Abstract
A global regulator of chromatin remodelling and gene expression, special AT-rich-binding protein 1 (SATB1) has been implicated in promotion of growth and metastasis of a number of cancers. Here, we demonstrate that the principal oncogene of Epstein–Barr virus (EBV), latent membrane protein 1 (LMP1) upregulates SATB1 RNA and protein expression in human nasopharyngeal cell lines. Silencing of endogenously expressed SATB1 with specific short hairpin RNA decreases cell proliferation and resistance to apoptosis induced by growth factor withdrawal. Additionally, we provide evidence that LMP1-mediated expression of Survivin, a multifunctional protein involved in promoting cell growth and survival, is mediated at least in part by SATB1 in human nasopharyngeal cells. Finally, we show that SATB1 protein levels are elevated in tissue samples from patients with nasopharyngeal carcinoma (NPC), and are directly correlated with the expression of LMP1. Taken together, our results suggest that SATB1 functions as a pro-metastatic effector of LMP1 signalling in EBV-positive NPC.
Special AT-rich sequence-binding protein 1 (SATB1) was originally identified as protein binding to matrix attachment regions (MARs) of unpaired DNA (Dickinson et al., 1992; Sun et al., 2006). SATB1 was shown to regulate distant genes by selectively tethering MARs to the nuclear matrix, resulting in the formation of a characteristic ‘cage-like’ network that circumscribes heterochromatin (Cai et al., 2003). Furthermore, SATB1 acts as a ‘docking site’ for several chromatin modifiers (Kumar et al., 2005; Yasui et al., 2002), and these chromatin modifiers were suggested to suppress gene expression through histone deacetylation and nucleosome remodelling at SATB1-bound MARs (Yasui et al., 2002). Phosphorylation of SATB1 might act as a molecular switch in determining whether the protein acts as a transcriptional activator or repressor (Pavan Kumar et al., 2006). Recently, SATB1 has attracted considerable attention due to its elevated expression in advanced stages of various types of malignant tumours, such as breast, gastric, colorectal and laryngeal carcinomas, which suggests a crucial role in promoting tumour invasion and metastasis (Cheng et al., 2010; Han et al., 2008; Iorns et al., 2010; Lu et al., 2011; Meng et al., 2012; Patani et al., 2009; Shushan et al., 2009; Zhao et al., 2010; Zheng, 2008). However, although there are reports that SATB1 regulates transcription of genes that have direct impact on cellular proliferation and apoptosis (Alvarez et al., 2000; Kumar et al., 2007), the mechanism by which SATB1 regulates these phenomena is still unclear and even controversial (Iorns et al., 2010; Li et al., 2007).
Nasopharyngeal carcinoma (NPC) is the most common Epstein–Barr virus (EBV)-associated tumour, and it is noteworthy for its proclivity to early invasion and metastasis (Burgos, 2005; Pagano, 2009; Wakisaka & Pagano, 2003; Zheng et al., 2007). The principal EBV oncoprotein LMP1, a member of the tumour necrosis factor receptor superfamily, is detected in at least 70 % of NPC cases, and is considered to be the main mediator of the oncogenic properties of EBV in the development of NPC and its metastasis (Marquitz & Raab-Traub, 2012; Tsao et al., 2002b; Wakisaka & Pagano, 2003; Yoshizaki et al., 2005). In a series of reports we have shown that LMP1 induces expression of a variety of cell-invasiveness and angiogenic factors and explored their effects in nasopharyngeal (NP) cells in culture (Yoshizaki et al., 1998; Kondo et al., 2005; Wakisaka et al., 2002; Murono et al., 2001; Wakisaka et al., 2004; Kondo et al., 2006, 2007; Horikawa et al., 2007). Since recent studies demonstrate that SATB1 promotes tumour growth and metastasis in several carcinomas (Lu et al., 2011; Meng et al., 2012; Patani et al., 2009; Tu et al., 2012; Zhao et al., 2010), we hypothesized that LMP1 upregulates expression of SATB1 in EBV-positive NPC cells.
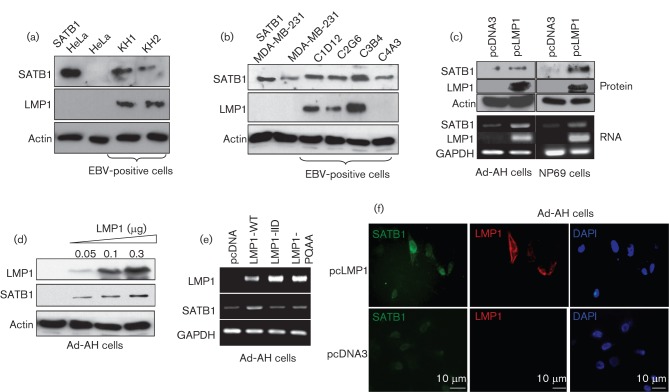
First, we examined the endogenous levels of SATB1 and LMP1 in two sets of EBV-infected cell lines: one set, epithelial-like cell lines with type II EBV latency obtained by fusion of a human type III EBV latency lymphoblastoid cell line with epithelial Hela cells (Contreras-Brodin et al., 1991); and another, EBV-infected breast cancer cell lines that express different levels of LMP1 (Arbach et al., 2006; Horikawa et al., 2007). Western blot analysis shows that the levels of SATB1 protein are significantly higher in the fused LMP1-expressing cells KH-1 and KH-2, than in the EBV-negative parental Hela cells (Fig. 1a), and that the expression of SATB1 is also upregulated in the LMP1-positive breast cancer clones C1D12, C2G6 and C3B4, compared with either the EBV-infected, but LMP1-negative, C4A3 cells or the uninfected parental line, MDA-MB-231 (Fig. 1b). These data illustrate the direct correlation between endogenous LMP1 and SATB1 protein levels in different EBV-positive carcinoma-derived cell lines.
