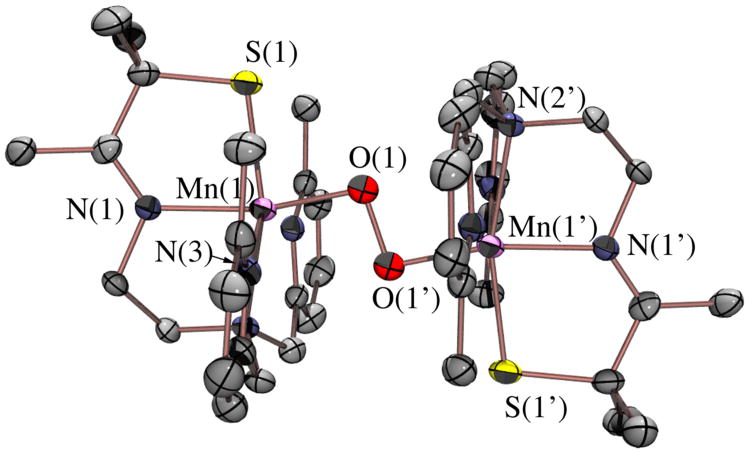
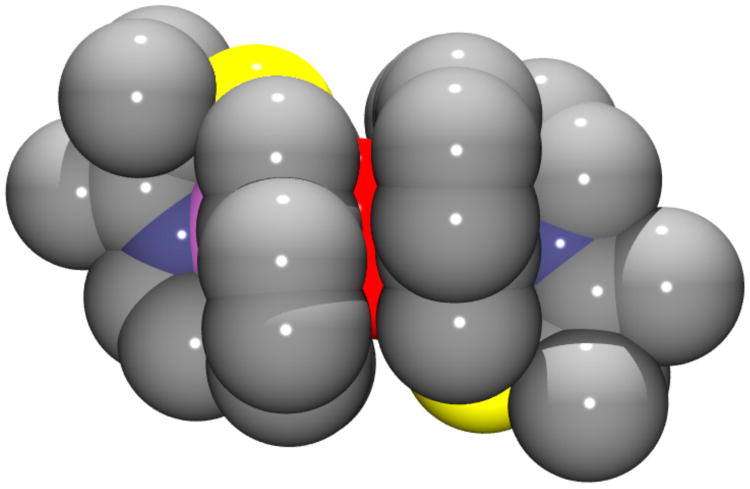
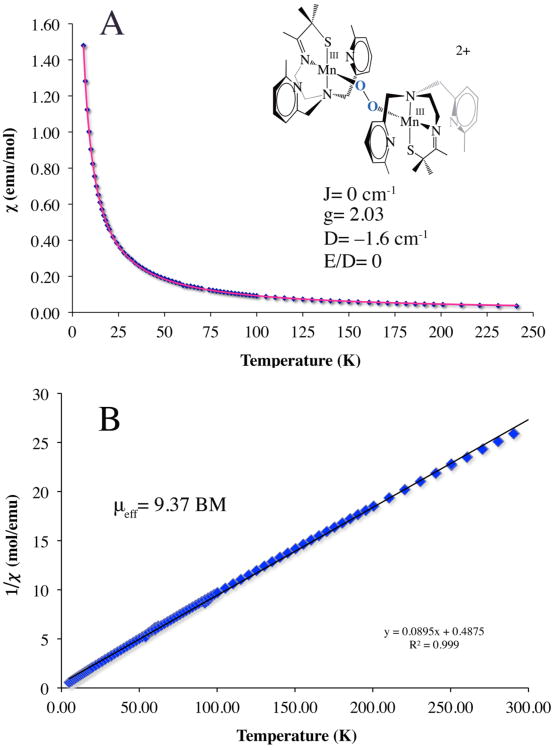
Verification of the bimetallic nature of peroxo-bound 3 was obtained by X-ray crystallography. Single crystals of the metastable intermediate were obtained by cooling a propionitrile (CH3CH2CN) solution of the tetraphenylborate salt of 1 to −80 °C, introducing O2, and then layering it with Et2O. As shown by the R-factor (6.1%; Table 1), and estimated standard deviations (Table 3), the structure of 3 is of high-quality. As shown in the ORTEP diagram of Figure 3, [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)(BPh4)2•2CH3CH2CN (3) contains an O2 ligand bridging two manganese ions in an end-on trans configuration (Figure S-3). Each Mn ion is ligated by a thiolate sulfur (S(1)), an O2-derived oxygen (O(1)), an imine (N(1)) and tertiary amine (N(2)), and two N-heterocyclic amine nitrogens (N(3, 4)). The distance separating the pyridine nitrogens N(4) and N(4′) from the Mn ions (2.492(3) Å) is significantly greater than the sum of their covalent radii (2.105 Å).61 Most likely this is due to Jahn-Teller-like distortion which allows for the stabilization of an antibonding electron. Steric constraints involving the 6-Me substituent and the gem-dimethyls adjacent to the sulfur likely contribute as well.50 The O–O bond length in 3 (1.452(5) Å, Table 3) is significantly longer than that (1.21 Å) of dioxygen (O=O), and close to that of reduced O–O2− (1.49 Å),48 consistent with reductive activation of the O–O bond. Dioxygen reduction is accompanied by oxidation of both Mn ions in 3 (from the +2 to the +3 oxidation state), as shown by comparison of the Mn–X (X= N, S) bond lengths with those of the reduced starting material 1 and final oxidized product 2 (Table 3).50 The Mn(III)-S bond (2.2747(12) Å) of 3 is 0.096 Å shorter than that of reduced 1, but comparable to that of oxidized mono-oxo bridged {[MnIII(SMe2N4(6-MeDPEN)]2-(μ-O)}2+ (2), {[MnIII(SMe2N4(2-QuinoEN)]2(μ-O)}2+ (2.292(1) Å), [MnIII(SMe2N4(6-Me-DPPN))]2(μ-O)(BPh4)2 (2.255(2) Å), and {[MnIII(SMe2N4(6-H-DPEN)]2(μ-O)}2+ (2.2968(15) Å),50 as well as alkylperoxo-ligated [MnIII(SMe2N4(2-QuinoEN)(OOtBu)]+ (2.270(3) Å).33 The peroxo in 3 is best described as binding in an end-on trans–μ–1,2–O2 fashion (Figure S-3), as opposed to an asymmetric side-on μ-η2: η2 fashion as shown by the significantly shorter Mn(1)–O(1)(1.832(3) Å) vs Mn(1)•••O(1′) (2.48 Å) distance. Peroxo-bridged 3 represents the first example of a binuclear Mn(III)-peroxo complex, structurally, or otherwise, characterized. Of the six structurally characterized metastable Mn(III)-peroxo compounds,33,36,37,39,40 all but one49 are mononuclear, and five contain the peroxo in a side-on (η2) binding mode. The only other multinuclear Mn-peroxo compound, [MnIII3(dien)3(μ-OAc)2(μ3-O)(cis μ-1,2-O2)]3+,49 contains a bridging cis μ-1,2-peroxo. Aside from this cis μ-1,2-peroxo compound, the only other crystallographically characterized end-on manganese-peroxo compound contains an alkyl-peroxo ligand.33 The Mn–O(1) peroxo bond of 3 is noticebly shorter than all other reported structurally characterized Mn(III)-peroxo compounds (reported range: Mn–O= 1.850(6)–1.901(4) Å).33,36,37,39,40 The acute Mn-O(1)-O(2) angle (97.5(6)˚) indicates that the Mn–O(1) peroxo bond of 3 does not have significant double bond character, however. The peroxo O–O bond of 3 (1.452(5) Å), is significantly longer (Table 2) than all of the structurally characterized side-on Mn(III)-η2-O22− (reported range: O–O= 1.410(4)–1.428(7) Å),36,37,39 but similar in length to the only structurally characterized end-on peroxo compounds, [MnIII(SMe2N4(QuinoEN))(OOtBu)]+ (1.457(7) Å),33 and within the error limits of [MnIII3(dien)3(μ-OAc)2(μ3-O)(cis μ-1,2-O2)]3+ (1.6(1) Å).49 The long peroxo bond length of 3 implies that the O–O bond should readily cleave, consistent with its observed conversion to binuclear mono-oxo bridged 2. Comparison of the peroxo O–O bond length in binuclear Mn-3 with selected examples of binuclear Fe and Cu peroxo complexes (Table 2) illustrates how the O–O bond length in 3 falls at the high end of that observed in the majority of reactive binuclear metal-peroxo complexes regardless of the peroxo binding mode. This suggests that the peroxo in 3 is highly activated. As shown by a recent analysis of the factors which influence the extremely long (1.540(5) Å) peroxo bond in [Cu2(MeAN)2(O2)]2+,57 a variety of factors (i.e. extent of overlap with the peroxo σ* vs π*) influence the peroxo O–O bond length which do not necessarily reflect the extent of O-O bond activation. Another physical parameter which is predictive of the level of O–O bond activation is the vibrational force constant. The force constant associated with the νO-O of 3 (k= 3.02 mdyn/Å), calculated using normal coordinate analysis, is comparable to those of reactive binuclear Fe– and Cu–peroxo compounds, [Fe2(cis-μ,-1,2-O2)(OBz)2{HB(pz′)3}2] (k= 3.1 mdyn/Å) and [{Cu(TMPA)}2(trans-μ-1,2-O2)]2+ (k= 3.103 mdyn/Å),55,62 suggesting that 3 should be reactive as well. The experimentally observed νO-O stretching frequencies associated with the above Fe- and Cu-peroxo compounds (832 and 876 cm−1),55,62 on the other hand, are significantly higher than that of 3 (due to the mechanical coupling between the M-O and O-O vibrations) showing that νO-O stretching frequency can not be used alone to predict levels of O–O bond activation.57 The low-temperature (−40 °C) half-life of 3 both in the presence (τ1/2= 11(2) sec; Figure S-4) and absence (τ1/2= 14(2) sec) of cyclohexane carboxaldehyde indicates 3 is unreactive towards electrophiles.35,43 This is in contrast to side-on mononuclear peroxo complexes, [MnIII(tmc)(O2)],43 and [MnIII(H3bupa)(O2)]1−.35 This lack of reactivity is perhaps not surprising, however, given the bridging, and therefore inaccessible and less nucleophilic, nature of the peroxo in 3. As shown by the space–filling diagram of Figure 4, the aromatic pyridine rings provide a protective cavity for the peroxo, thus explaining the stability (albeit limited) of what appears to be a highly activated O–O bond. The aromatic rings are roughly coplanar (deviation= 7.95°) with an average C•••C separation of 4.16 Å, suggesting that π-stacking interactions might help to stabilize the structure. The crystallographic data show that the Mn3+ ions in 3 are significantly more separated (4.113 Å) than would be expected for a bridging side-on μ–η2:η2–peroxo (∼3.5 Å),23,25,26,47 which is again consistent with an end-on trans–μ–1,2–peroxo description. This is a much a less commonly observed peroxo binding mode.63 Fits to the magnetic susceptibility vs temperature (J= 0 cm−1, D= −1.6 cm−1; E/D= 0) curves (Figure 5A), and inverse magnetic susceptibility vs temperature data (μeff= 9.37 BM; Figure 5B), indicate that the Mn3+ ions of 3 are magnetically uncoupled and high–spin (S= 2). Peroxo ligands often mediate strong exchange coupling,24,55,64,65 however DFT single point calculations on the crystal structure of 3 reproduce the low exchange coupling, and show that this mainly reflects the fact that the Mn d orbital forming a strong σ-bond with the peroxide π* orbital is unoccupied. Also, the π-interaction between peroxide and Mn d orbitals is very weak and therefore does not contribute to coupling between the two metal centers.
Table 3.
