Abstract
Chronic wasting disease (CWD) of cervids is almost certainly transmitted by mucosal contact with the causative prion, whether by direct (animal-to-animal) or indirect (environmental) means. Yet the sites and mechanisms of prion entry remain to be further understood. This study sought to extend this understanding by demonstrating that ferrets exposed to CWD via several mucosal routes developed infection, CWD prion protein (PrPCWD) amplification in lymphoid tissues, neural invasion and florid transmissible spongiform encephalopathy lesions resembling those in native cervid hosts. The ferrets developed extensive PrPCWD accumulation in the nervous system, retina and olfactory epithelium, with lesser deposition in tongue, muscle, salivary gland and the vomeronasal organ. PrPCWD accumulation in mucosal sites, including upper respiratory tract epithelium, olfactory epithelium and intestinal Peyer’s patches, make the shedding of prions by infected ferrets plausible. It was also observed that regionally targeted exposure of the nasopharyngeal mucosa resulted in an increased attack rate when compared with oral exposure. The latter finding suggests that nasal exposure enhances permissiveness to CWD infection. The ferret model has further potential for investigation of portals for initiation of CWD infection.
Introduction
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) affecting mule deer, white-tailed deer, elk and moose (Baeten et al., 2007; Sigurdson, 2008; Williams, 2005; Williams & Miller, 2002). CWD has been detected in both farmed and free-ranging cervid species in 22 states in the USA and two Canadian provinces, and in South Korea (Kim et al., 2005; Sohn et al., 2002). Although first detected in northern Colorado and southern Wyoming, its aetiological origin remains uncertain (Williams, 2005; Williams & Young, 1980). The facile progressive spread of CWD has called attention to its mechanism of transmission and its potential to transgress species barriers. Whilst the capacity of CWD prions to infect several non-cervid species (including sheep, goats, voles, field mice, hamsters, ferrets, cynomolgus macaques and squirrel monkeys) after intracerebral (i.c.) inoculation has been reported (Bartz et al., 1998; Hamir et al., 2005, 2006; Heisey et al., 2010; Kurt et al., 2011; Marsh et al., 2005; Race et al., 2009; Raymond et al., 2007), much less is known regarding the susceptibility of predator/scavenger species such as mustelids, canids and felids to CWD following presumed natural, oral–nasal exposure.
CWD has helped to increase interest in mucosal and haematogenous routes of prion infection (Andréoletti et al., 2000; Haley et al., 2009a; Haybaeck et al., 2011; Kincaid et al., 2012; Maignien et al., 1999; Sigurdson et al., 1999). Studies of CWD susceptibility in sympatric rodent and mustelid species suggest their potential to contribute to CWD epidemiology (Heisey et al., 2010; Kurt et al., 2009). Moreover, several studies in the transmissible mink encephalopathy (TME) and scrapie systems have focused attention on the nasal cavity and associated epithelia (Bessen et al., 2010; Denkers et al., 2010; Kincaid & Bartz, 2007; Kincaid et al., 2012; Sbriccoli et al., 2009) as conduits for prion entry and exit. Thus, the pharyngeal cavity, including its communications with the upper respiratory tract and associated lymphoid structures, presents a variety of possibilities for transduction, excretion and transmission of CWD prions.
Bartz et al. (1998) first demonstrated that ferrets are susceptible to CWD following i.c. inoculation. Sigurdson et al. (2008) demonstrated a species barrier following oral challenge with deer-origin CWD prions. Subsequent work by Perrott et al. (2012) demonstrated that this barrier was obviated upon second passage, as judged by enhanced susceptibility via mucosal exposure and decreased survival time. Two strains of ferret CWD were described that are readily transmissible by the oral route (Perrott et al., 2012). Only one strain showed accumulation of CWD prion protein (PrPCWD) in lymphoid tissues and, unexpectedly, no PrPCWD accumulated in the pharyngeal tonsil of inoculated ferrets. This observation led us to challenge the assumption that the tonsil is an important site for initial transduction of CWD and to investigate further the transmission and pathogenesis of CWD infection in ferrets after mucosal routes of exposure. Here, we report the broad distribution of PrPCWD in ferrets inoculated with the lymphotropic strain of ferret-adapted CWD and the most likely mucosal surfaces to contribute to the acquisition and shedding of ferret-adapted CWD.
Results
Summary data
The strain of ferret-adapted CWD [University of Wisconsin isolate (UWI)] used in these studies demonstrated broad prion distribution in lymphoid tissues following inoculation by the i.c. and oral per os (p.o.) routes (Perrott et al., 2012). The p.o. and oropharyngeal (o.ph.) versus nasopharyngeal (n.ph.) routes of challenge provided the most pertinent information on the peripheral distribution of PrPCWD and are thus emphasized in this report (Tables 1 and S1, available in JGV Online).
Table 1. Passage history and mucosal transmission of CWD (UWI isolate) in ferrets.
na, Not applicable; DR, dorsal recumbency; SR, sternal recumbency.
| Passage no. | Inoculation route | Attack rate* | Mean survival period in days (range) | Clinical course progression† |
| 4 | P.o. | 3/3 (100 %) | 474 (456–483) | Steady |
| 5 | I.c. | 4/4 (100 %) | 265 (256–276) | Steady |
| 5 | P.o. | 2/4 (50 %) | 438 (394–482) | Variable |
| 5 | I.g. | 2/3 (66 %) | 502 (472–532) | Variable |
| 5 | Pharyngeal | 2/4 (50 %) | 540 (450–630) | Variable |
| 5 | Contact | 0/4 (0 %) | na | None |
| 5 | N.ph. (DR) | 4/4 (100 %) | 489 (394–630) | Variable |
| 5 | O.ph. (SR) | 0/4 (0 %) | na | None |
Number of ferrets affected/number of ferrets inoculated.
The clinical course was described as steady if a uniform onset and progressive neurological deficit was observed, and as variable if the onset was spread out and/or the clinical signs appeared non-progressive for periods of the clinical illness.
Mucosal exposures
Variations in mucosal exposure (passage 5) were studied by inoculations that targeted either the upper or lower gastrointestinal tract. Anaesthetized ferrets were positioned for safety and to maximize the effectiveness of the fluid-tight seal in the oesophagus and trachea, thus preventing exposure of the lower respiratory or digestive tracts. Ferrets inoculated via the oral (p.o.), pharyngeal (p.h.) or intragastric (i.g.) route developed CWD with attack rates of 50 % (2/4), 50 % (2/4) and 66 % (2/3), respectively (Table 1). One i.g.-inoculated ferret died for unknown reasons. In comparison with progressive CWD in passage 4 (p.o.) and passage 5 (i.c.) ferrets, mucosal exposure in passage 5 (p.o., p.h. and i.g.) resulted in more variable clinical manifestations of disease characterized by a subtle onset and periods of clinical illness that appeared to be non-progressive. However, neither χ2 nor t-test statistics demonstrated significant differences in attack rate or incubation period among the inoculation groups.
