Abstract
FAAH is the main degrading enzyme of the fatty acid ethanolamides anandamide (AEA) and oleoylethanolamide (OEA), which have opposite effects on food intake and energy balance. AEA, an endogenous ligand of CB1 cannabinoid receptors, enhances food intake and energy storage, whereas OEA binds to PPAR-α receptors to reduce food intake and promoting lipolysis. To elucidate the role of FAAH in food intake and energy balance, we have evaluated different metabolic and behavioral responses related to feeding in FAAH-deficient (FAAH−/−) mice and their wild-type littermates. Total daily food intake was similar in both genotypes, but high-fat food consumption was enhanced during the dark hours and decreased during the light hours in FAAH−/− mice. The reinforcing and motivational effects of food were also enhanced in FAAH−/− mice as revealed by operant behavioral paradigms. These behavioral responses were reversed by the administration of the selective CB1 cannabinoid antagonist rimonabant. Furthermore, body weight, total amount of adipose tissue, plasmatic free fatty acids and triglyceride content in plasma, liver, skeletal muscle and adipose tissue, were increased in FAAH−/− mice. Accordingly, leptin levels were increased and adiponectin levels decreased in these mutant FAAH−/− mice also showed enhanced plasmatic insulin and blood glucose levels revealing an insulin resistance. As expected, both AEA and OEA levels were increased in hypothalamus, small intestine and liver of FAAH−/− mice. These results indicate that the lack of FAAH predominantly promotes energy storage by food intake-independent mechanisms, through the enhancement of AEA levels rather than promoting the anorexic effects of OEA.
Keywords: fatty acid amide hydrolase, anandamide, oleoylethanolamide, food intake, body weight, lipid turnover, food reinforcement
Introduction
The orexigenic properties of Cannabis sativa derivatives are mediated by CB1 cannabinoid receptors, which are involved in the regulation of food intake and energy balance (1). The selective CB1 receptor antagonist rimonabant reduces body weight and improves several metabolic parameters in animals (2) and humans (3). CB1 receptors are expressed in central and peripheral tissues involved in the control of food intake and metabolism (4). Thus, activation of CB1 receptors in the paraventricular nucleus of the hypothalamus (PVH) increases appetite (5), and in the limbic system enhances the incentive value of food (6;7). Conversely, the stimulation of CB1 receptor in the small intestine inhibits the peripheral satiety signals transmitted through vagal sensory fibers to the PVH (8;9). CB1 receptor activation in peripheral tissues also promotes energy storage by food intake-independent mechanisms. Hence, the activation of CB1 receptors facilitates fatty acid storage in adipocytes (4;10), and liponeogenesis in the liver (11).
One of the endogenous ligands of CB1 receptors is anandamide (AEA). AEA stimulates appetite when administered systemically (12) and locally in the hypothalamus (5), and enhances the incentive value of food when administered into the nucleus accumbens (13). AEA is degraded intracellularly by the enzyme fatty acid amide hydrolase (FAAH) (14), which is widely distributed in organs involved in food intake and energy balance such as brain, liver and small intestine (15). The endogenous levels of AEA are regulated by food intake so that feeding decreases AEA levels in the small intestine whereas fasting increases them (16). FAAH also hydrolyzes another fatty acid ethanolamide with opposite effects to AEA, namely oleoylethanolamide (OEA). OEA is a feeding-controlled signal, which activates peroxisome proliferator-activated receptor-α (PPAR-α). OEA administration produces anorexic effects by activating satiety signals forwarded from the vagal afferent neurons to the paraventricular hypothalamic nucleus (17), and stimulates peripheral lipolysis by activating PPAR-α in adipocytes (18). Previous studies have reported the specific role of AEA and OEA in food intake and metabolism (17;19). However, the role of FAAH in the regulation of feeding and energy expenditure remains only partially understood. In the present study mutant mice deficient in FAAH have been used to investigate the role of this enzyme in the control of body weight, feeding behavior, motivation for food, and lipid turnover.
Materials and Methods
Animals
FAAH knockout mice were generated by intercrossing 129SvJ-C57BL/6 FAAH heterozygous mice, and backcrossed into the C57BL/6 for at least five generations (20). FAAH−/− mice and their wild-type littermates were backcrossed into the C57BL/6 for at least five generations to limit potential strain-dependent allelic variations that might contribute to behavioral and physiological differences. Male FAAH−/− and wild-type littermates weighing 18–25 g at the beginning of the experiment were individually housed at a controlled temperature (22 ± 2°C) and humidity (55% to 65%) room with a 12 h light/dark cycle (lights on at 7:00 AM and off at 7:00 PM). They were kept on a standard chow diet (Pro-lab RMH 2500; PMI Nutrition International, Brentwood, MO) or a high-fat diet (60 kcal % fat; D12492; Research Diets, New Brunswick, NJ) for 12 weeks. Body weight and food intake were measured weekly. Water and food were available ad libitum except for the operant self-administration studies. In operant studies, food access was restricted keeping body weight to 85±5% from original. All experimental procedures were approved by the local ethical committees (Institutional Animal Care and Use Committee of the University of California, Irvine and CEEA-IMAS-UPF) and carried out in strict accordance with the National Institutes of Health and the European Communities Directive 86/609/EEC guidelines for care and use of experimental animals.
Drugs
Rimonabant was a kind gift of Sanofi-Aventis (Montpellier, France). It was administered at the dose of 5 mg/kg and prepared in a solution of 5% polyethylene glycol (PEG-400) (Sigma-Aldrich, Spain), 5% Tween-80 (Sigma-Aldrich, Spain) and 90% saline (0.9%). The same solution was used as vehicle. It was injected by the intraperitoneal route (i.p.) in a volume of injection of 0.1 ml/10 g body weight.
Analysis of Feeding Behavior
Apparatus
Food intake parameters and locomotor activity were recorded with an automated system (Scipro Inc, New York, NY, USA), as previously described (21). The system consisted of 24 cages equipped with baskets connected to weight sensors. The baskets contained standard or high-fat pellets and were accessible to the mice through a hole in the wire lid of the cage. Each time food was removed from the basket, the computer recorded the duration of the event, the amount of food retrieved, and the time at which the event occurred.
Feeding behavior
Mice were habituated to the test cages for 3 days and average feeding behavior was calculated. Food intake was recorded for 20 h and the following parameters were measured: locomotor activity, hourly food intake, first meal latency, meal size, first and average post-meal interval measured as the time interval separating two consecutive meals, first and average satiety ratio measured as the ratio between post-meal interval and meal size, and number of meals.
Tissue dissection
Mice exposed to a high-fat diet for 12 weeks were slightly anesthetized with halothane and decapitated. Plasma, hypothalamus, liver, duodenum, jejunum and soleus muscle were removed within approximately 30 sec from decapitation, frozen in dry ice, and stored at −80°C until analyses. Retroperitoneal fat tissue was removed and weighed to determine differences in adipose tissue accumulation.