Selected Bond Distances (Å) and Bond Angles (deg) for Five Coordinate [MnII(SMe2N4(6-Me-DPEN)](PF6) (1), oxo bridged [MnIII(SMe2N4(6-MeDPEN)]2-(μ-O)(BF4)2•2MeOH (2), Peroxo-Bound [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)(BPh4)2 •2CH3CH2CN (3)
| 1 | 2 | 3 | |
|---|---|---|---|
| Mn(1)–S(1) | 2.3710(6) | 2.2767(7) | 2.2747(12) |
| Mn(1)–N(1) | 2.186(2) | 1.999(3) | 2.040(3) |
| Mn(1)–N(2) | 2.297(2) | 2.151(2) | 2.203(3) |
| Mn(1)–N(3) | 2.222(2) | 2.581(2) | 2.410(3) |
| Mn(1)–N(4) | 2.239(2) | 2.501(2) | 2.492(3) |
| Mn(1)–O(1) | N/A | 1.7602(4) | 1.832(3) |
| O(1)–O(1′) | N/A | N/A | 1.452(5) |
| Mn(1)•••O(1′) | N/A | N/A | 2.481 |
| Mn(1)•••Mn(1′) | N/A | 3.520 | 4.113 |
| Mn(1)-O(1)-O(1′) | N/A | N/A | 97.5(6) |
| O(1)-Mn(1)-S(1) | N/A | 95.37 | 85.12(9) |
| O(1)-Mn(1)-N(3) | N/A | 92.79 | 92.4(1) |
| O(1)-Mn(1)-N(1) | N/A | 177.21(9) | 165.5(1) |
| O(1)-Mn(1)-N(2) | N/A | 100.44(6) | 111.1(1) |
| S(1)-Mn-N(2) | 156.98(5) | 164.17(7) | 163.3(1) |
| S(1)-Mn-N(3) | 117.36(5) | 106.2 (1) | 109.0(1) |
| S(1)-Mn-N(4) | 117.38(5) | 106.84(6) | 105.6(1) |
| N(1)-Mn-N(4) | 120.58(7) | 92.68(1) | 101.7(1) |
| Mn(1)–O–Mn(1′) | N/A | 180.0 | N/A |
Figure 3.
ORTEP of peroxo-bridged {[MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)}2+(3) showing 50% probability ellipsoids and the atom labeling scheme. Hydrogens have been omitted for clarity.
Figure 4.
Space filling diagram of peroxo-bridged {[MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)}2+ showing that the peroxo (red) is nestled in a protective cavity provided by the pyridine rings.
Figure 5.
Magnetic susceptibility vs temperature data (A, blue diamonds) obtained for a solid sample of peroxo-bridged [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2-O2)(BPh4)2•2CH3CH2CN (3). Fits to the chi vs T data (A; pink line; J= 0 cm-1, D= −1.6 cm-1, and E/D= 0)66, as well as the inverse magnetic susceptibility vs T data (B) are consistent with high–spin (S= 2) magnetically uncoupled (down to 5 K) Mn3+ ions in an axial environment.
Kinetics of the Reaction Between [MnII(SMe2N4(6-MeDPEN))](BPh4) (1) and O2
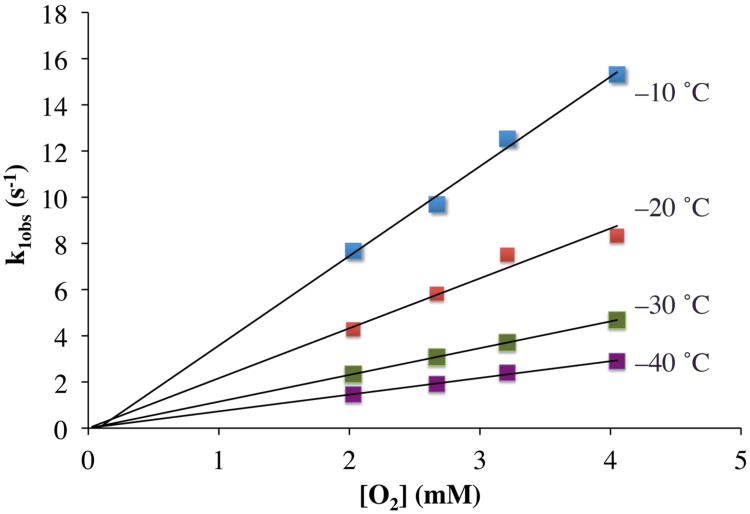
In order to determine the mechanism by which peroxo-bridged [MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)2+ (3) forms, we examined the kinetics of low-temperature reactions between [MnII(SMe2N4(6-MeDPEN))](BPh4) (1) and dioxygen. Given the widely accepted proposed mechanisms for binuclear metal-peroxo and μ-oxo formation,67 the likely mechanism was hypothesized to involve a mononuclear Mn-O2 intermediate. An intermediate (with λmax = 515 nm) is in fact observed to form en route to peroxo 3 on the msec time-scale using a stopped-flow instrument. This intermediate, [Mn(SMe2N4(6- MeDPEN))(O2)]+ (4; Scheme 2), which is presumed to be an η1- superoxo, accumulates rapidly (within fractions of a second) in MeCN at low temperatures, and then more slowly converts to μ-peroxo-bridged 3 (λmax = 640 nm). Kinetics for both of these steps (shown in Scheme 2) were measured by the stopped-flow technique over the temperature range −40 °C to − 10 °C. A representative spectral overlay is shown in Figure 6, and the general kinetic scheme is shown in equations 1 and 2:
Scheme 2.
Figure 6.