Position during exposure is significant
Observations made during exposure indicated that positioning in dorsal versus sternal recumbency influenced recovery of the inoculum. Redistribution of the inoculum onto the nasal cavity mucosa occurred in dorsal recumbency (n.ph. exposure) but not in sternal recumbency (o.ph. exposure). As a consequence, ferrets in the p.o. versus p.h. exposure groups were redesignated as n.ph. versus o.ph. The attack rate was 100 % (4/4) in the n.ph. exposure group (dorsal recumbency), whereas in the o.ph. exposure group (sternal recumbency), no animals (0/4) developed CWD (significant difference at P = 0.0455, χ2 test) (Fig. 1b). The shorter survival time in animals exposed via the n.ph. versus the i.g. route was not statistically significant (Fig. 1b). Thus, it appeared that the regions of the pharynx most exposed to the inoculum impacted strongly on the efficacy of CWD transmission (Fig. 1).
Fig. 1.
Survival of ferrets following challenge by mucosal surface exposure to PrPCWD. (a) Ferret survival for the p.h. (p.h._inoc), i.g. (i.g._inoc) and p.o. (p.o._inoc) routes of exposure. (b) Survival data for the same ferrets after redesignation (see Results) to n.ph. (p.o._dorsal) and o.ph. (p.o._sternal) (dorsal and sternal recumbancy, respectively) exposure versus i.g. exposure (i.g._inoc). The i.c. (i.c._inoc) group was invariant. Ferret survival for the p.o._sternal and control groups overlapped.
PrPCWD in the brain of infected ferrets
PrPCWD was demonstrated by both immunohistochemistry (IHC) and Western blotting (WB) in the brains of all ferrets that developed clinical signs of disease. In all groups, PrPCWD occurred as coarse to punctate granular aggregates similar to those described previously (Sigurdson et al., 2008). PrPCWD was more prominent in the grey matter, associated with neuron support cells and the glia limitans. PrPCWD was distributed symmetrically in ferrets inoculated by mucosal routes (Table S1, Fig. 2). In some situations, WB gave clearer results in the forebrain than IHC. Differences between IHC and WB scores arose when cellular prion protein (PrPC) staining was identified by IHC. PrPC lacks a granular, punctate appearance, and PrPCWD was always assessed in the context of control slides. PrPCWD could usually be discriminated by IHC, even when superimposed on the uniform background ‘blush’ contributed by PrPC. IHC was especially sensitive in non-neural tissues, whereas WB seemed more sensitive in the forebrain and in some sensory structures that contained abundant PrPC. The distribution and discrimination of PrPCWD by IHC was enhanced by perfusion fixation.
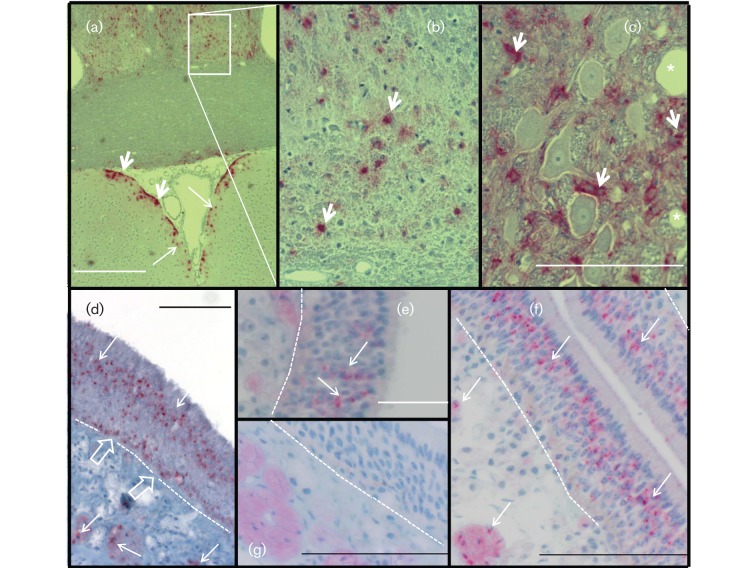
Fig. 2.
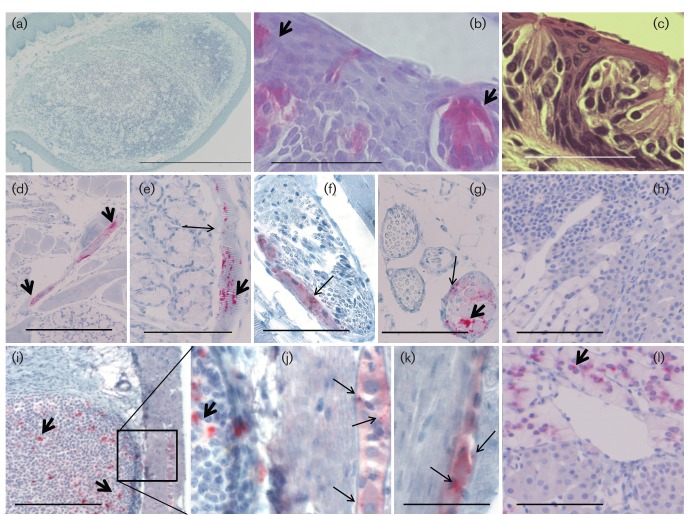
PrPCWD in the brain and upper respiratory tract epithelia. (a–c) IHC detection (RedMapstaining) demonstrated PrPCWD as punctate dots (long arrows) and coarse aggregates (short arrows) in the midbrain (a, b) and brainstem ganglion (c) of a ferret inoculated by the n.ph. route. There was symmetrical distribution of PrPCWD in the thalamic region. PrPCWD was associated with neurone support cells (short arrows) and a spongiform lesion (*) of brainstem ganglion. (d) PrPCWD in the olfactory cell layer and in unmyelinated nerves (long arrows) beneath the olfactory epithelium of ferret no. 538 inoculated by the i.c. route. PrPCWD was associated with the basal cell layer (open arrows) adjacent to the basement membrane (dotted line). (e, f) PrPCWD associated with the olfactory cell layer of ferret nos 521 (e) and 540 (f), inoculated by the i.g. and n.ph. routes, respectively. (g) Olfactory epithelium of a CWD-negative ferret. Bars, 500 µm (a); 100 µm (c, f, g); 50 µm (d, e).