Lipid quantification
Total triacylglycerol (TAG) levels were measured in retroperitoneal adipose tissue, liver and muscle homogenates and plasma. Tissue TAG were extracted from adipose tissue, liver and muscle with chloroform:methanol:NaCl, and both tissue and plasma TAG were and measured using the Infinity TAG kit (Thermo Electron Corporation, Melbourne, Australia). Plasma free fatty acids (FFA) were measured using the NEFA C kit (Wako Chemicals, Neuss, Germany). Finally, AEA, OEA and 2-AG were extracted from frozen liver, hypothalamus, duodenum and jejunum with methanol:chloroform and analyzed by HPLC/MS, as previously reported (22).
Blood glucose and plasmatic, insulin, adiponectin and leptin determination
Blood glucose was measured by Glucometer Elite® (Bayer Diagnostics, Tarrytown, NY). Plasma insulin, adiponectin and leptin were evaluated by ELISA (Millipore, Billerica, MA).
Operant Food Self-Administration
Apparatus
The food self-administration experiments were conducted in mouse operant chambers (Model ENV-307A-CT, Medical Associates, Georgia, VT, USA) equipped with an active and inactive lever. Responding on the active lever resulted in a food pellet delivery, while responding on the inactive lever had no consequences. A stimulus light, located above the active lever, was paired contingently with the delivery of the food.
Food-maintained operant behavior
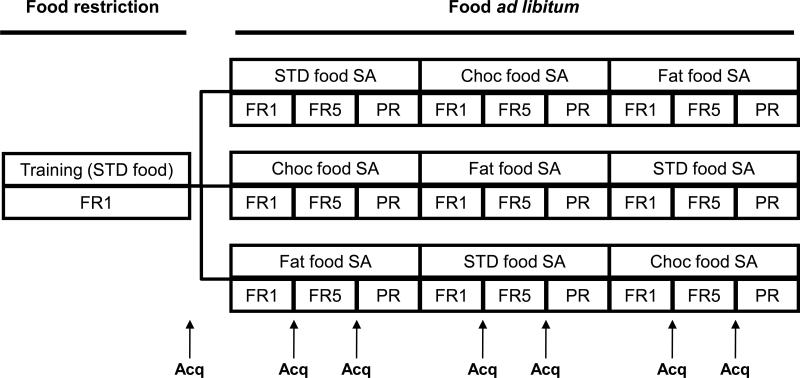
FAAH−/− and wild-type littermates were food restricted (keeping weigh to 85±5% from original) for the whole acquisition period of food-maintained operant behavior. Mice were daily trained in the operant chambers to respond for food pellets (Noyes Precision Pellets, Research Diets Inc, USA). First, mice were trained under a fixed ratio 1 (FR1) schedule of reinforcement for 1h. Mice were considered to acquire a self-administration behavior when fulfilled the following criteria: 1) a minimum of 10 reinforcers per session, 2) at least 75% responding on the active lever, and 3) a stable responding with less than 20% deviation from the mean of the total number of reinforcers earned in three consecutive sessions. Once acquisition criteria were achieved, food restriction was ended and animals were distributed in 3 different counterbalanced groups that were trained to obtain chocolate, fat or standard pellets. An FR1-FR5-progressive ratio (PR) series was followed changing mice from one schedule to the following when the above acquisition criteria were achieved. In PR schedule, the response requirement to earn a pellet escalated according to the following series: 1-2-3-5-12-18-27-40-60-90-135-200-300-450-675-1000. The PR session lasted for 4 h or until mice did not complete the ratio for delivery of one reinforcer within 1 h, and was performed only once. The breaking point to extinguish self-administration behavior was determined in each animal (Figure 1).
Figure 1.
Experimental design for food-maintained operant behavior. STD: Standard, Choc: Chocolate, FR1: Fixed ratio 1, FR5: Fixed ratio 5, PR: Progressive ratio, Acq: Acquisition.
Effect of rimonabant on chocolate-maintained operant behavior
In a second experiment, the effects of the CB1 receptor antagonist rimonabant on the reinforcing properties and motivational strength of chocolate pellets were evaluated in FAAH−/− and wild-type littermates. After the acquisition of food-maintained self-administration under an FR5 schedule, animals were pretreated with vehicle 30 min before a new FR5 or a PR self-administration session. On the next day, animals received an acute injection of rimonabant (5 mg/kg, i.p.) 30 min before starting the FR5 or PR session.
Statistical analyses
Body weight, weekly food intake, hourly food intake, meal parameters, incentive values of the different types of food, and rimonabant effects on the reinforcing and motivational effects of chocolate pellets between FAAH−/− and wild-type littermates were compared by a within-subjects two-way ANOVA, followed by one-way ANOVA or post hoc comparisons (Dunnett's test) for individual differences when required. Unpaired two-tailed Student t-test was used to evaluate differences between genotypes in the initial body weight, locomotor activity, fat mass, adipose tissue, liver, muscle and plasma TAG levels, plasma FFA levels, hypothalamus, duodenum, jejunum and liver AEA, OEA and 2-AG levels, glucemia and insulin, adiponectin and leptin levels. In all the experiments, differences were considered significant if the probability of error was less than 5%.
Results
FAAH−/− mice show similar food intake but enhanced body weight compared to wild-type littermates
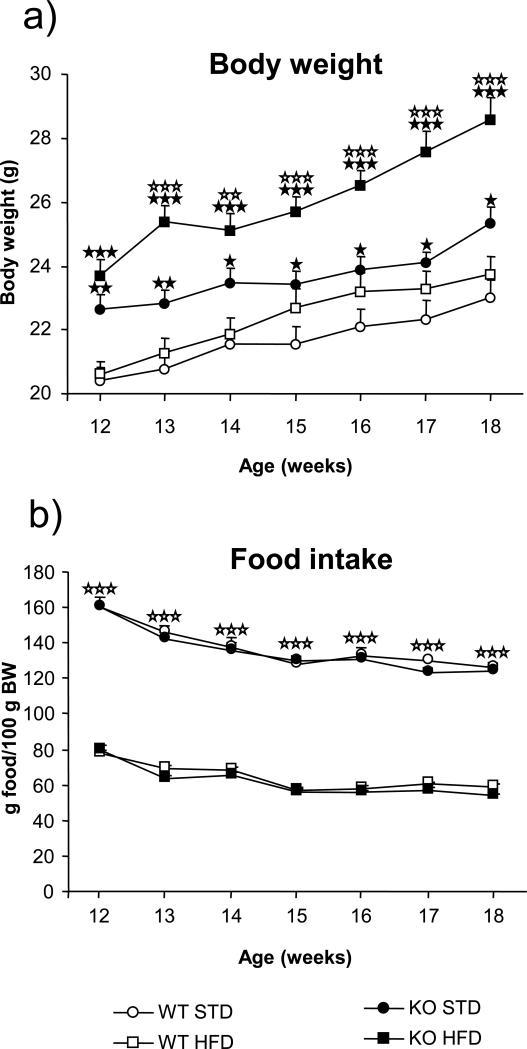
FAAH−/− mice and their wild-type littermates were fed ad libitum on a standard or a high-fat diet for 7 weeks, and body weights were recorded weekly. FAAH−/− mice showed higher body weight than wild-type littermates from the beginning of the experiment (p < 0.001). Differences in body weight between FAAH−/− and wild-type animals were maintained when exposed to standard and high-fat diet, as revealed by two-way ANOVA (Figure 2a; Table 1). In animals under standard diet this difference in body weigh was similar from the beginning to the end of the experiment. In contrast, body weight differences between FAAH−/− and wild-type mice under high-fat diet were increasing with time. Furthermore, the body weight increase resulting from the exposure to high-fat diet is significantly enhanced in FAAH−/− mice than in wild-type animals, when compared to the same genotype under a standard diet. This result suggests a higher sensitivity of FAAH−/− mice to gain weight under a high-fat diet when compared to their wild-type littermates.