Fig. 1.
Expression of SATB1 is increased in EBV latently infected cells. Increase in endogenous SATB1 protein levels correlates with the levels of the EBV primary oncogene LMP1 in EBV-positive fusion cervical carcinoma cells (a) and in EBV-infected breast cancer cell lines (b). Transient transfection of SATB1 served as a positive control. EBV primary oncogene LMP1 induces expression of endogenous SATB1 in human NP epithelial cell lines. Stable exogenous expression of LMP1 in Ad-AH and NP69 cells increases endogenous levels of SATB1 protein (c, top panel) and RNA (c, bottom panel). Dose-dependent upregulation of endogenous SATB1 by LMP1 overexpression in Ad-AH cells (d). Both CTAR1 and CTAR2 regions of LMP1 are involved in LMP1-mediated induction of SATB1 gene expression (e). Immunofluorescence analysis showing elevated levels of endogenous SATB1 protein in LMP1-transfected Ad-AH cells (f).
To investigate whether LMP1 alone can induce SATB1 expression, we transiently transfected LMP1 into two EBV-negative human NP cell lines, Ad-AH (Takimoto et al., 1988) and NP69 (Tsao et al., 2002b) and observed significant increases in levels of SATB1 protein and RNA in both sets of cell lines (Fig. 1c). To confirm these observations further we showed that transient overexpression of LMP1 into Ad-AH cells induces SATB1 in a dose-dependent manner (Fig. 1d). Moreover, fluorescence immunostaining revealed much higher levels of SATB1 protein in LMP1-expressing cells (Fig. 1e) (we did not observe any effects of the empty vector on SATB1 endogenous levels). Taken together these results demonstrate that LMP1 upregulates SATB1 RNA and protein expression in NP cells.
Two LMP1 intracellular signalling domains, the C-terminal regulatory regions of the protein, CTAR1 and CTAR2, are well established as major transmitters of LMP1-induced signalling pathways involved in initiation and maintenance of malignant potential in EBV-associated cancers including NPC (Brinkmann & Schulz, 2006; Hatzivassiliou & Mosialos, 2002; Izumi, 2004; Lam & Sugden, 2003; Li & Chang, 2003; Morris et al., 2009; Soni et al., 2007; Tsao et al., 2002a; Zheng et al., 2007). Fig. 1(e) shows that transfections of LMP1 inactive mutants with point mutations in CTAR1 (LMP1-PQAA) and CTAR2 (LMP-IID) (Mainou et al., 2007) reduced SATB1 RNA levels, indicating that LMP1-dependent expression of SATB1 most probably depends on activation of multiple signalling pathways from both CTAR1 and CTAR2 domains of LMP1.
Many studies, including ours, demonstrate that LMP1 expression induces an invasive phenotype in NP cells (Pagano, 2009; Tsao et al., 2002a; Zheng et al., 2007). To determine whether SATB1 is required for oncogenic properties of LMP1-expressing NP cells we generated a stable NP cell line in which SATB1 expression was suppressed with short hairpin RNA (shRNA) targeting SATB1 [pSuper-puro vector with SATB1 shRNA was the kind gift of Dr Hye-Jung Han (Han et al., 2008)]. First, we analysed cell growth with the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) assay. Growth curves are presented with the standard deviations (sd) as error bars. As shown in Fig. 2(a), silencing of the SATB1 gene with specific shRNA significantly reduced growth of LMP1-expressing NP69 cells, but had minimal effect on the parental NP69 cell line.
Fig. 2.