Time resolved spectral changes obtained upon mixing acetonitrile solutions of Mn(II) complex 1 (1.5 mM) and O2 (4.05 mM) at −10 °C. Inset: kinetic trace (λ = 515 nm) showing formation and subsequent decay of the Mn-O2 intermediate 4. All reported concentrations are after mixing in the stopped-flow cell.
| (1) |
| (2) |
| (3) |
Kinetic traces (λ = 515 nm) obtained under pseudo first-order conditions with excess O2 (Figure 6) were fit to the general two-term rate law shown in equation (3), which represents a general solution to the mechanism outlined in equations (1) and (2). Pseudo first-order rate constants, k1obs and k2obs, were obtained (see Supplemental material) by fitting the kinetic traces to equation (3). The observed rate constant associated with formation of intermediate 4, k1obs, was found to increase linearly with increasing O2 concentrations, allowing us to determine the second order rate constant (k1) from the slope of k1obs vs [O2] plots. Rate constants were obtained in this manner at four different temperatures (Table 4). The intercepts of the k1obs versus O2 plots were found to be close to zero (Figure 7) indicating that reaction (1) in Scheme 2 is essentially irreversible. The observed rate constant, k2obs associated with the conversion of superoxo-4 to peroxo-3 (rxn (2) in Scheme 2; eqn (2)), on the other hand, was found to be essentially independent of O2 concentration over the entire temperature range studied (Figure S-5). This indicates that the rate of formation of the μ-peroxo 3 is zero order in [O2]
Table 4.
Experimentally Measured Temperature-Dependent Rate Constants for the Formation of η1-Superoxo 4 (k1) and Binculear Peroxo-Bridged 3 (k2) in the Reaction Between [MnII(SMe2N4(6-MeDPEN))](BPh4) (1) and O2 in MeCN.
| Temperature (°C) | k1 (M−1 s−1) | k2 (M−1 s−1) |
|---|---|---|
|
| ||
| − 40 °C | 717 ± 45 | 22. 7 ± 0. 2 |
| − 30 °C | 1151 ± 24 | 60. 7 ± 0. 3 |
| − 20 °C | 2106 ± 259 | 153. 6 ± 1. 5 |
| − 10 °C | 3779 ± 180 | 416.9 ± 3.2 |
Figure 7.
Plots of the observed rate constants (k1obs) for the formation of superoxo intermediate 4 versus O2 concentration over the temperature range − 40 °C to − 10 °C in the reaction between Mn(II) complex 1 (1.5 mM) and O2 in CH3CN. The slope of each line represents the value of the second-order rate constant for superoxo 4 formation (k1) at each temperature.
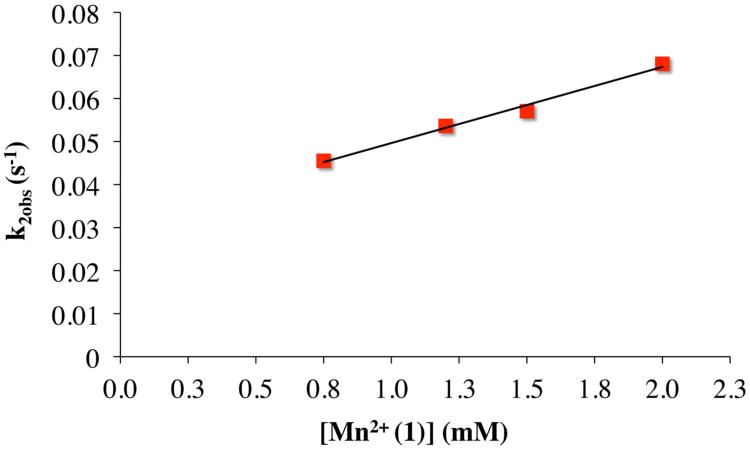
In order to determine the reaction order with respect to Mn(II) for steps (1) and (2) of Scheme 2 at −30 °C, the [MnII(SMe2N4(6-MeDPEN))]+(1) concentration was varied (from 0.8, 1.1, 1.5 to 2.0 mM after mixing) while maintaining a constant amount of excess oxygen (4.1 mM after mixing). The rates of formation of η1-superoxo 4 and binuclear peroxo-bridged 3 were determined as described above, by following the build-up and decay of 4 at λ = 515 nm. The observed rate constants (k′1obs and k′2obs) were obtained by fitting the kinetic traces to equation (3). The observed pseudo-first-order rate constant for η1-superoxo intermediate 4 formation (k′1obs) was found to be independent of [Mn(II)] in the presence of excess oxygen, consistent with first-order behavior with respect to [Mn(II)] for step (1) (Figure S-6). Observed rate constants for the formation of μ-peroxo 3 (k′2obs) showed a linear dependence on [Mn(II)] in excess oxygen, confirming second-order behavior with respect to [Mn(II)] overall (Figure 8). This supports the reaction mechanism outlined in equations (1) and (2) and Scheme 2. Taken together, the [O2]- and [Mn(II)]-dependency
Figure 8.
Plot of the observed rate constants (k′2obs) for formation of the binuclear peroxo complex 3 versus [Mn(II)L](BPh4) at − 30 °C in CH3CN. [O2] after mixing = 4.1 mM.
| (4) |
| (5) |
experiments yield the overall rate law for the formation of superoxo-4 (eqn (4)) and peroxo-3 (eqn (5)), respectively. Based on the effective second-order dependence on [Mn(II)] for μ-peroxo 3 formation (eqn 5), the decay event in kinetic traces at 515 nm was refit to a second-order equation (eqn (6)) from which k2
| (6) |
was obtained, and used for the calculation of activation parameters. The second-order rate constants k1 and k2 are summarized over the temperature range − 40 °C to − 10 °C in Table 4. Activation parameters for the formation of superoxo 4 (Δ H1‡ = 26.4±1.7 kJ mol−1, ΔS1‡ = −75.6±6.8 mol−1 K−1) and μ-peroxo 3(ΔH2‡ = 47.1±1.4 kJ mol−1, ΔS2‡ = − 15.0±5.7 J mol −1 K −1) were obtained from the Eyring plots shown in Figure S-7 The negative entropy of activation would be consistent with an associative process in both steps. The relatively small absolute value of the activation entropy, especially for the second step, may be attributed, at least in part, to desolvation of one (or both) reactants.