Figure 2.
Body weight (a) and food intake (b) in FAAH−/− mice (black) (n = 10 to 12) and wild-type littermates (white) (n = 9 to 12) under standard (circles) and high-fat (squares) diet. Body weight and food intake were determined once a week during 7 consecutive weeks. Body weight data are expressed as mean ± SEM of grams of body weight. ☆☆ p < 0.01, ☆☆☆ p < 0.001, when compared with the standard diet of the same genotype. ★ p < 0.05, ★★ p < 0.01, ★★★ p < 0.001, comparisons between genotypes (one-way ANOVA). Food intake data are expressed as mean ± SEM of grams of food consumed per 100 g of body weight. ☆☆☆ p < 0.001, when compared with standard diet (two-way ANOVA).
Table 1.
Two-way ANOVA calculated for body weight and weekly and hourly food intake, feeding parameters, locomotor activity, standard, chocolate and fat food self-administration, and effects of rimonabant on chocolate pellets self-administration in FAAH knockout and wild-type mice.
| Two-way ANOVA | ||||
|---|---|---|---|---|
| Age |
Genotype |
Interaction |
||
| Body weight | Regular diet (WT vs KO) | F(6, 90) = 94.974*** | F(1, 15) = 9.734** | F(6, 90) = 1.667 |
| High-fat diet (WT vs KO) | F(6, 126) = 82.260*** | F(1, 21) = 27.534*** | F(6, 126) = 5.294*** | |
| Age |
Diet |
Interaction |
||
|---|---|---|---|---|
| WT (regular vs high-fat diet) | F(6, 108) = 71.441*** | F(1, 18) = 0.892 | F(, 108) = 2.398* | |
| KO (regular vs high-fat diet) | F(6, 108) = 68.844*** | F(1, 18) = 1.689** | F(6, 108) = 7.697*** |
| Age |
Genotype |
Interaction |
||
|---|---|---|---|---|
| Food intake | Regular diet (WT vs KO) | F(6, 102) = 37.680*** | F(1, 17) = 0.239 | F(6, 102) = 2.035 |
| High-fat diet (WT vs KO) | F(6, 132) = 111.845*** | F(1, 22) = 1.544 | F(6, 132) = 2.772* |
| Age |
Diet |
Interaction |
||
|---|---|---|---|---|
| WT (regular vs high-fat diet) | F(6, 114) = 49.988*** | F(1, 19) = 321.495*** | F(6, 114) = 14.080*** | |
| KO (regular vs high-fat diet) | F(6, 120) = 55.230*** | F(1, 20) = 321.495*** | F(6, 120) = 3.699*** |
| Time |
Genotype |
Interaction |
||
|---|---|---|---|---|
| Hourly food intake | Regular diet (WT vs KO) | F(19, 323) = 280.471*** | F(1, 17) = 0.093 | F(19, 323) = 0.484 |
| High-fat diet (WT vs KO) | F(19, 418) = 293.304*** | F(1, 22) = 3.599 | F(19, 418) = 3.502*** |
| Time |
Diet |
Interaction |
||
|---|---|---|---|---|
| WT (regular vs high-fat diet) | F(19, 361) = 302.551*** | F(1, 19) = 160.292*** | F(19, 361) = 32.629*** | |
| KO (regular vs high-fat diet) | F(19, 380) = 281.401*** | F(1, 20) = 78.186*** | F(19, 380) = 31.894*** |
| Genotype |
Diet |
Interaction |
||
|---|---|---|---|---|
| Feeding parameters | Latency | F(1, 43) = 5.190* | F(1, 43) = 27.645*** | F(1, 43) = 1.059 |
| First meal size | F(1, 43) = 0.388 | F(1, 43) = 0.113 | F(1, 43) = 1.880 | |
| First post-meal interval | F(1, 43) = 0.848 | F(1, 43) = 16.183*** | F(1, 43) = 0.619 | |
| First satiety ratio | F(1, 43) = 0.839 | F(1, 43) = 2.933 | F(1, 43) = 3.491 | |
| Average meal size | F(1, 43) = 0.969 | F(1, 43) = 26.059*** | F(1, 43) = 0.028 | |
| Average post-meal interval | F(1, 43) = 0.204 | F(1, 43) = 18.587*** | F(1, 43) = 0.133 | |
| Average satiety ratio | F(1, 43) = 0.007 | F(1, 43) = 18.387*** | F(1, 43) = 0.230 | |
| Number of meals | F(1, 43) = 1.472 | F(1, 43) = 72.029*** | F(1, 43) = 0.228 |
| Time |
Genotype |
Interaction |
||
|---|---|---|---|---|
| Locomotor activity | F(19, 418) = 37.544*** | F(1, 22) = 0.02 | F(19, 418) = 0.034 |
| Food type |
Genotype |
Interaction |
||
|---|---|---|---|---|
| Self-administration | FR1 | F(2, 81) = 4.762* | F(1, 81) = 26.551*** | F(2, 81) = 0.621 |
| FR5 | F(2, 81) = 20.446*** | F(1, 81) = 13.236*** | F(2, 81) = 0.898 | |
| PR | F(2, 81) = 15,652*** | F(1, 81) = 33.827*** | F(2, 81) = 2.875 |
| Treatment |
Genotype |
Interaction |
||
|---|---|---|---|---|
| Rimonabant treatment | FR5 | F(1, 25) = 178.864*** | F(1, 25) = 9.358** | F(1, 25) = 6.175* |
| PR | F(1, 23) = 63.844*** | F(1, 23) = 4.523* | F(1, 23) = 4.306* |
Two-way ANOVA with age (body weight and weekly food intake), time (hourly food intake and locomotor activity), food type (self-administration), and treatment (rimonabant treatment) as within-subject, and genotype or diet as between-subject factors.
p<0,05
p<0,01
p<0,001.