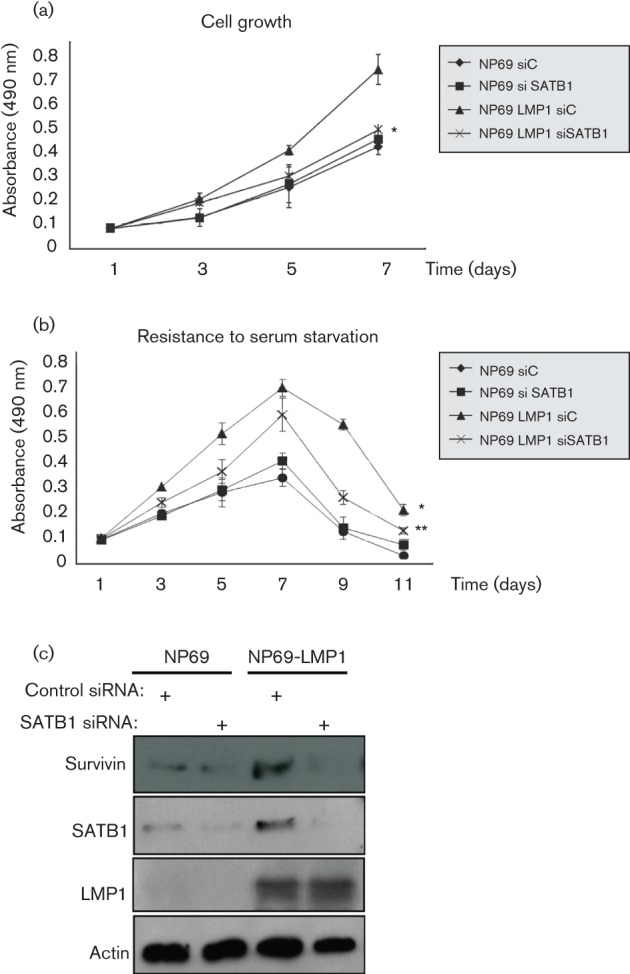
Expression of SATB1 is required for growth and survival of NP cells. Reduction of SATB1 expression with specific siRNA inhibits proliferation of NP69 and NP69-LMP1 cells (a). Reduction of SATB1 levels results in reduced resistance of NP69 and NP-LMP1 cells to growth factor withdrawal (b). Inhibition of SATB1 expression correlates with the reduction of LMP1-induced levels of anti-apoptotic protein Survivin in NP69 cells (c). Student’s t test: *, P = 0.0019, compared with NP69 control siRNA cells. **, P = 0.0097, compared with NP69-LMP1 control siRNA cells.
Deprivation of growth factors leads to cell-cycle arrest following the induction of apoptosis (Lo et al., 2003; Zhang et al., 2008a). We tested the ability of SATB1 to protect the cells from effects induced by growth factor withdrawal by measuring cell viability of control and NP69 cells expressing SATB1 shRNA, cultivated in medium without growth-factor supplements (Fig. 2b). For the first 7 days all cell lines demonstrated some increase in cell growth, but eventually underwent starvation-induced cell-cycle arrest. As expected, LMP1-expressing cells were more resistant to growth factor withdrawal than parental NP69 cells, which is consistent with a previous report (Lo et al., 2003). While the difference in survival between parental NP69 cells expressing control and SATB1 shRNAs was minimal, resistance to growth factor withdrawal was significantly decreased by SATB1 shRNA in NP69-LMP1 cells (Fig. 2b). The differences in cell growth and apoptosis between NP69 cell clones were analysed by the paired Student’s t-test. Error bars indicate sd of the mean and significance was defined at the level of P<0.05.
Our results indicate that SATB1 participates in the proliferation and survival of NP epithelial cells, most probably through induction of pro-mitotic and anti-apoptotic factors. One such factor that mediates proliferation, invasion and metastasis of many cancers is Survivin. Originally discovered as an inhibitor of apoptosis, Survivin also promotes cell proliferation and enhances invasion (Cheung et al., 2011; Church & Talbot, 2012; Kanwar et al., 2010, 2011; Lladser et al., 2011). Absent in most adult tissues, Survivin is selectively upregulated in many human carcinomas including NPC and correlates with poor prognosis (Fukayama et al., 2008; Hino et al., 2008; Lippert et al., 2007; Margulis et al., 2008; McGuire & Fitzpatrick, 2009; Mori et al., 2007; Shi et al., 2006; Zhang et al., 2008b). Survivin is also a well known target of LMP1-mediated activation of cell signalling: LMP1 activates the survivin gene through several signalling pathways, and both CTAR1 and CTAR2 domains are required (Brinkmann & Schulz, 2006).
To investigate whether the inhibitory effects on cell growth and survival we observed with suppression of SATB1 (Fig. 2a and b) correlate with the expression of Survivin, we compared endogenous levels of Survivin protein in NP NP69 cells under control conditions and with suppressed levels of SATB1 (Fig. 2c). Western blot analysis shows that inhibition of SATB1 expression with shRNA resulted in some reduction in Survivin levels, and this effect was much more profound in the presence of LMP1: inhibition of SATB1 expression resulted in a significant reduction of Survivin in LMP1-positive NP69 cells. Different EBV products induce the expression of Survivin through different mechanisms (Bai et al., 2005; Lu et al., 2011; Paydas et al., 2009; Slovin et al., 1981), and our data indicate that SATB1 is directly or indirectly involved in LMP1-mediated upregulation of Survivin. In the future we plan to investigate the mechanisms of such upregulation.