The distribution of PrPCWD in the brains of ferrets inoculated by the i.g. and n.ph. routes was also investigated by regional dissection and WB. PrPCWD was consistently demonstrated in the brainstem, cerebellar peduncle, ventral midbrain pons, dorsal midbrain colliculus, thalamus/hypothalamus and olfactory bulb (Fig. 3). PrPCWD was weakly demonstrated or absent in the hippocampus, posterior cerebral cortex, cerebral cortex, anterior olfactory cortex and lateral olfactory tract. Accumulation of PrPCWD was usually greater in hindbrain than forebrain regions. Although the attack rate suggested that CWD infection resulted from non-overlapping exposure routes (p.h. vs i.g.), PrPCWD distribution patterns were not sufficiently uniform within or between groups for the routes of exposure to be linked with characteristic distribution patterns (Table S1). In some animals inoculated by differing routes, however, prominent regional depositions of PrPCWD were observed, such as localization in the olfactory bulb or olfactory epithelium (see below). Ferret no. 532 (Fig. 3, Table S1) with n.ph. exposure had large amounts of PrPCWD in the olfactory bulb, whereas the hindbrain versus forebrain PrPCWD distribution seen in the majority of ferrets is illustrated by ferret no. 521 with i.g. exposure (Fig. 3, Table S1).
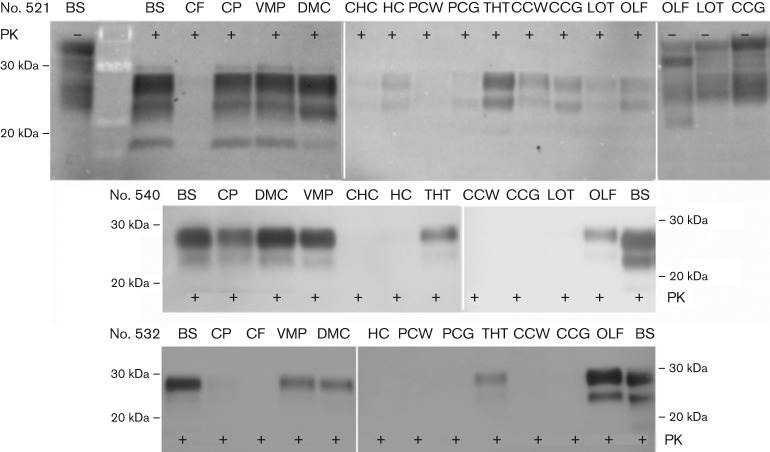
Fig. 3.
Distribution of PrPCWD in the brain of ferrets inoculated by mucosal routes. Distribution of PrPCWD was compared in the brains of ferrets inoculated by the i.g. (no. 521) versus n.ph. (nos 540 and 532) route. Brainstem (BS), cerebellar peduncle (CP), ventral midbrain pons (VMP), dorsal midbrain colliculus (DMC), thalamus/hypothalamus (THT) and olfactory bulb (OLF) accumulated PrPCWD. PrPCWD was absent or less prominent in cerebellar folia (CF), caudal (CHC) and forward hippocampus (HC), posterior cortex white matter (PCW) and grey matter (PCG), cerebral cortex white matter (CCW) and grey matter (CCG) and lateral olfactory tract (LOT). Proteinase K (PK) digestion was carried out (+) or not (−), as indicated. PrPCWD (PK+) and PrPC (PK−) comparisons were based on 800 and 200 µg tissue equivalents (TEs), respectively.
PrPCWD in the upper respiratory tract and nasopharygeal mucosae
PrPCWD was detected in upper respiratory tract mucosae (Fig. 4). CWD prions were prominent in the olfactory epithelium and to a lesser extent in respiratory epithelium. Punctate immunoreactivity, the hallmark of protease-resistant PrPCWD, was observed in ferret no. 538, inoculated by the i.c. route (Fig. 2). Three glycoforms of ferret PrPCWD have been described previously (Bartz et al.,1998; Sigurdson et al., 2008). The predominant diglycoslyated isoform, relative molecular mass 27–30 kDa, was the most sensitive marker for PK-resistant PrPCWD. The most prominent signal corresponded with the olfactory cell layer. Some evidence for a separate layer of PrPCWD was seen adjacent to the basement membrane, the location of brush cells and progenitor cells. Unmyelinated nerve fibres in the lamina propria also accumulated PrPCWD, as did the frontal sinus mucosa and the mucosa of the nasal turbinate (Fig. 4). Ferrets inoculated by mucosal routes showed accumulation in the central region of the olfactory epithelium, corresponding with the olfactory cell layer. Dissection and WB confirmed that PrPCWD was more strongly associated with olfactory than respiratory mucosae (data not shown). Ferret no. 540 (n.ph. exposure) had relatively large amounts of PrPCWD in the olfactory epithelium (Fig. 2).
Fig. 4.
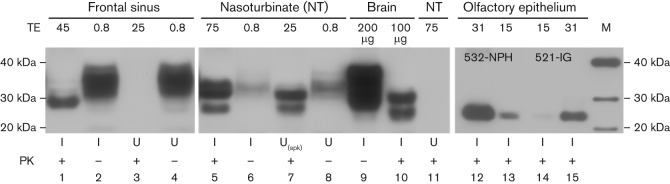
PrPCWD associated with upper respiratory tract and olfactory epithelia. PrPC and PrPCWD in frontal sinus, nasoturbinate (NT) and brainstem of ferrets inoculated (I) by the IC route (lanes 1–11) were compared, as shown in this composite WB. Uninoculated (U) CWD-negative ferrets showed no PrPCWD accumulation. TEs used to demonstrate PrPC and PrPCWD are given in mg unless indicated otherwise. A negative sample, spiked (spk) with 200 µg TE of CWD-positive brain, showed recovery of PrPCWD by sodium phosphotungstic acid (NaPTA) precipitation (lane 7). Dissection separation of respiratory from olfactory tissue demonstrated that PrPCWD was more associated with olfactory tissue (data not shown). More PrPCWD was demonstrated in the olfactory epithelium of ferret no. 532 (lanes 12 and 13) inoculated by the n.ph. route than this location in ferret no. 521 (lanes 14 and 15) inoculated by the i.g. route. M, Molecular mass markers (kDa).