See Materials and methods for details. WT: wild-type; KO: knockout.
Food intake of FAAH−/− and wild-type animals under standard and high-fat diet was also monitored. Two-way ANOVA indicated significant effect of diet in both genotypes and significant interaction between age and genotype in animals under high-fat diet. However, subsequent one-way ANOVA revealed significant differences only between diets but not between genotypes (Figure 2b; Table 1). To determine whether differences in physical activity were contributing to the enhanced body weight of FAAH−/− mice, locomotor activity was measured in both genotypes. FAAH−/− and wild-type mice showed circadian variations in locomotor activity, both increasing the activity during the dark period. However, no differences between genotypes were revealed in any of the locomotor measurements (Table 1).
FAAH−/− mice show increased food intake during the dark period and decreased food intake during the light period
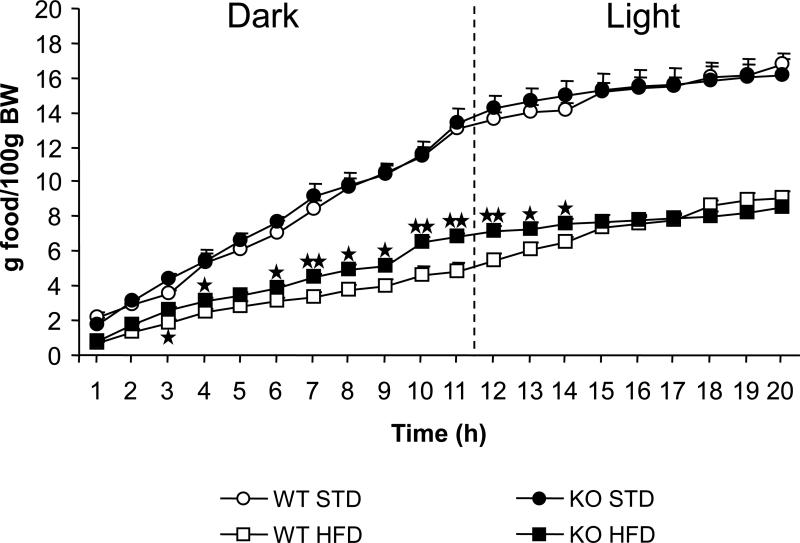
Hourly food intake and meal parameters were evaluated in FAAH−/− and wild-type littermates. As expected, animals under a standard diet consumed a higher amount of food during the dark period than during the light period, but no differences between genotypes were observed. However, significant differences between genotypes were observed when animals were fed with high-fat diet. Wild-type mice consumed a similar amount of high-fat food during the dark and light hours. On the contary, FAAH−/− mice consumed higher amounts of food during the dark than during the light hours, as occurred with standard diet (Figure 3; Table 1).
Figure 3.
Hourly food intake in FAAH−/− mice (black) (n = 10 to 12) and wild-type littermates (white) (n = 9 to 12) under standard (circles) and high-fat (squares) diet. Food intake was measured once per hour during 20 consecutive hours. Data are expressed as mean ± SEM of grams of food consumed per 100 g of body weight. p < 0.01 in all comparisons with standard diet in both FAAH−/− mice and wild-type littermates. ★ p < 0.05, ★★ p < 0.01, comparisons between genotypes (one-way ANOVA).
Analysis of first meal parameters by two-way ANOVA (Table 1) revealed a significant increase in the latency, average meal size, and first and average post-meal interval, and a decrease in average satiety ratio and number of meals in animals under a high-fat diet when compared to animals under a standard diet. However, only a significant decrease in the latency was observed in FAAH−/− animals when compared to wild-type littermates. No differences between genotypes were revealed in any of the other parameters evaluated (data not shown).
Enhanced operant performance and motivation for food in FAAH−/− mice
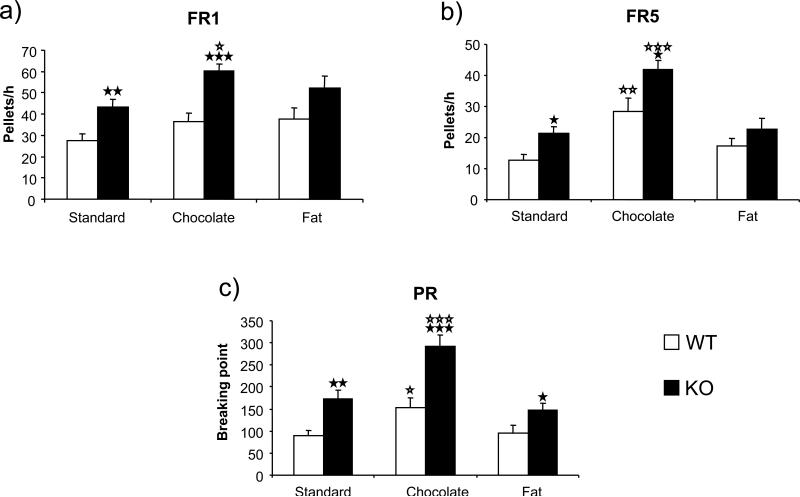
FAAH−/− mice and wild-type littermates were kept under food restriction and trained to self-administer food under an FR1 schedule of reinforcement for 20 days. The reinforcing effects and the motivation to obtain different types of food (standard pellets, fat pellets or chocolate pellets) were evaluated under FR1, FR5 and PR schedules of reinforcement in mice that had previously satisfied self-administration criteria (Figure 4; Table 1). The time of re-acquisition was similar for the different types of food in both genotypes. In wild-type animals, the number of responses during the achievement of the FR1 criteria was similar for standard, chocolate and fat pellets (F(2, 36) = 1.763, n.s.). When wild-type animals were trained under an FR5 schedule, the number of active responses during the achievement of the acquisition criteria was different depending on the type of food (F(2, 36) = 6.846, p < 0.01). Thus, post hoc analysis showed that the number of responses for chocolate was significantly higher than for standard pellets (p < 0.01). In wild-type animals, significant differences were also revealed in the breaking point for the different types of food obtained in the PR schedule (F(2, 36) = 3.738, p < 0.05). Subsequent post hoc analysis showed that the breaking point for chocolate was significantly higher than for standard pellets (p < 0.05).
Figure 4.