Finally, we tested whether there is a correlation between SATB1 and LMP1 expression in EBV-positive tumour tissue samples from NPC patients. Ten randomly selected NPC paraffin-embedded specimens from Kanazawa University Hospital (Ishikawa, Japan) were used for the detection of LMP1 and SATB1. Specimens obtained at biopsy were from eight men and two women, ages 45–78 years (mean 58.7 years), and classified histologically as follows: five non-keratinizing carcinoma (type II) and five undifferentiated carcinoma (type III) [5th TNM classification system of the International Union Against Cancer (1997)]. There were two stage IIB, seven stage III and one stage IVC. SATB1 levels were examined by immunohistochemistry in the NPC tissues of the ten patients (Fig. 3). Pearson’s correlation coefficient was used to detect correlations among the expression scores of each protein analysed in NPC. The expression score of each protein in relation to clinical data was analysed with the Mann–Whitney U-test as described previously (Horikawa et al., 2011). Expression scores for SATB1 and LMP1 are closely correlated (r = 0.731; P<0.001). These data demonstrate a significant in-vivo correlation between LMP1 and SATB1 levels in NPC, which supports the hypothesis that expression of SATB1 is associated with a highly aggressive and metastatic phenotype of several human carcinomas (Han et al., 2008; Kuo & Chao, 2010; Peng et al., 2011; Tu et al., 2012).
Fig. 3.
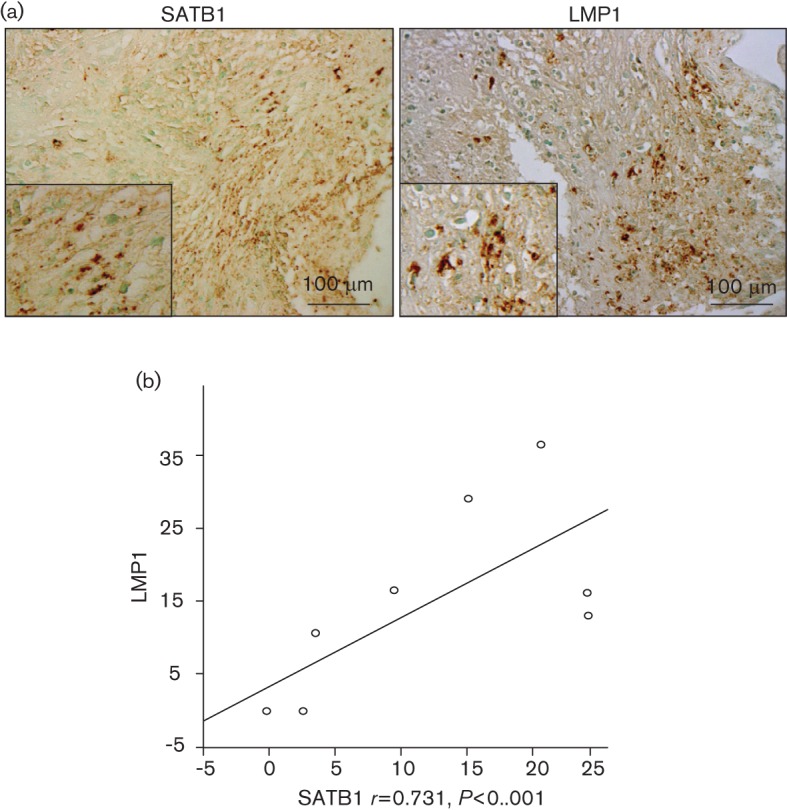
Correlation between SATB1 and LMP1 expression in EBV-positive NPC tissues. Immunohistochemical detection of SATB1 and EBV LMP1 in human NP carcinoma tissues (a). Regression analysis shows the significant correlation between expression of SATB1 and LMP1 in the ten NPC specimens obtained at biopsy (b).
Recently, we showed that LMP1 contributes to the cancer progenitor-like phenotype of NPC (Kondo et al., 2011), and that SATB1 is required for proper stem-cell differentiation and negatively regulates pluripotency genes (Savarese et al., 2009). Considering that SATB1 expression is lost as the progenitor cells further differentiate (Agrelo & Wutz, 2009; Wen et al., 2005), it is possible that expression of SATB1 is part of an embryonic pathway for establishing epigenetic patterns that are reactivated in somatic progenitors (Agrelo & Wutz, 2009). Upregulation of SATB1 by LMP1 strengthens these recent findings, and SATB1 could have some role in the context of the cancer stem/progenitor cell axis in the oncogenesis of NPC. The data presented here underscore also a series of findings that support the contribution of LMP1 to upregulation of metastasis via inducing epithelial mesenchymal transition (EMT) (Horikawa et al., 2007, 2011).
SATB1 expression and the ability of Xist induction to initiate X chromosome inactivation are two aspects that provide a link between the epigenetic context of embryonic cells with that of somatic progenitors and tumour cells (Agrelo et al., 2009). Haematopoietic progenitors are probably at a critical transition when cell identity is unstable. This epigenetic context thus defines the biology of a class of cancer progenitors that distinguishes them from normal cells and could be a novel target for cancer therapies. The data presented here underscore also a series of findings that support the contribution of LMP1 to upregulation of metastasis via inducing EMT (Horikawa et al., 2007, 2011). Induction of SATB1 by LMP1 in epithelial cells sheds light on a more precise role of LMP1 in the cancer stem cell theory (Kondo et al., 2011). It will be of interest to investigate the clinical implications of LMP1-mediated SATB1 induction in future studies.