Dioxygen binding to iron(II), cobalt(II), and copper(II) is particularly relevant to bioinorganic chemistry, and synthetic model complexes provide important mechanistic insights.51,68,79 Manganese dioxygen chemistry is, on the other hand, less well developed,32,35 and no kinetic information is available for comparison to our results. The overall reaction pathway of oxygenation of [MnII(SMe2N4(6-MeDPEN))]+ (1) is similar to well-established mechanisms of dioxygen reactivity with other biologically-relevant first-row transition-metal complexes.51,68-74,80-82 Initial coordination of dioxygen to a mononuclear metal complex (Table 5) is often followed by the formation of a dinuclear peroxo species, which in many cases undergoes O-O bond cleavage, to afford either a high-valent mononuclear metal-oxo (M=O) species (Scheme 2) or a high-valent bimetallic bis μ-oxo (M(μ-O)2M) species.8,26,67,75,83 Steric bulk may, in some cases, stabilize the mononuclear dioxygen adducts and prevent the formation of a dinuclear peroxo complex.47,71,80,84 The relative rates of individual steps tend to vary as a function of the metal ion and ligand. Kinetic data for O2 binding to selected examples of aminopyridine or polyamine ligated copper(I), cobalt(II), or iron(II) complexes are listed in Table 5. Although not directly comparable to Mn(II)-containing 1 due to differences in ligand structure and solvent, the activation barrier to O2 binding to five-coordinate 1 is notably higher than that of coordinatively unsaturated complexes (Table 5). It is comparable, on the other hand, to that of six-coordinate diiron(II) complexes, such as [Fe2(HPTP)(O2CPh)2]+, [Fe2(OH)2(TPA)2]2+, or [Fe2(OH)2(BQPA)2]2+.51,86 Oxygen binding to six-coordinate iron(II) further decrease upon increasing the steric bulk of the ligand (e.g., [Fe2(OH)2(6-Me3TPA)2]2+).75,86 Oxygen binding to coordinatively unsaturated Co(II) or Cu(I) is even more rapid (Table 5),76,87-89 but it can be also retarded by steric hindrance (e.g. [Cu(HIPT3)tren]+).90
Table 5.
Comparison of Kinetic Parameters for Dioxygen Binding to Mn(II), Fe(II), Cu(I) and Co(II) Complexes.
| Complex | k1, M−1 s−1 | ΔH‡, kJ/mol | ΔS‡, J/K mol | Ref |
|---|---|---|---|---|
|
| ||||
| [MnII(SMe2N4(6-Me-DPEN)]+ (1) | 3.78(18) × 103 (233 K)a | 26(2) | −76(7) | This work |
| [FeII2(HPTP)(O2CPh)]2 + | 9.6(3) × 102 (233 K)c | 16.5(4)3 | −114(2) | (85) |
| [FeII (OH)2 (TPA)2]2+ | 1.21(6) × 101 (233 K)b | 30(4)4 | −94(10) | (60) |
| [FeII2(OH)2(6-Me3TPA)2]2+ | 1.94(11) (233 K)a | 16(2) | −167(10) | (60), (86) |
| [FeII2(OH)2(BQPA)2]2+ | 3.2(2) (233 K)b | 36(4) | −80(10) | (60) |
| [FeII2(OH)2(BnBQA)2]2 | 2.67(10) × 103 (233 K)a | 16(2) | −108(10) | (60) |
| [CuI(Me6tren)(EtCN)]+ | 9.5(4) × 104 (183 K)c2 | 17.1(6) | −52(3) | (93) |
| [CuI(TPA)]+ | 1.50(2) × 108 (193 K)d1 | 7.62 | −45 | (76), (87), (88) |
| [CuI(TPA)(EtCN)]+ | 5.0(3) × 105 (223 K)c | 31.6(5) | 10.0(3) | (76) |
| [CuI(HIPT)3tren]+ | 2.3(2)e | N/A | N/A | (90) |
| [CoII(SalMeDPT) | 8.8 × 104 (203 K)e | 12.7 | −80 | (89) |
MeCN.
CH2Cl2.
CH3CH2CN.
THF.
Acetone.

Conclusions
In conclusion, we describe a rare instance in which two metastable intermediates are observed in the low-temperature dioxygen chemistry of a coordinatively unsaturated manganese complex, [MnII(SMe2N4(6-MeDPEN))] + (1). Although oxygen binding to biologically relevant Fe(II), Cu(I), and Co(II) has been extensively studied,62,91,92 the observation of more than one intermediate in these processes is relatively rare.76,87,88,93 Manganese dioxygen chemistry, on the other hand, remains largely unexplored.32,35,40 Low-temperature stopped-flow experiments reported herein show that a dioxygen intermediate, [MnIII(SMe2N4(6-MeDPEN))(O2)]+ (4), rapidly forms upon the addition of O2 to coordinatively unsaturated 1 consistent with O2 binding to an open coordination site. The thiolate ligand helps to maintain an open coordination site.94 O2 binding to 1 is found to be relatively slow compared to related biologically relevant Fe and Cu complexes. In the absence of any other kinetic data for O2 binding to Mn(II), it would be difficult to draw any conclusions regarding the role of the metal ion in this reaction. Dioxygen intermediate 4 was shown to convert more slowly to a species, 3, with isotopically sensitive stretches in the resonance Raman spectrum (νO-O(Δ18O) = 819(47) cm−1, kO–O= 3.02 mdyn/Å, νMn-O(Δ18O) = 611(25) cm−1) consistent with a peroxo. The frequency of this νO-O stretch is compared with that of related binuclear Cu- and Fe-peroxo compounds. Roughly ½ equiv H2O2 was shown to be released per Mn ion of 3, and the rate of conversion of 4 to 3 was shown to be dependent on [Mn(II)] concentration, consistent with its formulation as a binuclear Mn-peroxo. This was verified by X-ray crystallography, where the peroxo of {[MnIII(SMe2N4(6-Me-DPEN)]2(trans–μ–1,2–O2)}2+ (3) is shown to be bridging between two Mn(III) ions in an end-on trans-μ-1,2-fashion. This represents the first characterized example of a binuclear Mn(III)-peroxo similar to intermediates proposed to be involved in both H2O oxidation and DNA biosynthesis, arguably two of the most fundamental processes of life. Neither of these biological intermediates has been observed. The peroxo O–O bond of 3 is shown to be is considerably longer than that of all crystallographically characterized mononuclear side-on peroxo compounds, and fall at the high end of reported bond lengths for binuclear Cu- and Fe-peroxo compounds, regardless of the peroxo binding mode.