Operant self-administration of standard, chocolate and high-fat pellets in FAAH−/− mice (black bars) (n = 14) and wild-type littermates (white bars) (n = 15) under a fixed ratio 1 (FR1) (a), fixed ratio 5 (FR5) (b) and progressive ratio (PR) (c) schedules of reinforcement. FR1 and FR5 data are expressed as mean ± SEM of the average number of pellets obtained during the 3 days of the acquisition criteria. PR data are expressed as mean ± SEM of the breaking point achieved. ☆ p < 0.05, ☆☆ p < 0.01, ☆☆☆ p < 0.001, when compared with the standard pellets of the same genotype (Dunnett's test).★ p < 0.05, ★★ p < 0.01, ★★★ p < 0.001, comparisons between genotypes (one-way ANOVA).
In FAAH−/− animals, the number of responses during the achievement of the FR1 criteria was different for standard, chocolate and fat pellets (F(2, 45) = 3.745, p < 0.05). Subsequent post hoc analysis indicated that the number of active responses for chocolate was significantly enhanced compared to standard pellets (p < 0.05). When FAAH−/− mice were trained under an FR5 schedule, the number of active responses during the achievement of the acquisition criteria was also different depending on the type of food (F(2, 45) = 15.182, p < 0.001). Thus, subsequent post hoc analysis also showed that the number of responses to obtain chocolate was significantly higher than for standard pellets (p < 0.001). In FAAH−/− animals significant differences were also revealed in the breaking point for the different types of food during the PR schedule (F(2, 45) = 14.083, p < 0.001). Subsequent post hoc analysis showed that the breaking point for chocolate was significantly higher than for standard pellets (p < 0.001).
Comparisons between genotypes revealed that the number of active responses to obtain chocolate (F(1, 27) = 20.564, p < 0.001) and standard (F(1, 27) = 11.592, p < 0.01) pellets was significantly higher in FAAH−/− mice in comparison to wild-type littermates under an FR1 schedule (Figure 4a). No differences between genotypes were revealed under an FR1 schedule in the number of active responses to obtain fat pellets. Similar differences between genotypes were revealed under an FR5 schedule, where FAAH−/− mice also showed a higher responding for standard (F(1, 27) = 7.469, p < 0.05) and chocolate (F(1, 27) = 6.794, p < 0.05) pellets, whereas no differences were shown when responding for fat pellets (Figure 4b). Under a PR schedule, significantly higher breaking points were observed in FAAH−/− than in wild-type mice, when trained to obtain chocolate (F(1, 27) = 18.598, p < 0.001), high-fat (F(1, 27) = 4.745, p < 0.05) or standard (F(1, 27) = 11.058, p < 0.01) pellets (Figure 4c).
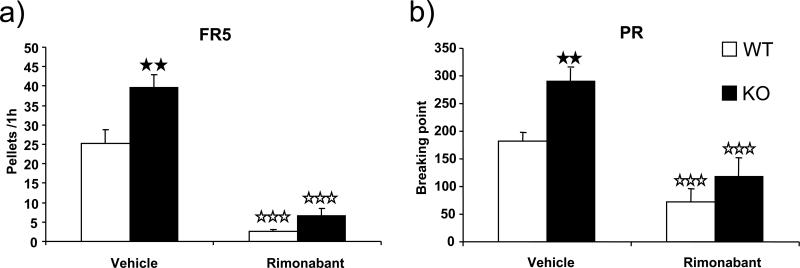
Rimonabant decreases operant performance and motivation for food in FAAH−/− and wild-type mice
The reinforcing properties of chocolate pellets were evaluated in FAAH−/− mice and wild-type littermates under FR5 and PR schedules after vehicle and rimonabant acute administration (Figure 5). The administration of CB1 antagonist rimonabant reduced the performance of FAAH−/− in the food-rewarding operant tasks to levels comparable to those of wild-types, as indicated by the significant interaction of treatment and genotype (Table 1). As in the previous experiment, FAAH−/− mice showed a higher number of responses under an FR5 schedule and a higher breaking point under a PR schedule than wild-type mice after vehicle administration. Rimonabant administration significantly decreased the number of active responses in both wild-type (F(1, 11) = 58.010, p < 0.001) and FAAH−/− mice (F(1, 14) = 157.073, p < 0.001) under an FR5 schedule (Figure 5a). Rimonabant also reduced the breaking point obtained under PR schedule in FAAH−/− mice (F(1, 13) = 44.822, p < 0.001) and wild-type littermates (F(1, 10) = 24.777, p < 0.001) (Figure 5b). No significant differences between genotypes were observed after the administration of rimonabant.
Figure 5.
Effects of acute rimonabant (5 mg/kg, i.p.) on operant self-administration of chocolate pellets in FAAH−/− mice (black bars) (n = 15) and wild-type littermates (white bars) (n = 12) under FR5 (a) and PR (b) schedules of reinforcement. Data are expressed as mean ± SEM of reinforcers obtained during 1 h in animals under FR5, and the breaking point achieved under PR. ☆☆☆ p < 0.001, when compared with the vehicle group of the same genotype, ★★ p < 0.01, comparisons between genotypes (one-way ANOVA).
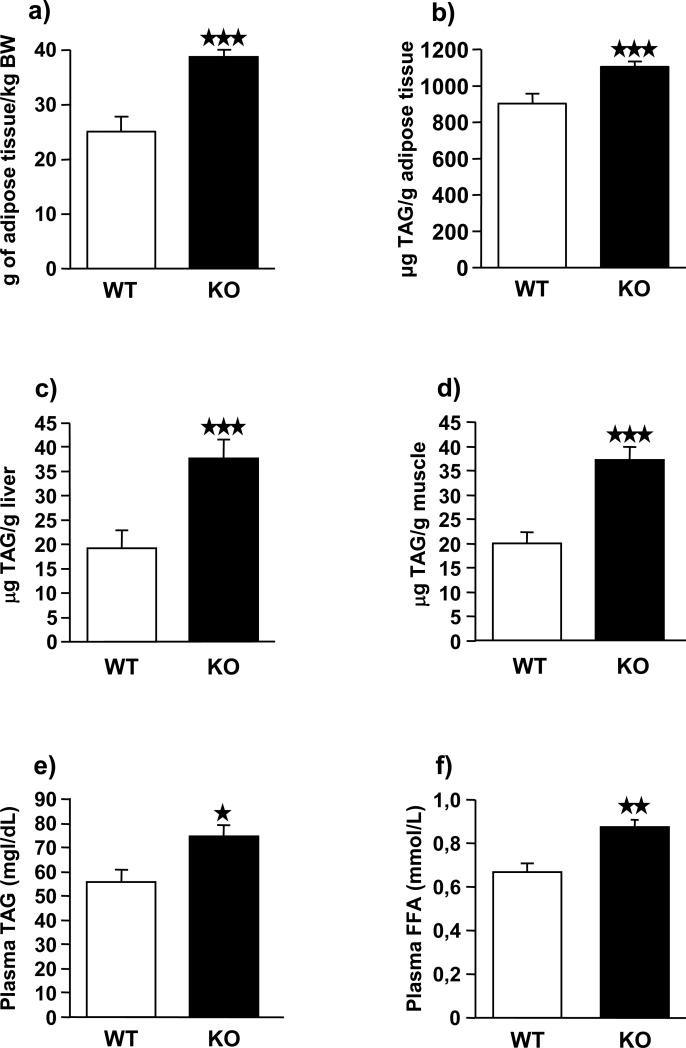
FAAH−/− mice have increased fat mass and lipid content
The total amount of retroperitoneal fat mass, TAG content in adipose tissue, liver, skeletal muscle, and plasma, as well as plasma FFAs were measured in FAAH−/− and wild-type mice exposed to a high-fat diet (Figure 6). FAAH−/− mice exhibited a higher amount of total visceral fat mass when compared to wild-type mice (p < 0.001) (Figure 6a). FAAH−/− animals also showed significantly higher levels of TAG in fat tissue (p < 0.001) (Figure 6b), liver (p < 0.001) (Figure 6c), skeletal muscle (p < 0.001) (Figure 6d) and plasma (p < 0.05) (Figure 6e) than wild-type littermates. Similarly, FFAs levels in FAAH−/− animals were significantly increased when compared to FAAH+/+ animals (p < 0.05) (Figure 6f).