PrPCWD in the tongue and oropharyngeal mucosae
PrPCWD was demonstrated in the dorsal mucosal surface of the tongue by dissection and WB (Fig. 5). When an area containing obvious taste buds was not included in the dissection, the body of the tongue, but not the pharyngeal surface, demonstrated PrPCWD (Fig. 5). In the superficial layer of the tongue, PrPC and PrPCWD were demonstrated by IHC mainly in the taste buds (Fig. 6). PrPCWD was also demonstrated sparsely in individual muscle fibres of the tongue and occasionally in nerves and small nerve ganglia (Fig. 6).
Fig. 5.
PrPCWD associated with the digestive tract, salivary gland and vomeronasal organ. PrPCWD was detected in the vomeronasal organ (VNO, lane 1), brainstem (lanes 2–4), salivary gland (Sal-Gl, lane 5) and the tongue (lanes 7–10) of ferrets inoculated (I) by the i.c. route, as shown in this composite WB. An uninoculated (U) CWD-negative ferret showed no PrPCWD accumulation (lane 6). Tongue epithelium from a negative ferret, spiked (spk) with 200 µg TE of CWD-positive brain, showed recovery of PrPCWD by NaPTA precipitation (lane 8). PrPCWD was detected in Peyer’s patches (PP) of ferrets inoculated by the p.o. and i.c. routes (lanes 11, 12 and 17), in the gut wall opposite Peyer’s patches (OPP) in ferrets inoculated by the p.o., i.c. and n.ph. routes (lanes 13, 14 and 18) and equally in the serosal (JEJS) and mesenteric (JEJM) borders of the jejunum of an i.c.-inoculated ferret (lanes 15 and 16, respectively). PrPCWD was recovered from tongue muscle (TM) but not tongue epithelium (TEP) in a trial that evaluated the most superficial layer of epithelium (lanes 9 and 10). TEs are given in mg unless indicated otherwise. Mr, Molecular mass markers (kDa). The molecular mass markers were also included in lane 2 together with the brainstem sample.
Fig. 6.
Location of PrPCWD in peripheral tissues. PrPCWD was revealed as punctate dots (long arrows) and coarse aggregates (short arrows) by IHC (RedMap staining). (a) CWD-positive ferrets, inoculated by the n.ph. or any other route, did not accumulate PrPCWD in the tonsil. (c) Haematoxylin and eosin staining of taste bud and taste pore. (b, d–g, i–l) PrPCWD was seen in tastebuds (b), muscle (d, e), nerve bundles (f), ganglia (g), Peyer’s patches [i, j (amplification of inset)], myenteric plexus (j, k) and adrenal medulla (l) of ferrets inoculated by the n.ph. route. (h) PrPCWD was not present in uninoculated ferrets, for example the adrenal medulla of a negative ferret. Disease-specific PrPCWD, with a punctate appearance, was superimposed on the PrPC uniform ‘blush’ in sensory structures/nervous tissue (b, f, g, j, k). Bars, 1.0 mm (a); 100 µm (b, c, k); 400 µm (d, i); 200 µm (e–h, l).
PrPCWD in salivary glands and other epithelial/glandular tissues
In the parotid, mandibular and lingual salivary glands, PrPCWD accumulated in the nerve cell bodies of ganglia. In the adrenal gland, small nerve ganglia and chromaffin cells in the medulla accumulated PrPCWD (Fig. 6). In the pancreas, PrPCWD mainly targeted small nerve ganglia and cells in the islets of Langerhans (data not shown). Thus, PrPCWD principally targeted neural and neuroendocrine rather than epithelial elements of glandular tissues.
PrPCWD in lymph nodes and peripheral lymphoid tissues
PrPCWD was detected in the germinal centres and lymphoid follicles of retropharyngeal, mandibular, mesenteric, colonic, duodenal, gastric and ileocecal lymph nodes. PrPCWD was widespread in the lymphoid system, accumulating in the spleen and Peyer’s patches (Table S1, Fig. 6). PrPCWD was also associated with foci of inflammatory cells in seemingly ectopic locations such as the lachrymal gland (data not shown). Interestingly, in no animal was PrPCWD demonstrated in the palatolingual tonsil, a prominent feature of the ferret caudal pharynx (Fig. 6).
Other sensory epithelia
PrPCWD was demonstrated but close to the limit of detection by NaPTA-enhanced WB in the vomeronasal organ (Fig. 5), a location with implications for transmission. In contrast, PrPCWD was abundant in the retina (data not shown).
PrPCWD in peripheral nerves and ganglia
Although PrPCWD was rarely demonstrated in peripheral nerves by IHC, it was detected in dorsal root ganglia associated with the pectoral (cervical cord C4–C6) and pelvic (lumbosacral cord L5–S1) limbs and in ganglia of the vagus nerve. PrPCWD was also seen in submucosal ganglia and myenteric ganglia (Fig. 6) and in the vagus and sciatic nerve (data not shown).
Discussion
An interesting finding of these studies was the apparent enhanced permissiveness to infection associated with exposure targeted to the nasopharyngeal mucosa. Other studies employing either nasal or aerosol prion exposure have also inferred enhanced efficacy over oral inoculation (Bessen et al., 2010; Denkers et al., 2010; Kincaid & Bartz, 2007; Kincaid et al., 2012). Nasal regions adjacent to the pharynx, exposed through anaesthetic position variation, implicated the upper respiratory tract mucosa as the site most likely to facilitate prion entry. Whilst these were clear- cut results (100 vs 0 %), relatively few ferrets were challenged in experiments not originally designed to show an effect due to positioning. Thus, there was some statistical support for differentiating nasopharyngeal entry from oropharyngeal entry but only a trend to suggest that the n.ph. route was more permissive than the lower digestive tract (100 vs 50 %), by i.g. or oral routes. The limited number of ferrets in the study and the end-point-based assessment of PrPCWD distribution warrant a cautious interpretation of results.
Whilst superficial lesions in the lingual mucosa have been shown to greatly facilitate oral infection by CWD, scrapie and TME prions (Bartz et al., 2005; Carp, 1982; Denkers et al., 2011), no trauma to the mucosal surface was required for efficient CWD prion infection in ferrets exposed by the n.ph. route. However, studies by Bessen et al. (2012) have shown that damage to nasal epithelial integrity can enhance susceptibility by this route of prion exposure.