Supplementary Material
Acknowledgments
JAK gratefully acknowledges the NIH (#RO1GM45881), EIS gratefully acknowledges NSF Biochemistry MCB0919027, and E.Rybak-Akimova thanks the US Department of Energy, Office of Basic Energy Science (DE-FG02-06ER15799) for funding.
Footnotes
Supporting Information Available. Details regarding the fits to the kinetic traces, quantitative detection of H2O2, a quantitative UV/vis of peroxo 3, ORTEP diagram showing connectivity within the dimanganese peroxo core, half-life dependence on CCA, plots of k2obs vs [O2], k′;1obs vs [Mn(II)], Eyring plots for second-order rate constants k1 and k2, and crystallographic tables for peroxo 3. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Tinberg CE, Lippard SJ. Acc Chem Res. 2011;44:280–288. doi: 10.1021/ar1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovaleva EG, Lipscomb JD. Science. 2007;316:453–456. doi: 10.1126/science.1134697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisvert L, Goldberg KI. Acc Chem Res. 2012;45:899–910. doi: 10.1021/ar2003072. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Xu Z, Shen T, Wu G, Zhang L, Jiao N. Organic Lett. 2012;14:2362–2365. doi: 10.1021/ol300781s. [DOI] [PubMed] [Google Scholar]
- 5.Timokhin VI, Anastasi NR, Stahl SS. J Am Chem Soc. 2003;125:12996–12997. doi: 10.1021/ja0362149. [DOI] [PubMed] [Google Scholar]
- 6.Gottumukkala AL, Teichert JF, Heijnen D, Eisink N, van Dijk S, Ferrer C, van den Hoogenband A, Minnaard AJ. J Org Chem. 2011;76:3498–3501. doi: 10.1021/jo101942f. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Lippard SL. J Am Chem Soc. 1996;118:4914–4915. [Google Scholar]
- 8.Decker A, Solomon EI. Curr Op Chem Biol. 2005;9:152–163. doi: 10.1016/j.cbpa.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Umena Y, Kawakami K, Shen JR, Kamiya N. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 10.Yano J, Kern J, Sauer K, Latimer M, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messigner J, Zouni A, Yachandra VK. Science. 2006;314:821–825. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy JP, Brudvig GW. Chem Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 12.Kanady JS, Tsui EY, Day MW, Agapie T. Science. 2011;333:733–736. doi: 10.1126/science.1206036. [DOI] [PubMed] [Google Scholar]
- 13.Yachandra VK, Sauer K, Klein MP. Chem Rev. 1996;96:2927–2950. doi: 10.1021/cr950052k. [DOI] [PubMed] [Google Scholar]
- 14.Lewis NS, Nocera DG. Proc Natl Acad Sci, USA. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong FA. Phil Trans R Soc B. 2008;363:1263–1270. doi: 10.1098/rstb.2007.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Stubbe J. Biochemistry. 2011;50:5615–5623. doi: 10.1021/bi200348q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boal AK, Cotruvo JA, Jr, Stubbe J, Rosenzweig AC. Science. 2010;329:1526–1530. doi: 10.1126/science.1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotruvo JA, Jr, Stubbe J. Biochemistry. 2010;49:1297–1309. doi: 10.1021/bi902106n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen J, Junge W. Nature. 2004;430:480–483. doi: 10.1038/nature02676. [DOI] [PubMed] [Google Scholar]
- 20.Skulan AJ, Brunold TC, Baldwin J, Saleh L, Bollinger JM, Jr, Solomon EI. J Am Chem Soc. 2004;126:8842–8855. doi: 10.1021/ja049106a. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg R, Gray HB. Inorg Chem. 2008;47:1697–1699. doi: 10.1021/ic800155g. [DOI] [PubMed] [Google Scholar]
- 22.Magnuson A, Anderlund M, Johansson O, Lindblad P, Lomoth R, Plivka T, Ott S, Stensjo K, Styring S, Sundstron V, Hammarstrom L. Acc Chem Res. 2009;42:1899–1909. doi: 10.1021/ar900127h. [DOI] [PubMed] [Google Scholar]
- 23.Tolman WB. Acc Chem Res. 1997;30:227–237. [Google Scholar]
- 24.Solomon EI, Tuczek F, Root DE, Brown CA. Chem Rev. 1994;94:827–856. [Google Scholar]
- 25.Kitajima N, Fujisawa K, Fujimoto C, Moro-oka Y, Hashimoto S, Kitagawa T, Toriumi K, Tatsumi K, Nakamura A. J Am Chem Soc. 1992;114:1277–1291. [Google Scholar]
- 26.Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. doi: 10.1021/cr020632z. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs JA. Science. 2003;299:1024–1025. doi: 10.1126/science.1081792. [DOI] [PubMed] [Google Scholar]
- 28.Neese F, Solomon EI. J Am Chem Soc. 1998;120:12829–12848. [Google Scholar]
- 29.Roelfes G, Vrajmasu V, chen K, Ho RYN, Rohde JU, Zondervan C, Crois RM, Schudde EP, Lutz M, Spek AL, Hage R, Feringa BL, Munck E, Que L., Jr Inorg Chem. 2003;42:2639–2653. doi: 10.1021/ic034065p. [DOI] [PubMed] [Google Scholar]