Acknowledgements
We thank Drs Sai Wah Tsao and Erik K. Flemington, respectively, for the NP69SV40T and the Ad-AH cell lines. This work was supported by the National Cancer Institute (Bethesda, MD) (CA 19014) (J. S. P.) and the Nakayama Foundation for Human Science (Tokyo, Japan) (K. E.).
References
- Agrelo R., Wutz A. (2009). Cancer progenitors and epigenetic contexts: an Xisting connection. Epigenetics 4, 568–570 10.4161/epi.4.8.10186 [DOI] [PubMed] [Google Scholar]
- Agrelo R., Souabni A., Novatchkova M., Haslinger C., Leeb M., Komnenovic V., Kishimoto H., Gresh L., Kohwi-Shigematsu T., Kenner L. (2009). SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells. Dev Cell 16, 507–516 10.1016/j.devcel.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J. D., Yasui D. H., Niida H., Joh T., Loh D. Y., Kohwi-Shigematsu T. (2000). The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev 14, 521–535 [PMC free article] [PubMed] [Google Scholar]
- Arbach H., Viglasky V., Lefeu F., Guinebretière J. M., Ramirez V., Bride N., Boualaga N., Bauchet T., Peyrat J. P. & other authors (2006). Epstein–Barr virus (EBV) genome and expression in breast cancer tissue: effect of EBV infection of breast cancer cells on resistance to paclitaxel (Taxol). J Virol 80, 845–853 10.1128/JVI.80.2.845-853.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M., Papoudou-Bai A., Kitsoulis P., Horianopoulos N., Kamina S., Agnantis N. J., Kanavaros P. (2005). Cell cycle and apoptosis deregulation in classical Hodgkin lymphomas. In Vivo 19, 439–453 [PubMed] [Google Scholar]
- Brinkmann M. M., Schulz T. F. (2006). Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol 87, 1047–1074 10.1099/vir.0.81598-0 [DOI] [PubMed] [Google Scholar]
- Burgos J. S. (2005). Involvement of the Epstein–Barr virus in the nasopharyngeal carcinoma pathogenesis. Med Oncol 22, 113–122 10.1385/MO:22:2:113 [DOI] [PubMed] [Google Scholar]
- Cai S., Han H. J., Kohwi-Shigematsu T. (2003). Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat Genet 34, 42–51 10.1038/ng1146 [DOI] [PubMed] [Google Scholar]
- Cheng C., Lu X., Wang G., Zheng L., Shu X., Zhu S., Liu K., Wu K., Tong Q. (2010). Expression of SATB1 and heparanase in gastric cancer and its relationship to clinicopathologic features. APMIS 118, 855–863 10.1111/j.1600-0463.2010.02673.x [DOI] [PubMed] [Google Scholar]
- Cheung C. H., Cheng L., Chang K. Y., Chen H. H., Chang J. Y. (2011). Investigations of survivin: the past, present and future. Front Biosci 16, 952–961 10.2741/3728 [DOI] [PubMed] [Google Scholar]
- Church D. N., Talbot D. C. (2012). Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep 14, 120–128 10.1007/s11912-012-0215-2 [DOI] [PubMed] [Google Scholar]
- Contreras-Brodin B. A., Anvret M., Imreh S., Altiok E., Klein G., Masucci M. G. (1991). B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J Gen Virol 72, 3025–3033 10.1099/0022-1317-72-12-3025 [DOI] [PubMed] [Google Scholar]
- Dickinson L. A., Joh T., Kohwi Y., Kohwi-Shigematsu T. (1992). A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell 70, 631–645 10.1016/0092-8674(92)90432-C [DOI] [PubMed] [Google Scholar]
- Fukayama M., Hino R., Uozaki H. (2008). Epstein–Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Sci 99, 1726–1733 10.1111/j.1349-7006.2008.00888.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H. J., Russo J., Kohwi Y., Kohwi-Shigematsu T. (2008). SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature 452, 187–193 10.1038/nature06781 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou E., Mosialos G. (2002). Cellular signaling pathways engaged by the Epstein–Barr virus transforming protein LMP1. Front Biosci 7, d319–d329 10.2741/hatziva [DOI] [PubMed] [Google Scholar]
- Hino R., Uozaki H., Inoue Y., Shintani Y., Ushiku T., Sakatani T., Takada K., Fukayama M. (2008). Survival advantage of EBV-associated gastric carcinoma: survivin up-regulation by viral latent membrane protein 2A. Cancer Res 68, 1427–1435 10.1158/0008-5472.CAN-07-3027 [DOI] [PubMed] [Google Scholar]
- Horikawa T., Yang J., Kondo S., Yoshizaki T., Joab I., Furukawa M., Pagano J. S. (2007). Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res 67, 1970–1978 10.1158/0008-5472.CAN-06-3933 [DOI] [PubMed] [Google Scholar]