In contrast to our observations in ferrets, the tonsils of deer show early and sustained accumulation of PrPCWD, affording reliable antemortem diagnosis (O’Rourke et al., 2003; Schuler et al., 2005; Sigurdson et al., 1999; Spraker et al., 2002c; Wild et al., 2002) and suggesting the tonsil as a site for initiation of infection (Sigurdson et al., 1999; Spraker et al., 2002a). Our studies suggest that adjacent anatomical locations in addition to the tonsil are likely to be permissive for prion entry in the native host. Tonsillar tissue in cervids is extensively folded and recessed in crypts, whereas the ferret tonsil is prominent and exposed in the caudal pharynx. Whilst the tonsil-associated epithelial barrier may differ between ferrets and deer, a reason for the lack of PrPCWD in the ferret tonsil was not determined.
Lack of infection after o.ph. exposure was most likely a mucosal barrier effect due to stratified squamous epithelium. In contrast, permissive respiratory mucosa is pseudo-stratified, extensive and endowed with nerves and nasal-associated lymphoid tissue. IHC confirmed the presence of PrPCWD in taste buds and the possibility of PrPCWD shedding from this epithelial surface. Taste buds accumulate prions centrifugally following i.c. inoculation of hamsters with the HY strain of TME (DeJoia et al., 2006). Our study extends these findings to mucosal challenge and another host. Lack of disease transmission following o.ph. exposure alone indicates that taste buds were not an entry portal under our challenge conditions.
The trend for a longer incubation period following i.g. exposure may have resulted from a longer migration distance for PrPCWD, prior to entry to the CNS, if trafficking of PrPCWD occurred in nerves. If PrPCWD was distributed by lymphatics/blood, then the trend towards longer incubation may have been dose related, as evidenced by the attack rate. Variable accumulation of PrPCWD in Peyer’s patches, mucosal ganglia and the mesenteric and root of mesentery lymph nodes of these ferrets did not enable the initiation site to be resolved.
The distribution of PrPCWD in ferrets inoculated with the UWI isolate of ferret CWD (Perrott et al., 2012) was similar to that in deer (Sigurdson et al., 2001, 2002; Spraker et al., 2002a). PrPCWD was demonstrated along vagal pathways and in dorsal root ganglia, mucosal ganglia, neuroendocrine tissues and peripheral nerves, indicating that nerve transport may also be an important means of PrPCWD trafficking in ferrets. PrPCWD was seen in germinal centres in lymphoid tissues, ectopic mononuclear cell foci, muscle, ganglia within exocrine and endocrine glands, and specialized sensory epithelia. CWD prions are broadly distributed in peripheral tissues, body fluids and excreta of infected cervids (Angers et al., 2006, 2009; Haley et al., 2009a, b; Mathiason et al., 2006; Sigurdson et al., 2001, 2002; Spraker et al., 2002a), facilitating environmental contamination. Although we did not examine secreta or excreta, the widespread distribution of PrPCWD in ferrets resembles that in deer.
Other prion transmission studies (Bessen et al., 2010, 2012; Denkers et al., 2010; Haybaeck et al., 2011; Kincaid & Bartz, 2007; Kincaid et al., 2012; Sbriccoli et al., 2009) provide a basis for further examination of prion transmission via the nasal mucosa. The greater efficacy of droplet application of TME prions to the external nares of hamsters compared with conventional oral inoculation provided some of the first evidence for prion entry by the respiratory route (Kincaid & Bartz, 2007). The earliest site of prion replication was in the nasal-associated lymphoid tissue (Kincaid & Bartz, 2007). Sbriccoli et al. (2009) extended these observations in hamster scrapie, ruling out direct extension from the olfactory mucosa to the brain (cranial nerve 1) but opening up the possibility of trigeminal (cranial nerve 5) involvement. The efficient transmission of prions by aerosols (Denkers et al., 2010; Haybaeck et al., 2011) reinforces the concept of a permissive nasal mucosal portal of prion entry. Whilst the exact channel(s) of prion entry for aerosolized versus droplet challenge may differ, the recent studies of Kincaid et al. (2012) demonstrated both paracellular and transcellular (M cell) mechanisms of transport across an initial entry site in the nasal mucosa of hamsters.
Ferret nos 540 and 532 (n.ph. exposure) in the current study had relatively large amounts of PrPCWD in the olfactory epithelium and olfactory bulb, respectively. Although we investigated the lateral olfactory tract projection(s) described in ferrets (Dennis & Kerr, 1975) in order to show distribution patterns reflective of n.ph. versus i.g. exposure, no strong conclusions regarding the initiation of CWD infection could be drawn. It was tenable that these animals may have acquired CWD via the olfactory mucosa. It was also possible that initiation of infection was different for ferrets in the same exposure group, given that nasal cavity mucosae contain nociceptor-like cells (brush cells) that interact with the trigeminal nerve and defensive host-cell populations. The hamster model of TME implicates regional lymphocentre involvement (Kincaid & Bartz, 2007), yet the very early observations in this challenge system did not implicate nasal-associated lymphoid tissue (Kincaid et al., 2012). Sbriccoli et al. (2009) concluded that, in hamsters, the brainstem nuclei acquire PrPCWD first, and centripetal spread from the nares to the brain via the olfactory nerve does not occur (Sbriccoli et al., 2009). However, as the studies of Bessen et al. (2010) demonstrated centrifugal excretion of prions via nasal olfactory receptor (neuroepithelial) cells, it is clear that more work is merited to understand better these intriguing aspects of prion pathogenesis.
Our results indicated that CWD prions in the ferret behave similarly to those in the hamster model of TME and support the likelihood that deer potentially shed prions from olfactory mucosae, as distribution of PrPCWD to olfactory regions of the brain has been described by Spraker et al. (1997, 2002b). PrPCWD has also been described in the nasal septum of mule deer in association with lymphoid follicles (Spraker et al., 2002a). Interestingly, in sheep with scrapie, the olfactory mucosa appears to be spared (Corona et al., 2009), in contrast to hamsters with sheep scrapie, which show prions in the olfactory mucosa (Sbriccoli et al., 2009). With ruminant hosts seemingly differing in terms of the distribution of prions in the olfactory mucosa, it is interesting to note that ferret CWD appears to share similarities with hamster TME and hamster scrapie.
Ferret PrPCWD is highly associated with mucosal surfaces, giving rise to communicable olfactory secretions and intestinal excretions. Intestinal proteases do not fully degrade PrPCWD, even after prolonged incubation (M. R. Perrott, unpublished data), suggesting that environmental contamination via excreta is feasible. It would be interesting to investigate whether or not ferret CWD is transmissible by natural contact, using a trial design with a wide range of ferret-to-ferret interactions and fomite exposure. Notwithstanding uncertainty regarding lateral or environmental spread of ferret CWD, this host is a tractable species with which to investigate phenomena associated with prion transmission and CWD.
Methods
Inocula.