- 30.Brown CD, Neidig ML, Neibergall MB, Lipscomb JD, Solomon EI. J Am Chem Soc. 2007;129:7427–7438. doi: 10.1021/ja071364v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacs JA, Brines LM. Acc Chem Res. 2007;40:501–509. doi: 10.1021/ar600059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shook RL, Peterson SM, Greaves J, Moore C, Rheingold AL, Borovik AS. J Am Chem Soc. 2011;133:5810–5817. doi: 10.1021/ja106564a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coggins MK, Kovacs JA. J Am Chem Soc. 2011;133:12470–12473. doi: 10.1021/ja205520u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger RA, Chattopadhyay S, Day VW, Jackson TA. J Am Chem Soc. 2010;132:2821–2831. doi: 10.1021/ja910235g. [DOI] [PubMed] [Google Scholar]
- 35.Shook RL, Gunderson WA, Greaves J, Ziller JW, Hendrich MP, Borovik ASJ. Am Chem Soc. 2008;130:8888–8889. doi: 10.1021/ja802775e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanAtta RB, Strouse CE, Hanson LK, Valentine JS. J Am Chem Soc. 1987;109:1425–1434. [Google Scholar]
- 37.Kitajima N, Komatsuzaki H, Hikichi S, Osawa M, Moro-oka Y. J Am Chem Soc. 1994;116:11596–11597. [Google Scholar]
- 38.Singh UP, Sharma AK, Hikichi S, Komatsuzaki H, Moro-oka Y, Akita M. Inorg Chim Acta. 2006;359:4407–4411. [Google Scholar]
- 39.Annaraj J, Cho J, Lee YM, Kim SY, Latifi R, de Visser SP, Nam W. Angew Chem lnt Ed EngI. 2009;48:4150–4153. doi: 10.1002/anie.200900118. [DOI] [PubMed] [Google Scholar]
- 40.Bossek U, Weyhermuller T, Wieghardt K, Nuber B, Weiss J. J Am Chem Soc. 1990;112:6387–6388. [Google Scholar]
- 41.Geiger RA, Leto DF, Chattopadhyay S, Dorlet P, Anxolabéhère-Mallart E, Jackson TA. Inorg Chem. 2011;50:10190–10203. doi: 10.1021/ic201168j. [DOI] [PubMed] [Google Scholar]
- 42.Geiger RA, Wijeratne G, Day VW, Jackson TA. Eur J Inorg Chem. 2012;10:1598–1608. [Google Scholar]
- 43.Seo MS, Kim JY, Annaraj J, Kim Y, Lee YM, Kim SJ, Nam W. Angew Chem lnl Ed EngI. 2007;46:377–380. doi: 10.1002/anie.200603414. [DOI] [PubMed] [Google Scholar]
- 44.Bull C, Niederhoffer EC, Yoshida T, Fee JA. J Am Chem Soc. 1991;113:4069–4076. [Google Scholar]
- 45.Hearn AS, Tu CK, Nick HS, Silverman DN. J Biol Chem. 1999;274:24457–24460. doi: 10.1074/jbc.274.35.24457. [DOI] [PubMed] [Google Scholar]
- 46.Groni S, Dorlet P, Blain G, Bourcier S, Guillot R, Anxolabéhère-Mallart E. Inorg Chem. 2008;47:3166–3172. doi: 10.1021/ic702238z. [DOI] [PubMed] [Google Scholar]
- 47.Chufan EE, Puiu SC, Karlin KD. Acc Chem Res. 2007;40:563–572. doi: 10.1021/ar700031t. [DOI] [PubMed] [Google Scholar]
- 48.Vaska L. Acc Chem Res. 1976;9:175–182. [Google Scholar]
- 49.Bhula R, Gainsford GJ, Weatherburn DC. J Am Chem Soc. 1988;110:7550–7552. [Google Scholar]
- 50.Coggins MK, Toledo S, Shaffer E, Kaminsky W, Shearer J, Kovacs JA. Inorg Chem. 2012;51:6633–6644. doi: 10.1021/ic300192q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kryatov SV, Rybak-Akimova EV, Schindler S. Chem Rev. 2005;105:2175–2226. doi: 10.1021/cr030709z. [DOI] [PubMed] [Google Scholar]
- 52.Sheldrick GM. SHELXL-97: Program for the Refinement of Crystal Structures. University of Gottingen; Germany: 1997. [Google Scholar]
- 53.Mackay S, Edwards C, Henderson A, Gilmore C, Stewart N, Shankland K, Donald A. MaXus: a computer program for the solution and refinement of crystal structures from diffraction data. University of Glasgow; Scotland: 1997. [Google Scholar]
- 54.Waasmaier D, Kirfel A. Acta Crystallogr A. 1995;51:416. [Google Scholar]
- 55.Brunold TC, Tamura N, Kitajima N, Moro-oka Y, Solomon EI. J Am Chem Soc. 1998;120:5674–5690. [Google Scholar]
- 56.Kitajima N, Fujisawa K, Moro-oka Y. J Am Chem Soc. 1989;111:8975–8976. [Google Scholar]
- 57.Park GY, Qayym MF, Woertink J, Hodgson KO, Hedman B, Narducci Sarjeant AA, Solomon EI, Karlin KD. J Am Chem Soc. 2012;134:8513–8524. doi: 10.1021/ja300674m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Do LH, Hayashi T, Moenne-Loccoz P, Lippard SJ. J Am Chem Soc. 2010;132:1273–1275. doi: 10.1021/ja909718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Furutachi H, Fujinami S, Nagamoto S, Maeda Y, Watanabe Y, Kitagawa T, Suzuki M. J Am Chem Soc. 2005;127:826–827. doi: 10.1021/ja045594a. [DOI] [PubMed] [Google Scholar]
- 60.Kryatov SV, Taktak S, Korendovych IV, Rybak-Akimova EV, Kaizer J, Torelli S, Shan X, Mandal S, MacMurdo VL, Payeras AM, Que L., Jr Inorg Chem. 2005;44:85–99. doi: 10.1021/ic0485312. [DOI] [PubMed] [Google Scholar]
- 61.Shannon RD. Acta Cryst. 1976;A32:751–767. [Google Scholar]
- 62.Baldwin MJ, Ross PK, Pate JE, Tyeklar Z, Karlin K, Solomon EI. J Am Chem Soc. 1991;113:8671–8679. [Google Scholar]
- 63.Jacobson RR, Tyeklar Z, Farooq A, Karlin KD, Liu S, Zubieta J. J Am Chem Soc. 1988;110:3690–3692. [Google Scholar]
- 64.Dong Y, Menage S, Brennan BA, Elgren TE, Jang HG, Pearce LL, Que LJ. J Am Chem Soc. 1993;115:1851–1859. [Google Scholar]
- 65.Kitajima N, Tamura N, Amagai H, Fukui H, Moro-oka Y, Mizutani Y, Kitagawa H, Mather R, Heerwegh K, Reed CA, Randall CR, Que L, Jr, Tatsumi K. J Am Chem Soc. 1994;116:9071–9085. [Google Scholar]
- 66.Fits to the data were obtained using the program “julX” (http://www.mpibac.mpg.de/bac/logins/bill/julX_en.php), written by Eckard Bill, Max Planck Institute for Bioinorganic Chemistry.