- Horikawa T., Yoshizaki T., Kondo S., Furukawa M., Kaizaki Y., Pagano J. S. (2011). Epstein–Barr virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer 104, 1160–1167 10.1038/bjc.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorns E., Hnatyszyn H. J., Seo P., Clarke J., Ward T., Lippman M. (2010). The role of SATB1 in breast cancer pathogenesis. J Natl Cancer Inst 102, 1284–1296 10.1093/jnci/djq243 [DOI] [PubMed] [Google Scholar]
- Izumi K. M. (2004). Epstein–Barr virus signal transduction and B-lymphocyte growth transformation. Prog Mol Subcell Biol 36, 269–288 [DOI] [PubMed] [Google Scholar]
- Kanwar R. K., Cheung C. H., Chang J.-Y., Kanwar J. R. (2010). Recent advances in anti-survivin treatments for cancer. Curr Med Chem 17, 1509–1515 10.2174/092986710790979935 [DOI] [PubMed] [Google Scholar]
- Kanwar J. R., Kamalapuram S. K., Kanwar R. K. (2011). Targeting survivin in cancer: the cell-signalling perspective. Drug Discov Today 16, 485–494 10.1016/j.drudis.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Kondo S., Wakisaka N., Schell M. J., Horikawa T., Sheen T. S., Sato H., Furukawa M., Pagano J. S., Yoshizaki T. (2005). Epstein–Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int J Cancer 115, 368–376 10.1002/ijc.20849 [DOI] [PubMed] [Google Scholar]
- Kondo S., Seo S. Y., Yoshizaki T., Wakisaka N., Furukawa M., Joab I., Jang K. L., Pagano J. S. (2006). EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res 66, 9870–9877 10.1158/0008-5472.CAN-06-1679 [DOI] [PubMed] [Google Scholar]
- Kondo S., Yoshizaki T., Wakisaka N., Horikawa T., Murono S., Jang K. L., Joab I., Furukawa M., Pagano J. S. (2007). MUC1 induced by Epstein–Barr virus latent membrane protein 1 causes dissociation of the cell-matrix interaction and cellular invasiveness via STAT signaling. J Virol 81, 1554–1562 10.1128/JVI.02222-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Wakisaka N., Muramatsu M., Zen Y., Endo K., Murono S., Sugimoto H., Yamaoka S., Pagano J. S., Yoshizaki T. (2011). Epstein–Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol 85, 11255–11264 10.1128/JVI.00188-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. P., Purbey P. K., Ravi D. S., Mitra D., Galande S. (2005). Displacement of SATB1-bound histone deacetylase 1 corepressor by the human immunodeficiency virus type 1 transactivator induces expression of interleukin-2 and its receptor in T cells. Mol Cell Biol 25, 1620–1633 10.1128/MCB.25.5.1620-1633.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. P., Bischof O., Purbey P. K., Notani D., Urlaub H., Dejean A., Galande S. (2007). Functional interaction between PML and SATB1 regulates chromatin-loop architecture and transcription of the MHC class I locus. Nat Cell Biol 9, 45–56 10.1038/ncb1516 [DOI] [PubMed] [Google Scholar]
- Kuo T. C., Chao C. C. (2010). Hepatitis B virus X protein prevents apoptosis of hepatocellular carcinoma cells by upregulating SATB1 and HURP expression. Biochem Pharmacol 80, 1093–1102 10.1016/j.bcp.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Lam N., Sugden B. (2003). CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal 15, 9–16 10.1016/S0898-6568(02)00083-9 [DOI] [PubMed] [Google Scholar]
- Li H. P., Chang Y. S. (2003). Epstein–Barr virus latent membrane protein 1: structure and functions. J Biomed Sci 10, 490–504 10.1007/BF02256110 [DOI] [PubMed] [Google Scholar]
- Li K., Cai R., Dai B. B., Zhang X. Q., Wang H. J., Ge S. F., Xu W. R., Lu J. (2007). SATB1 regulates SPARC expression in K562 cell line through binding to a specific sequence in the third intron. Biochem Biophys Res Commun 356, 6–12 10.1016/j.bbrc.2007.01.201 [DOI] [PubMed] [Google Scholar]
- Lippert B. M., Knauer S. K., Fetz V., Mann W., Stauber R. H. (2007). Dynamic survivin in head and neck cancer: molecular mechanism and therapeutic potential. Int J Cancer 121, 1169–1174 10.1002/ijc.22941 [DOI] [PubMed] [Google Scholar]
- Lladser A., Sanhueza C., Kiessling R., Quest A. F. (2011). Is survivin the potential Achilles’ heel of cancer? Adv Cancer Res 111, 1–37 10.1016/B978-0-12-385524-4.00001-5 [DOI] [PubMed] [Google Scholar]
- Lo A. K., Liu Y., Wang X. H., Huang D. P., Yuen P. W., Wong Y. C., Tsao G. S. (2003). Alterations of biologic properties and gene expression in nasopharyngeal epithelial cells by the Epstein–Barr virus-encoded latent membrane protein 1. Lab Invest 83, 697–709 [DOI] [PubMed] [Google Scholar]