Brain homogenates from CWD-infected mule deer and the passages in ferrets have been described previously (Bartz et al., 1998; Perrott et al., 2012; Sigurdson et al., 2008). The infectious deer brain material was a gift from Drs Elizabeth Williams and Michael Miller (Colorado Division of Wildlife, CO, USA). Brain tissue from ferrets was harvested aseptically and homogenized (10 and 25 %, w/v), as described previously (Perrott et al., 2012).
Ferrets.
Domestic ferrets (Mustela putorius furo) were obtained from Marshall Farms (North Rose, NY, USA) as neutered weanlings at 6–8 weeks of age. The husbandry and passage histories of the ferrets prior to passage 5 for i.c., i.p. and p.o. inoculation have been described elsewhere (Perrott et al., 2012; Sigurdson et al., 2008). For general anaesthesia, ferrets were pre-medicated/induced with 0.05 mg atropine (Vedco) kg−1 and 20 mg telazol (Fort Dodge Laboratories) kg−1, intubated and maintained with isoflurane/O2. Approval for the studies was obtained from the Animal Care and Use Committee, Colorado State University.
Pharyngeal exposure.
This experimental strategy was designed to atraumatically expose the oral pharyngeal mucosa to CWD inoculum without exposure of the lower gastrointestinal tract. An endotracheal tube was placed and cuff inflated. A composite device – a no. 12 French feeding tube and inflatable Foley’s catheter – was inserted to prevent fluid from the pharynx entering the oesophagus. Briefly, this device employed a 4–10 mm diameter (‘feeding-type’) catheter such that when installed the flared end blocked the oesophageal entrance. The Foley catheter was installed inside the feeding tube, with the inflatable end extending from the feeding tube. The devices were glued together and dead space was eliminated with silicon. The composite feeding tube was lubricated, fitted and the Foley catheter inflated, ensuring retention of inoculum in the oral cavity. One millilitre of 25 % (w/v) brain inoculum was placed in the caudal pharynx and redistributed over the tongue to increase exposure. After 45 min of exposure, the inoculum was recovered and the oral cavity rinsed to remove residual inoculum. Ferrets were positioned in dorsal recumbency, which allowed exposure of the nasopharygeal mucosa, or in sternal recumbency, which did not.
I.g. exposure.
The lubricated cut end of a no. 12 French feeding catheter was passed as far as the distal oesophagus. A narrow paediatric catheter was introduced through the feeding catheter, into the stomach, and 1 ml of the 25 % (w/v) suspension of inoculum was injected and flushed into the stomach with saline.
Controls
Positive controls.
One group of ferrets (n = 4) for the pharyngeal exposure experiment was administered 1 ml 25 % (w/v) brain homogenate p.o. Juvenile ferrets were restrained while the suspension was administered to the caudal pharynx and until swallowing had occurred. A second group (n = 4) was inoculated with 300 µl 10 % (w/v) brain homogenate by the i.c. route.
Negative controls.
One group of ferrets (n = 4) was administered normal (CWD-negative) ferret brain suspension by both the i.c. and p.o. route.
Experimental groups.
The experimental groups were initially p.h. and i.g. (experimental) versus p.o. and i.c. (positive controls). After reassignment, the groups were n.ph., o.ph. and i.g. (experimental) versus i.c. (positive control).
Statistical analysis.
Survival charts and the results of statistical analyses for reporting significance were performed using GraphPad Prism version 4.03 for Windows (GraphPad Software, http://www.graphpad.com).
WB.
Tissues samples were weighed and homogenized to 10 % (w/v) in PBS (pH 7.4). Benzonase and MgCl2 (Sigma) were added to 100 U ml−1 and 1.5 mM, respectively, and incubated for 45 min at 37 °C. N-Lauroyl sarcosine (4 %, w/v) in PBS was added (1 : 1) to samples and incubated for 30 min at room temperature. PK (Invitrogen) was added (50 µg ml−1) and incubated for 1 h with constant agitation. Digestion was halted with Pefabloc SC (4 mM final concentration; Fluka). Brain region analysis was based on 800 µg TEs for PrPCWD and 125 µg TE for PrPC, as described previously (Perrott et al., 2012). Modifications to extraction protocols for nerves and solid tissues were required (Moya et al., 2004), and up to 62.5 mg TE was analysed using an extended protocol and NaPTA.
NaPTA modification.
After low-speed clarification (1000 g for 1–2 min), PrPCWD was precipitated using 0.3 % (w/v) NaPTA and centrifugation (18 000 g for 30 min). Pellets were resuspended in 15 µl 0.1 % (w/v) N-lauroyl sarcosine and 6.25 µl 4× sample buffer (Invitrogen) was added to the resuspended pellets, which were then boiled and separated by electrophoresis (12 % NuPAGE Bis-Tris polyacrylamide; Invitrogen). Proteins were transferred to PVDF membranes (Bio-Rad) and detected used standard methods (Wadsworth et al., 2001).
Detection.
The primary mAb BAR-224 (0.066 µg ml−1; kindly supplied by Jacques Grassi, CEA, France) was detected using secondary goat anti-mouse HRP-conjugated Fab fragment (0.045 µg ml−1; Jackson Laboratories) and a chemiluminescent kit (ECL plus; GE Healthcare), followed by film development and fluorescent signal capture (STORM 860; Molecular Dynamics).
Brain region analysis.
Systematic dissection and semi-quantitative analysis of PrPCWD from the different brain regions of infected ferrets were performed as described previously (Perrott et al., 2012). Briefly, this involved harvesting a standard set of samples from precisely determined anatomical locations and preparing them for WB as described above.
IHC.
Tissue samples were fixed by immersion in 10 % (v/v) neutral buffered formalin, or the ferret was perfused with 4 % (w/v) paraformaldehyde-lysine-periodate (PLP) and post-fixed in PLP for 48–72 h (Liu et al., 2003; McLean & Nakane, 1974). Tissues were immersed in 88 % formic acid (1 h) and then rinsed overnight in water, equilibrated to 70 % ethanol and processed. Decalcification was by extended immersion in 10 % formic acid. Tissue sections were mounted onto positively charged glass slides, deparaffinized and rehydrated. Sections were treated using all or a subset of the methods listed below, optimized by tissue type: formic acid (88 %, v/v; Sigma) immersion for 5–15 min, hydrated autoclaving at 121 °C for 10–15 min in target antigen-retrieval solution (Dako) and proprietary CC1 and blocking solutions (Ventana Medical Systems). Automated IHC, performed on a Discovery immunostainer (Roche), used mAb BAR-224 (0.25–4.0 µg ml−1), biotinylated universal secondary antibody (Roche), alkaline phosphatase–streptavidin conjugate, substrate chromagen (RedMap; Roche) and a counterstain. Positive- and negative-control tissue sections were included in each run. Several mAb variations, including omission of the primary reagent, substitution of an irrelevant primary reagent and substitution of complementary PrP antibodies, targeting different epitopes, confirmed the specificity of the staining reaction for each tissue type.