- 67.Balch AL, Chan YW, Cheng RJ, La Mar GN, Latos-Grazynski L, Renner MW. J Am Chem Soc. 1984;106:7779–7785. [Google Scholar]
- 68.Fukuzumi S, Karlin KD. Coord Chem Rev. 2013;257:187–195. doi: 10.1016/j.ccr.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Eldik R, Hubbard CD. Coord Chem Rev. 2010;254:297–308. [Google Scholar]
- 70.Bakac A. Adv Inorg Chem. 2004;55:1–59. [Google Scholar]
- 71.Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. doi: 10.1021/cr020633r. [DOI] [PubMed] [Google Scholar]
- 72.Korendovych IV, Kryatov SV, Rybak-Akimova EV. Acc Chem Res. 2007;40:510–521. doi: 10.1021/ar600041x. [DOI] [PubMed] [Google Scholar]
- 73.Karlin KD, Kaderli S, Zuberbuhler AD. Acc Chem Res. 1997;30:139–147. [Google Scholar]
- 74.Tine MR. Coord Chem Rev. 2012;256:316–327. [Google Scholar]
- 75.Tshuva EY, Lippard SJ. Chem Rev. 2004;104:987–1012. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- 76.Zhang CX, Kaderli S, Costas M, Kim E, Neuhold YM, Karlin KD, Zuberbuhler AD. Inorg Chem. 2003;42:1807–1824. doi: 10.1021/ic0205684. [DOI] [PubMed] [Google Scholar]
- 77.Kieber-Emmons MT, Riordan CG. Acc Chem Res. 2007;40:609–617. doi: 10.1021/ar700043n. [DOI] [PubMed] [Google Scholar]
- 78.Hatcher LQ, Vance MA, Narducci Sarjeant AA, Solomon EI, Karlin KD. Inorg Chem. 2006:3004–3013. doi: 10.1021/ic052185m. [DOI] [PubMed] [Google Scholar]
- 79.Momenteau M, Reed CA. Chem Rev. 1994;94:659–698. [Google Scholar]
- 80.Rybak-Akimova EV. In: Physical Inorganic Chemistry. Bakac A, editor. Vol. 2. Wiley; 2012. pp. 109–188. [Google Scholar]
- 81.Bakac A. Progr Inorg Chem. 1995;43:267–351. [Google Scholar]
- 82.Jones RD, Summerville DA, Basolo F. Chem Rev. 1979;79:139–179. [Google Scholar]
- 83.Wu AJ, Penner-Hahn JE, Pecoraro VL. Chem Rev. 2004;104:903–938. doi: 10.1021/cr020627v. [DOI] [PubMed] [Google Scholar]
- 84.Würtele C, Gaoutchenova E, Harms K, Holthausen MC, Sundermeyer J, Schindler S. Angew Chem Int Ed. 2006;45:3867–3869. doi: 10.1002/anie.200600351. [DOI] [PubMed] [Google Scholar]
- 85.Feig AL, Becker M, Schindler S, van Eldik R, Lippard SJ. Inorg Chem. 1996;35:2590–2601. doi: 10.1021/ic951242g. [DOI] [PubMed] [Google Scholar]
- 86.Kryatov SV, Rybak-Akimova EV, MacMurdo VL, Que LJ. Inorg Chem. 2001;40:2220–2228. doi: 10.1021/ic001300k. [DOI] [PubMed] [Google Scholar]
- 87.Lucas HR, Meyer GJ, Karlin KDJ. Am Chem Soc. 2010;132:12927–12940. doi: 10.1021/ja104107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fry HC, Scaltrito DV, Karlin KD, Meyer GJJ. Am Chem Soc. 2003;125:4657–4663. doi: 10.1021/ja034911v. [DOI] [PubMed] [Google Scholar]
- 89.Rybak-Akimova EV, Otto W, Deardorf P, Roesner R, Busch DH. Inorg Chem. 1997;36:2746–2753. doi: 10.1021/ic961371c. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi Y, Ohkubo K, Nomura T, Kubo M, Fujieda N, Sugimoto H, Fukuzumi S, Goto K, Ogura T, Itoh S. Eur J Inorg Chem. 2012:4574–4578. [Google Scholar]
- 91.Dong Y, Yan S, Young VG, Jr, Que L., Jr Angew Chem Int Ed Engl. 1996;35:618–619. [Google Scholar]
- 92.Wurtele MC, Sander O, Lutz V, Waitz T, Tuczek F, Schindler S. J Am Chem Soc. 2009;131:7544–7545. doi: 10.1021/ja902327s. [DOI] [PubMed] [Google Scholar]
- 93.Weitzer M, Schindler S, Brehm G, Hörmann E, Jung B, Kaderli S, Zuberbühler A. Inorg Chem. 2003;42:1800–1806. doi: 10.1021/ic025941m. [DOI] [PubMed] [Google Scholar]
- 94.Brines LM, Villar-Acevedo G, Kitagawa T, Swartz RD, Lugo-Mas P, Kaminsky W, Benedict JB, Kovacs JA. Inorg Chim Acta. 2008;361:1070–1078. doi: 10.1016/j.ica.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.