- Lu X., Cheng C., Zhu S., Yang Y., Zheng L., Wang G., Shu X., Wu K., Liu K., Tong Q. (2010). SATB1 is an independent prognostic marker for gastric cancer in a Chinese population. Oncol Rep 24, 981–987 [DOI] [PubMed] [Google Scholar]
- Lu J., Murakami M., Verma S. C., Cai Q., Haldar S., Kaul R., Wasik M. A., Middeldorp J., Robertson E. S. (2011). Epstein–Barr virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology 410, 64–75 10.1016/j.virol.2010.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou B. A., Everly D. N., Jr, Raab-Traub N. (2007). Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol 81, 9680–9692 10.1128/JVI.01001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis V., Lotan Y., Shariat S. F. (2008). Survivin: a promising biomarker for detection and prognosis of bladder cancer. World J Urol 26, 59–65 10.1007/s00345-007-0219-y [DOI] [PubMed] [Google Scholar]
- Marquitz A. R., Raab-Traub N. (2012). The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol 22, 166–172 10.1016/j.semcancer.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. B., Fitzpatrick J. M. (2009). Biomarkers in renal cell carcinoma. Curr Opin Urol 19, 441–446 10.1097/MOU.0b013e32832f0c68 [DOI] [PubMed] [Google Scholar]
- Meng W. J., Yan H., Zhou B., Zhang W., Kong X. H., Wang R., Zhan L., Li Y., Zhou Z. G., Sun X. F. (2012). Correlation of SATB1 overexpression with the progression of human rectal cancer. Int J Colorectal Dis 27, 143–150 10.1007/s00384-011-1302-9 [DOI] [PubMed] [Google Scholar]
- Mori F., Piro F. R., Della Rocca C., Mesiti G., Giampaoli S., Silvestre G., Lazzaro D. (2007). Survivin and Cyclooxygenase-2 are co-expressed in human and mouse colon carcinoma and in terminally differentiated colonocytes. Histol Histopathol 22, 61–77 [DOI] [PubMed] [Google Scholar]
- Morris M. A., Dawson C. W., Young L. S. (2009). Role of the Epstein–Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol 5, 811–825 10.2217/fon.09.53 [DOI] [PubMed] [Google Scholar]
- Murono S., Inoue H., Tanabe T., Joab I., Yoshizaki T., Furukawa M., Pagano J. S. (2001). Induction of cyclooxygenase-2 by Epstein–Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci U S A 98, 6905–6910 10.1073/pnas.121016998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. (2009). EBV Diseases. In DNA Tumor Viruses, pp 217–240 New York: Springer [Google Scholar]
- Patani N., Jiang W., Mansel R., Newbold R., Mokbel K. (2009). The mRNA expression of SATB1 and SATB2 in human breast cancer. Cancer Cell Int 9, 18 10.1186/1475-2867-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan Kumar P., Purbey P. K., Sinha C. K., Notani D., Limaye A., Jayani R. S., Galande S. (2006). Phosphorylation of SATB1, a global gene regulator, acts as a molecular switch regulating its transcriptional activity in vivo. Mol Cell 22, 231–243 10.1016/j.molcel.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Paydas S., Ergin M., Seydaoglu G., Erdogan S., Yavuz S. (2009). Prognostic [corrected] significance of angiogenic/lymphangiogenic, anti-apoptotic, inflammatory and viral factors in 88 cases with diffuse large B cell lymphoma and review of the literature. Leuk Res 33, 1627–1635 10.1016/j.leukres.2009.02.015 [DOI] [PubMed] [Google Scholar]
- Peng Z. K., Yang D. H., Li X. H., Huang Y., Zhang G. Q., Zhong K. B., Bi M. P., Li G. H. (2011). [Expression of special AT-rich sequence-binding protein 1 mRNA in hepatocellular carcinoma and its clinical significance]. Nan Fang Yi Ke Da Xue Xue Bao 31, 1207–1211 (in Chinese). [PubMed] [Google Scholar]
- Savarese F., Dávila A., Nechanitzky R., De La Rosa-Velazquez I., Pereira C. F., Engelke R., Takahashi K., Jenuwein T., Kohwi-Shigematsu T. & other authors (2009). Satb1 and Satb2 regulate embryonic stem cell differentiation and Nanog expression. Genes Dev 23, 2625–2638 10.1101/gad.1815709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Bastianutto C., Li A., Perez-Ordonez B., Ng R., Chow K. Y., Zhang W., Jurisica I., Lo K. W. & other authors (2006). Multiple dysregulated pathways in nasopharyngeal carcinoma revealed by gene expression profiling. Int J Cancer 119, 2467–2475 10.1002/ijc.22107 [DOI] [PubMed] [Google Scholar]
- Shushan S., Cinamon U., Levy D., Sokolov M., Roth Y. (2009). Laryngeal cancer in acquired immunodeficiency syndrome. Int J STD AIDS 20, 582–584 10.1258/ijsa.2008.008345 [DOI] [PubMed] [Google Scholar]