Acknowledgements
The authors wish to acknowledge the help and contributions of Dr Jason Bartz, Mr Bruce Cummings, Dr Daniel Gould, Dr Anthony Kincaid, Dr Terry Spraker and Mr Robert Zink. BAR-224 was kindly supplied by Dr Jacques Grassi. Thanks are also due to the support personnel of the Prion and Retrovirus Research Laboratory, especially Ms Jeanette Hayes-Klug and Ms Sheila Hayes for coordinating the ferret care. The studies were funded by National Institutes of Health contracts NO1-AI-25491 and R01-NS-061902.
Footnotes
A supplementary table is available with the online version of this paper.
References
- Andréoletti O., Berthon P., Marc D., Sarradin P., Grosclaude J., van Keulen L., Schelcher F., Elsen J. M., Lantier F. (2000). Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81, 3115–3126 [DOI] [PubMed] [Google Scholar]
- Angers R. C., Browning S. R., Seward T. S., Sigurdson C. J., Miller M. W., Hoover E. A., Telling G. C. (2006). Prions in skeletal muscles of deer with chronic wasting disease. Science 311, 1117 10.1126/science.1122864 [DOI] [PubMed] [Google Scholar]
- Angers R. C., Seward T. S., Napier D., Green M., Hoover E., Spraker T., O’Rourke K., Balachandran A., Telling G. C. (2009). Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 15, 696–703 10.3201/eid1505.081458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten L. A., Powers B. E., Jewell J. E., Spraker T. R., Miller M. W. (2007). A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43, 309–314 [DOI] [PubMed] [Google Scholar]
- Bartz J. C., Marsh R. F., McKenzie D. I., Aiken J. M. (1998). The host range of chronic wasting disease is altered on passage in ferrets. Virology 251, 297–301 10.1006/viro.1998.9427 [DOI] [PubMed] [Google Scholar]
- Bartz J. C., Dejoia C., Tucker T., Kincaid A. E., Bessen R. A. (2005). Extraneural prion neuroinvasion without lymphoreticular system infection. J Virol 79, 11858–11863 10.1128/JVI.79.18.11858-11863.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen R. A., Shearin H., Martinka S., Boharski R., Lowe D., Wilham J. M., Caughey B., Wiley J. A. (2010). Prion shedding from olfactory neurons into nasal secretions. PLoS Pathog 6, e1000837 10.1371/journal.ppat.1000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen R. A., Wilham J. M., Lowe D., Watschke C. P., Shearin H., Martinka S., Caughey B., Wiley J. A. (2012). Accelerated shedding of prions following damage to the olfactory epithelium. J Virol 86, 1777–1788 10.1128/JVI.06626-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I. (1982). Transmission of scrapie by oral route: effect of gingival scarification. Lancet 319, 170–171 10.1016/S0140-6736(82)90421-4 [DOI] [PubMed] [Google Scholar]
- Corona C., Porcario C., Martucci F., Iulini B., Manea B., Gallo M., Palmitessa C., Maurella C., Mazza M. & other authors (2009). Olfactory system involvement in natural scrapie disease. J Virol 83, 3657–3667 10.1128/JVI.01966-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoia C., Moreaux B., O’Connell K., Bessen R. A. (2006). Prion infection of oral and nasal mucosa. J Virol 80, 4546–4556 10.1128/JVI.80.9.4546-4556.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers N. D., Seelig D. M., Telling G. C., Hoover E. A. (2010). Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol 91, 1651–1658 10.1099/vir.0.017335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers N. D., Telling G. C., Hoover E. A. (2011). Minor oral lesions facilitate transmission of chronic wasting disease. J Virol 85, 1396–1399 10.1128/JVI.01655-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis B. J., Kerr D. I. (1975). Olfactory bulb connections with basal rhinencephalon in the ferret: an evoked potential and neuroanatomical study. J Comp Neurol 159, 129–148 10.1002/cne.901590108 [DOI] [PubMed] [Google Scholar]
- Haley N. J., Mathiason C. K., Zabel M. D., Telling G. C., Hoover E. A. (2009a). Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS ONE 4, e7990 10.1371/journal.pone.0007990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Seelig D. M., Zabel M. D., Telling G. C., Hoover E. A. (2009b). Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE 4, e4848 10.1371/journal.pone.0004848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir A. N., Kunkle R. A., Cutlip R. C., Miller J. M., O’Rourke K. I., Williams E. S., Miller M. W., Stack M. J., Chaplin M. J., Richt J. A. (2005). Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J Vet Diagn Invest 17, 276–281 10.1177/104063870501700313 [DOI] [PubMed] [Google Scholar]
- Hamir A. N., Kunkle R. A., Cutlip R. C., Miller J. M., Williams E. S., Richt J. A. (2006). Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J Vet Diagn Invest 18, 558–565 10.1177/104063870601800606 [DOI] [PubMed] [Google Scholar]
- Haybaeck J., Heikenwalder M., Klevenz B., Schwarz P., Margalith I., Bridel C., Mertz K., Zirdum E., Petsch B. & other authors (2011). Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathog 7, e1001257 10.1371/journal.ppat.1001257 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Heisey D. M., Mickelsen N. A., Schneider J. R., Johnson C. J., Johnson C. J., Langenberg J. A., Bochsler P. N., Keane D. P., Barr D. J. (2010). Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 84, 210–215 10.1128/JVI.00560-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-Y., Shon H.-J., Joo Y.-S., Mun U.-K., Kang K.-S., Lee Y.-S. (2005). Additional cases of chronic wasting disease in imported deer in Korea. J Vet Med Sci 67, 753–759 10.1292/jvms.67.753 [DOI] [PubMed] [Google Scholar]
- Kincaid A. E., Bartz J. C. (2007). The nasal cavity is a route for prion infection in hamsters. J Virol 81, 4482–4491 10.1128/JVI.02649-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid A. E., Hudson K. F., Richey M. W., Bartz J. C. (2012). Rapid transepithelial transport of prions following inhalation. J Virol 86, 12731–12740 10.1128/JVI.01930-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt T. D., Telling G. C., Zabel M. D., Hoover E. A. (2009). Trans-species amplification of PrPCWD and correlation with rigid loop 170N. Virology 387, 235–243 [DOI] [PubMed] [Google Scholar]