Figure 6.
Total amount of fat content (a), analysis of triglycerides (TAG) levels in adipose tissue (b) and liver (c) soleus muscle (d) and plasma (e), and plasmatic free fatty acid (FFA) levels (f) in FAAH−/− mice (black bars) (n = 12) and wild-type littermates (white bars) (n = 12) under high-fat diet. Amount of adipose tissue data are expressed as mean ± SEM of grams of fat per kg of body weight, TAG tissue levels data are expressed as mean ± SEM of μg of TAG per grams of tissue, plasmatic TAG are expressed as mean ± SEM of mg TAG per dL of plasma, and FFA levels are expressed as mean ± SEM of mmol of FFA per L of plasma. ★ p < 0.05, ★★ p < 0.01, ★★★ p < 0.001, comparisons between genotypes (Student t-test).
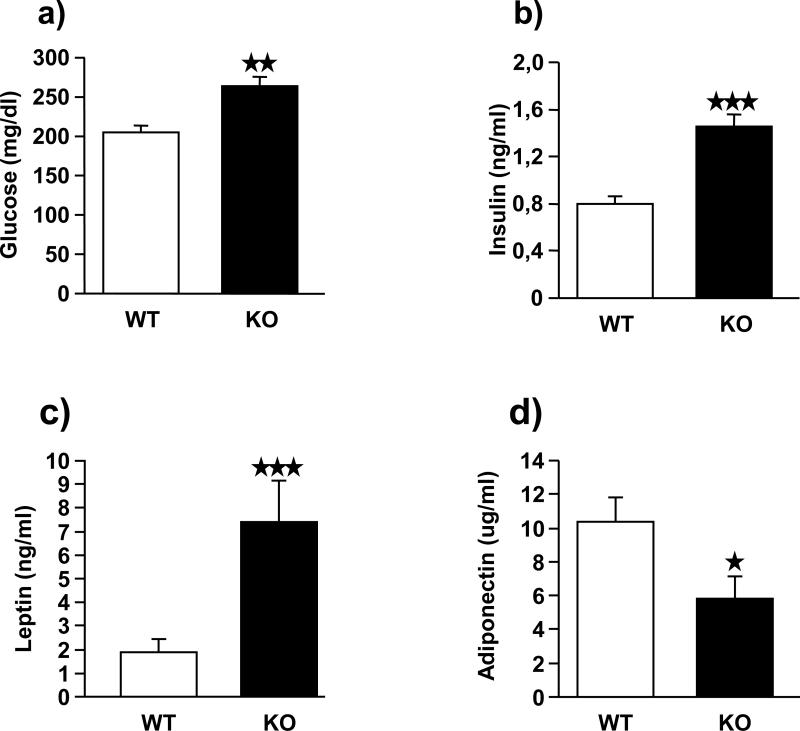
FAAH−/− mice have increased glucose, insulin and leptin levels but decreased adiponectin levels
Blood glucose and plasmatic, insulin, leptin and adiponectin levels were determined in FAAH−/− and wild-type mice under a high-fat diet (Figure 7). FAAH−/− animals showed significantly higher levels of glucose (p < 0.01) (Figure 7a), insulin (p < 0.001) (Figure 7b) and leptin (p < 0.001) (Figure 7c). In contrast, adiponectin levels (Figure 7d) were significantly reduced in FAAH−/− mice (p < 0.05).
Figure 7.
Plasmatic glucose, insulin, leptin and adiponectin levels in FAAH−/− mice (black bars) (n = 12) and wild-type littermates (white bars) (n = 12). Glucose levels (a) are expressed as mean ± SEM of mg of glucose per dL of plasma. Insulin (b) and leptin (c) levels are expressed as mean ± SEM of ng of hormone per mL of plasma. Adiponectin levels (d) are expressed as mean ± SEM of μg of hormone per mL of plasma. ★★ p < 0.01, ★★★ p < 0.001, comparisons between genotypes (Student t-test).