- Slovin S. F., Glassy M. C., Dambaugh T., Catalano M. A., Curry R. A., Ferrone S., Kieff E., Vaughan J. H., Carson D. A. (1981). Discordant expression of 2 Epstein–Barr virus-associated antigens, EBNA and RANA, in man-rodent somatic cell hybrids. J Immunol 127, 585–590 [PubMed] [Google Scholar]
- Soni V., Cahir-McFarland E., Kieff E. (2007). LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol 597, 173–187 10.1007/978-0-387-70630-6_14 [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang T., Su Y., Yin Y., Xu S., Ma C., Han X. (2006). The behavior of SATB1, a MAR-binding protein, in response to apoptosis stimulation. Cell Biol Int 30, 244–247 10.1016/j.cellbi.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Takimoto T., Sato H., Ogura H., Tanaka S., Umeda R. (1988). The difference in tumorigenicity between Epstein–Barr virus (EBV) genome-positive and genome-negative epithelial hybrid cell lines derived from the human nasopharynx. Laryngoscope 98, 1334–1338 10.1288/00005537-198812000-00010 [DOI] [PubMed] [Google Scholar]
- Tsao S. W., Tramoutanis G., Dawson C. W., Lo A. K., Huang D. P. (2002a). The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol 12, 473–487 10.1016/S1044579X02000901 [DOI] [PubMed] [Google Scholar]
- Tsao S. W., Wang X., Liu Y., Cheung Y. C., Feng H., Zheng Z., Wong N., Yuen P. W., Lo A. K. & other authors (2002b). Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta 1590, 150–158 10.1016/S0167-4889(02)00208-2 [DOI] [PubMed] [Google Scholar]
- Tu W., Luo M., Wang Z., Yan W., Xia Y., Deng H., He J., Han P., Tian D. (2012). Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int 32, 1064–1078 10.1111/j.1478-3231.2012.02815.x [DOI] [PubMed] [Google Scholar]
- Wakisaka N., Pagano J. S. (2003). Epstein–Barr virus induces invasion and metastasis factors. Anticancer Res 23 (3A), 2133–2138 [PubMed] [Google Scholar]
- Wakisaka N., Murono S., Yoshizaki T., Furukawa M., Pagano J. S. (2002). Epstein–Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res 62, 6337–6344 [PubMed] [Google Scholar]
- Wakisaka N., Kondo S., Yoshizaki T., Murono S., Furukawa M., Pagano J. S. (2004). Epstein–Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol 24, 5223–5234 10.1128/MCB.24.12.5223-5234.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Huang S., Rogers H., Dickinson L. A., Kohwi-Shigematsu T., Noguchi C. T. (2005). SATB1 family protein expressed during early erythroid differentiation modifies globin gene expression. Blood 105, 3330–3339 10.1182/blood-2004-08-2988 [DOI] [PubMed] [Google Scholar]
- Yasui D., Miyano M., Cai S., Varga-Weisz P., Kohwi-Shigematsu T. (2002). SATB1 targets chromatin remodelling to regulate genes over long distances. Nature 419, 641–645 10.1038/nature01084 [DOI] [PubMed] [Google Scholar]
- Yoshizaki T., Sato H., Furukawa M., Pagano J. S. (1998). The expression of matrix metalloproteinase 9 is enhanced by Epstein–Barr virus latent membrane protein 1. Proc Natl Acad Sci U S A 95, 3621–3626 10.1073/pnas.95.7.3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T., Wakisaka N., Pagano J. S. (2005). Epstein–Barr virus: Invasion and Metastasis. Norfolk, UK: Caister Academic Press [Google Scholar]
- Zhang Q., Zhang Z., Wang C., Xiao Z., Yu Y., Yang F., Chen Z., He Z. (2008a). Proteome analysis of the transformation potential of the Epstein–Barr virus-encoded latent membrane protein 1 in nasopharyngeal epithelial cells NP69. Mol Cell Biochem 314, 73–83 10.1007/s11010-008-9767-8 [DOI] [PubMed] [Google Scholar]
- Zhang R., Wang T., Li K. N., Qin W. W., Chen R., Wang K., Jia L. T., Zhao J., Wen W. H. & other authors (2008b). A survivin double point mutant has potent inhibitory effect on the growth of hepatocellular cancer cells. Cancer Biol Ther 7, 547–554 10.4161/cbt.7.4.5484 [DOI] [PubMed] [Google Scholar]
- Zhao X. D., Ji W. Y., Zhang W., He L. X., Yang J., Liang H. J., Wang L. L. (2010). Overexpression of SATB1 in laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 72, 1–5 10.1159/000264777 [DOI] [PubMed] [Google Scholar]
- Zheng J. (2008). Is SATB1 a master regulator in breast cancer growth and metastasis? Womens Health (Lond Engl) 4, 329–332 10.2217/17455057.4.4.329 [DOI] [PubMed] [Google Scholar]
- Zheng H., Li L. L., Hu D. S., Deng X. Y., Cao Y. (2007). Role of Epstein–Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol 4, 185–196 [PubMed] [Google Scholar]