- Kurt T. D., Seelig D. M., Schneider J. R., Johnson C. J., Telling G. C., Heisey D. M., Hoover E. A. (2011). Alteration of the chronic wasting disease species barrier by in vitro prion amplification. J Virol 85, 8528–8537 10.1128/JVI.00809-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. G., Brown D. A., Fraser J. R. (2003). Immunohistochemical comparison of anti-prion protein (PrP) antibodies in the CNS of mice infected with scrapie. J Histochem Cytochem 51, 1065–1071 10.1177/002215540305100810 [DOI] [PubMed] [Google Scholar]
- Maignien T., Lasmézas C. I., Beringue V., Dormont D., Deslys J. P. (1999). Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J Gen Virol 80, 3035–3042 [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Kincaid A. E., Bessen R. A., Bartz J. C. (2005). Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 79, 13794–13796 10.1128/JVI.79.21.13794-13796.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason C. K., Powers J. G., Dahmes S. J., Osborn D. A., Miller K. V., Warren R. J., Mason G. L., Hays S. A., Hayes-Klug J. & other authors (2006). Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314, 133–136 10.1126/science.1132661 [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. (1974). Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22, 1077–1083 10.1177/22.12.1077 [DOI] [PubMed] [Google Scholar]
- Moya K. L., Hässig R., Créminon C., Laffont I., Di Giamberardino L. (2004). Enhanced detection and retrograde axonal transport of PrPc in peripheral nerve. J Neurochem 88, 155–160 10.1046/j.1471-4159.2003.02150.x [DOI] [PubMed] [Google Scholar]
- O’Rourke K. I., Zhuang D., Lyda A., Gomez G., Williams E. S., Tuo W., Miller M. W. (2003). Abundant PrPCWD in tonsil from mule deer with preclinical chronic wasting disease. J Vet Diagn Invest 15, 320–323 10.1177/104063870301500403 [DOI] [PubMed] [Google Scholar]
- Perrott M. R., Sigurdson C. J., Mason G. L., Hoover E. A. (2012). Evidence for distinct chronic wasting disease (CWD) strains in experimental CWD in ferrets. J Gen Virol 93, 212–221 10.1099/vir.0.035006-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B., Meade-White K. D., Miller M. W., Barbian K. D., Rubenstein R., LaFauci G., Cervenakova L., Favara C., Gardner D. & other authors (2009). Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15, 1366–1376 10.3201/eid1509.090253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond G. J., Raymond L. D., Meade-White K. D., Hughson A. G., Favara C., Gardner D., Williams E. S., Miller M. W., Race R. E., Caughey B. (2007). Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J Virol 81, 4305–4314 10.1128/JVI.02474-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbriccoli M., Cardone F., Valanzano A., Lu M., Graziano S., De Pascalis A., Ingrosso L., Zanusso G., Monaco S. & other authors (2009). Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol 117, 175–184 10.1007/s00401-008-0474-z [DOI] [PubMed] [Google Scholar]
- Schuler K. L., Jenks J. A., DePerno C. S., Wild M. A., Swanson C. C. (2005). Tonsillar biopsy test for chronic wasting disease: two sampling approaches in mule deer and white-tailed deer. J Wildl Dis 41, 820–824 [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J. (2008). A prion disease of cervids: chronic wasting disease. Vet Res 39, 41 10.1051/vetres:2008018 [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J., Williams E. S., Miller M. W., Spraker T. R., O’Rourke K. I., Hoover E. A. (1999). Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80, 2757–2764 [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J., Spraker T. R., Miller M. W., Oesch B., Hoover E. A. (2001). PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 82, 2327–2334 [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J., Barillas-Mury C., Miller M. W., Oesch B., van Keulen L. J., Langeveld J. P., Hoover E. A. (2002). PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J Gen Virol 83, 2617–2628 [DOI] [PubMed] [Google Scholar]
- Sigurdson C. J., Mathiason C. K., Perrott M. R., Eliason G. A., Spraker T. R., Glatzel M., Manco G., Bartz J. C., Miller M. W., Hoover E. A. (2008). Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 138, 189–196 10.1016/j.jcpa.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Sohn H.-J., Kim J.-H., Choi K.-S., Nah J.-J., Joo Y.-S., Jean Y.-H., Ahn S.-W., Kim O.-K., Kim D.-Y., Balachandran A. (2002). A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci 64, 855–858 10.1292/jvms.64.855 [DOI] [PubMed] [Google Scholar]
- Spraker T. R., Miller M. W., Williams E. S., Getzy D. M., Adrian W. J., Schoonveld G. G., Spowart R. A., O’Rourke K. I., Miller J. M., Merz P. A. (1997). Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33, 1–6 [DOI] [PubMed] [Google Scholar]
- Spraker T. R., Zink R. R., Cummings B. A., Sigurdson C. J., Miller M. W., O’Rourke K. I. (2002a). Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet Pathol 39, 546–556 10.1354/vp.39-5-546 [DOI] [PubMed] [Google Scholar]
- Spraker T. R., Zink R. R., Cummings B. A., Wild M. A., Miller M. W., O’Rourke K. I. (2002b). Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet Pathol 39, 110–119 10.1354/vp.39-1-110 [DOI] [PubMed] [Google Scholar]
- Spraker T. R., O’Rourke K. I., Balachandran A., Zink R. R., Cummings B. A., Miller M. W., Powers B. E. (2002c). Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest 14, 3–7 10.1177/104063870201400102 [DOI] [PubMed] [Google Scholar]
- Wadsworth J. D., Joiner S., Hill A. F., Campbell T. A., Desbruslais M., Luthert P. J., Collinge J. (2001). Tissue distribution of protease resistant prion protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet 358, 171–180 10.1016/S0140-6736(01)05403-4 [DOI] [PubMed] [Google Scholar]
- Wild M. A., Spraker T. R., Sigurdson C. J., O’Rourke K. I., Miller M. W. (2002). Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J Gen Virol 83, 2629–2634 [DOI] [PubMed] [Google Scholar]
- Williams E. S. (2005). Chronic wasting disease. Vet Pathol 42, 530–549 10.1354/vp.42-5-530 [DOI] [PubMed] [Google Scholar]
- Williams E. S., Miller M. W. (2002). Chronic wasting disease in deer and elk in North America. Rev Sci Tech 21, 305–316 [DOI] [PubMed] [Google Scholar]
- Williams E. S., Young S. (1980). Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16, 89–98 [DOI] [PubMed] [Google Scholar]