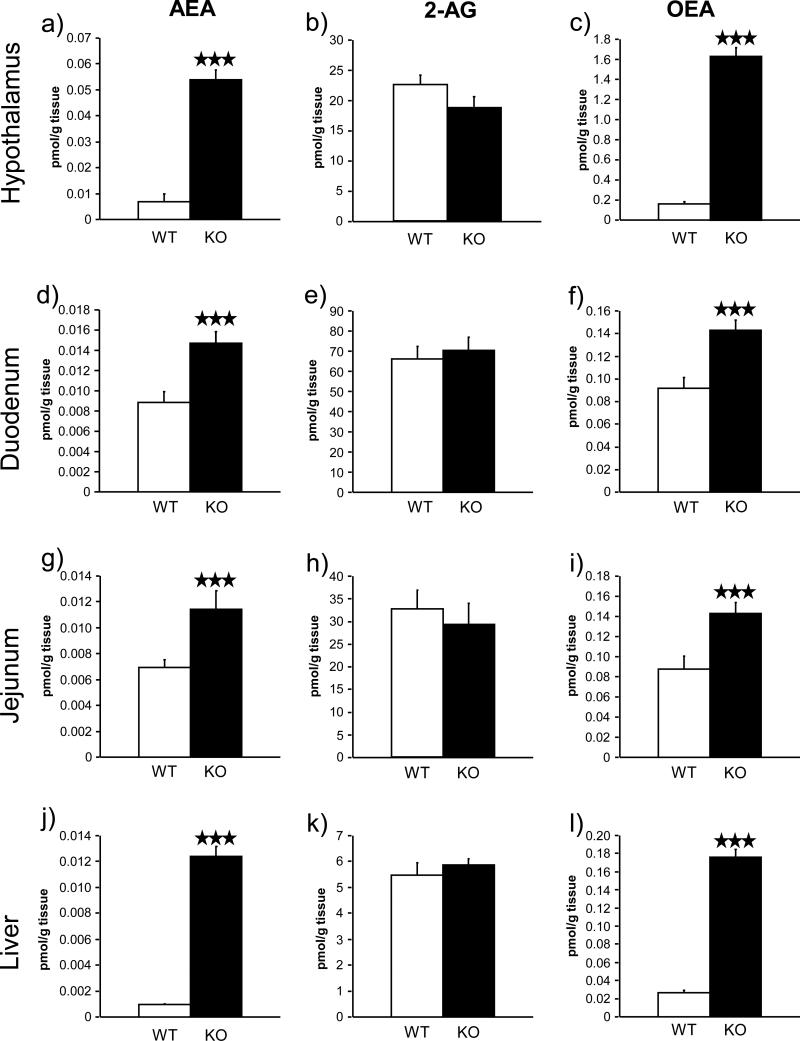
FAAH−/− mice have enhanced levels of AEA and OEA, but not 2-AG
The levels of the two main endocannabinoids AEA and 2-AG, and OEA were analyzed in different tissues involved in feeding behavior and metabolism (hypothalamus, duodenum, jejunum and liver) from FAAH knockout mice and wild-type littermates exposed to a high-fat diet. AEA and OEA, which are degraded by FAAH, showed significant enhanced levels in the hypothalamus (p < 0.001) (Figure 8 a & c), duodenum (p < 0.001) (Figure 8 d & f), jejunum (p < 0.001) (Figure 8 g & i) and liver (p < 0.001) (Figure 8 j & l) in FAAH−/− mice when compared to wild-type littermates. However, 2-AG, which is not metabolized by FAAH, showed similar levels in all the central and peripheral tissues evaluated in both FAAH−/− and wild-type mice (Figure 8 b, e, h & k).
Figure 8.
Anandamide (AEA), 2-arachidonoylglycerol (2-AG) and oleoylethanolamide (OEA) levels in hypothalamus, duodenum, jejunum and liver in FAAH−/− mice (black bars) (n = 12) and wild-type littermates (white bars) (n = 12) under a high-fat diet. Data are expressed as mean ± SEM of picomols of AEA, 2-AG or OEA per gram of tissue. ★★★ p < 0.001, comparisons between genotypes (Student t-test).
Discussion
In the present study knockout mice were used to determine the involvement of FAAH in the regulation of feeding behavior and energy balance. Pharmacological inhibition or genetic deletion of FAAH was reported to enhance the levels of two fatty acid ethanolamides, AEA and OEA (20;23), which play an opposite role in the control of food intake and metabolism. AEA promotes food intake (5;12) and energy storage (24), whereas OEA exerts anorexic and lipolytic effects (25;26). The levels of both fatty acid ethanolamides were increased in the hypothalamus and small intestine of FAAH−/− animals. However, these mutants and their wild-type littermates showed similar food intake. AEA enhances feeding through the activation of CB1 receptors located both in central nervous system and peripheral tissues (4;27). On the other hand, OEA released in the small intestine activates PPAR-α (Fu, et al 2003), and transmits through the vagus nerve a satiety signal to the nucleus of the solitary tract and then to the hypothalamus (17). Our results suggest that the effects of high levels of AEA and OEA on food intake compensate each other in FAAH−/− mice, resulting in similar food consumption when compared to wild-type animals. Despite the similar food intake observed in both genotypes, a decrease in the latency was revealed in FAAH−/− mice under a high-fat diet, but no differences were revealed in the other feeding parameters evaluated. In addition, hourly standard food intake was similar between genotypes, both showing a higher food consumption in the dark hours than in the light hours. On the contrary, significant differences in the pattern of hourly high-fat food consumption were observed between genotypes. Wild-type animals exposed to a high-fat diet exhibited similar food intake in the dark and in the light hours, leading to a consumption of extra calories during the light period, the usual restcaloric intake period, in agreement with previous studies (28). Interestingly, FAAH−/− animals under high-fat diet kept food intake differences between dark and light hours, as animals under standard diet. Several studies have reported enhanced levels of AEA and decreased FAAH activity during the dark period (29). Indeed, the endocannabinoid system modulates several orexigenic and anorexic hypothalamic peptides that are under a circadian control (4;30-32). Interestingly, the circadian control of these neuropeptides is altered by the exposure to a high-fat diet (28). Together, these results suggest that AEA plays an important role in the modulation of food intake in the light and in the dark hours, probably through the control of feeding-regulating neuropeptides.
FAAH−/− mice showed an enhanced body weight in comparison to wild-type littermates from early age. Furthermore, FAAH−/− animals were more sensitive than wild-types to gain weight when fed with high-fat diet. The effects of high-fat diet exposure were mild in wild-type animals since a period of at least 12 weeks of exposure to HFD is required to induce important changes of body weight. However, exposure to a high-fat diet caused a marked body weight enhancement in FAAH−/− mice. Both genotypes showed equivalent caloric intake, body temperature (20) and spontaneous locomotor activity. Hence, FAAH seems to enhance energy expenditure by food intake-independent mechanisms. FAAH−/− mice showed an opposite phenotype to that of CB1 knockouts, which are leaner than wild-types under both standard and high-fat diet by food intake-independent mechanism (4;33). By contrast, PPAR-α−/− mice were heavier than wild-types only when exposed to high-fat diet (25). Then, OEA administration reduced body weight by a mechanism directly involving a reduction of caloric intake (26). Therefore, FAAH may play an important role in the control of metabolism by mechanisms independent from food intake, where AEA would exert a predominant effect, leading to an enhanced body weight in FAAH−/− mice. In agreement with this idea, the amount of adipose tissue and the TAG levels in adipose tissue, liver, skeletal muscle and plasma were higher in FAAH−/− mice than in wild-types. As expected, the enhancement of the adipose content was associated to an important increase of plasmatic leptin in FAAH−/− mice. The enhanced weight and the biochemical changes found in the plasma, adipose tissue, liver and skeletal muscle of FAAH−/− animals suggest an increased lipogenesis in the peripheral organs. Interestingly, adiponectin levels were decreased in FAAH−/− mice, which could participate in the alteration of the lipid metabolism and could also promote insulin resistance in these mutants. In agreement with the high TAG content in the liver and the low adiponectin levels, the plasmatic insulin levels and blood glucose concentration were enhanced in FAAH−/− mice revealing an insulin resistance in these mutant mice. In spite of the increased lipogenesis, the plasmatic levels of FFA were also enhanced in FAAH−/− mice suggesting an enhancement of lipid oxidation as an alternative energy resource due to the presence of insulin resistance in these mice. Both endocannabinoids and OEA modulate lipid turnover (4;18), and CB1 receptors (11) and PPAR-α (34) participate in fatty acid metabolism in the liver, the main organ involved in the neosynthesis of lipids. AEA levels are increased in adipose tissue (27) and liver (11) of animals under a high-fat diet, whereas OEA levels in visceral adipose tissue were similar in obese and lean mice (35). Interestingly, animals lacking FAAH showed enhanced levels of both AEA and OEA in the liver. Therefore, AEA seems to play a predominant role over OEA in the control of liver lipogenesis, as suggested by these studies in FAAH−/− mice.
The reinforcing and motivational properties of food were studied in FAAH−/− mice by using an operant paradigm. The use of an operant paradigm allowed us to evaluate the incentive value of a standard food, a highly palatable food (chocolate pellets), and a high-caloric food (fat pellets) in FAAH−/− and wild-type animals. The better performance of animals to obtain chocolate pellets rather than other types of food indicates a predominant effect of palatability over the caloric value in maintaining operant behavior in both FAAH−/− and wild-type mice. Genetic deletion of FAAH enhanced the performance of an operant behavior to obtain standard and chocolate pellets under different effort requirements (FR1 and FR5), which suggests an enhancement of the reinforcing properties of these types of food. However, the performance on the operant behavior to obtain high-caloric fat pellets was not modified in FAAH−/−. On the other hand, the motivational strength for standard, chocolate and high-fat food evaluated as the breaking point obtained in PR sessions was enhanced in FAAH−/− mice. In spite of the increased motivation for food, the total food intake was not modified in FAAH−/− mice, suggesting a predominant effect of energy signals from both central and peripheral tissues over the motivational signals leading to food intake in these animals.
The specific involvement of CB1 receptors in the enhanced motivation for food in FAAH−/− mice was also investigated. The selective CB1 antagonist rimonabant was injected into animals trained to self-administer chocolate pellets, the type of food showing the highest reinforcing effect in the previous experiment. Rimonabant strongly reduced the number of responses on FR5 or PR schedules in both FAAH−/− and wild-type mice. CB1 receptors play a crucial role in the reinforcing effects of palatable food and in the behavioral phenotype of FAAH−/−. This involvement was indicated by a significant interaction between rimonabant treatment and genotype in the chocolate food self-administration and by the similar performance of both genotypes after rimonabant administration observed in the same paradigm. In agreement, CB1 receptor deletion and rimonabant administration were reported to markedly reduce the reinforcing effects of sweet, but not fat food (7;36). Reinforcing effects of palatable food are mediated by the nucleus accumbens, which contains high levels of CB1 receptors (37). Local administration of AEA in the nucleus accumbens enhances food rewarding effects (13). Thus, the increased levels of AEA could be responsible for the enhanced motivation for palatable food of FAAH−/− animals through the activation of CB1 receptors in the nucleus accumbens.
In conclusion, the present results reinforce the hypothesis that FAAH plays an important role in the control of energy balance. Targeted deletion of this enzyme increases the endogenous levels of AEA and OEA in central and peripheral organs involved in food intake and energy metabolism. The effects of AEA and OEA on food intake appear to compensate each other, leading to a normal food intake in FAAH−/− mice. However, these mutants resisted the alterations in the circadian pattern of food intake produced by high-fat diet, and showed an enhancement in the reinforcing effects and motivation to obtain food. In spite of the similar caloric intake, FAAH−/− displayed an enhanced body weight, fat content and insulin resistance associated to enhanced leptin levels and decreased adiponectin concentration, which was likely due to a predominant effect of AEA over OEA on lipogenesis in peripheral organs. The ability of OEA to induce satiety and lipolysis suggest that additional metabolizing enzymes such as NAPE-PLD (38) may regulate the action of OEA levels on feeding and lipid metabolism. Evidence supports the predominant role of CB1 receptors in the overweight, enhanced motivation for food, and increased lipogenesis of FAAH−/− mice. Nevertheless, the OEA receptor PPAR-α and the transient receptor potential vanilloid type 1 (TRPV1) activated by AEA and OEA might also participate in these effects (25;39) Therefore, the contribution of these receptors to the phenotype of FAAH−/− animals can not be completely excluded, and further studies using TRPV1 and PPAR-α antagonists are required in FAAH−/− mice to elucidate this issue.
Acknowledgments
This study was supported by grants from European Communities (GENADDICT LSHM-CT-2004-005166 and PHECOMP LHSM-CT-2006-037669), National Institute on Drug Abuse (NIDA) (DA012413), Instituto de Salud Carlos III (RD06/001/001), Spanish Ministry of Education (SAF2007-64062) and Generalitat de Catalunya (2005SGR00131). CT was financed by FI and BE fellowships from AGAUR (Generalitat de Catalunya).
Reference List
- 1.Cota D. CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev. 2007;23:507–517. doi: 10.1002/dmrr.764. [DOI] [PubMed] [Google Scholar]
- 2.Colombo G, Agabio R, Díaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–L117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 4.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 200; 134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- 8.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le FG, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 11.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 13.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 14.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 15.Ueda N, Yamamoto S. Anandamide amidohydrolase (fatty acid amide hydrolase). Prostaglandins Other Lipid Mediat. 2000;61:19–28. doi: 10.1016/s0090-6980(00)00052-6. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci. 2005;62:708–716. doi: 10.1007/s00018-004-4494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman M, Lo VJ, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- 19.Kunos G. Understanding metabolic homeostasis and imbalance: what is the role of the endocannabinoid system? Am J Med. 2007;120(Suppl 1):S18–S24. doi: 10.1016/j.amjmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology. 2003;28:1311–1316. doi: 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- 22.Giuffrida A, Rodríguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor. AM404. Eur J Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- 23.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 24.Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. doi: 10.1159/000115362. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Gaetani S, Oveisi F, Lo VJ, Serrano A, Rodríguez de Fonseca F, Rosengarth A, Luecke H, Di GB, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodríguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 27.Matias I, Bisogno T, Di Marzo V. Endogenous cannabinoids in the brain and peripheral tissues: regulation of their levels and control of food intake. Int J Obes (Lond) 2006;30(Suppl 1):S7–S12. doi: 10.1038/sj.ijo.0803271. [DOI] [PubMed] [Google Scholar]
- 28.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945–950. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamber KM, Macarthur H, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, cuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci. 2007;27:4870–4881. doi: 10.1523/JNEUROSCI.0732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osei-Hyiaman D, DePetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ, Mackie K, Palkovits M, Kunos G. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- 33.Ravinet TC, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matias I, Gonthier MP, Petrosino S, Docimo L, Capasso R, Hoareau L, Monteleone P, Roche R, Izzo AA, Di Marzo V. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol. 2007;152:676–690. doi: 10.1038/sj.bjp.0707424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940). Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R45–R50. doi: 10.1152/ajpregu.00126.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]